We present a 64-year-old man diagnosed with Hodgkin's lymphoma at 3 years, who received chemotherapy and several sessions of Mediastinal Radiotherapy (MRT), which caused torrential tricuspid regurgitation (TR), complete heart block (CHB) and porcelain aorta. The patient successfully underwent complex tricuspid repair without cardiac arrest and implantation of an epicardial pacemaker.
Presentamos el caso de un paciente de 64 años que recibió radioterapia mediastinal y quimioterapia por diagnóstico de linfoma a los tres años. Como secuela de la radiación desarrolló insuficiencia tricúspide torrencial, bloqueo cardiaco completo y aorta en porcelana. Tratamos exitosamente al paciente sometiéndolo a una reparación compleja de la válvula tricúspide a corazón latiendo e implantando un marcapasos epicárdico.
Cardiac lesions due to MRT are the product of the response to DNA damage, which activates cell apoptosis and causes a common effect: fibrosis. Classically, pericardium is the most common organ affected (90%, pericardial adhesion, excessive pericardial fluid); however, radiation damages can involve all cardiac structures causing accelerated coronary disease, rhythm alterations (in some cases CHB requiring pacemaker), heart valve disease (HVD), mainly aortic and mitral, vascular disorders including pulmonary artery stenosis, aortic calcification, etc.1,2
Case report64-Year-old male diagnosed with Hodgkin's lymphoma at 3 years old, which received chemotherapy and several sessions of MRT. 13 years ago a dual-chamber pacemaker was placed due to CHB and 11 years ago a pericardial window was performed due to severe pericardial effusion. Subsequently, the patient had recurrent hospitalizations for dyspnoea, ascites, and pleural effusion.
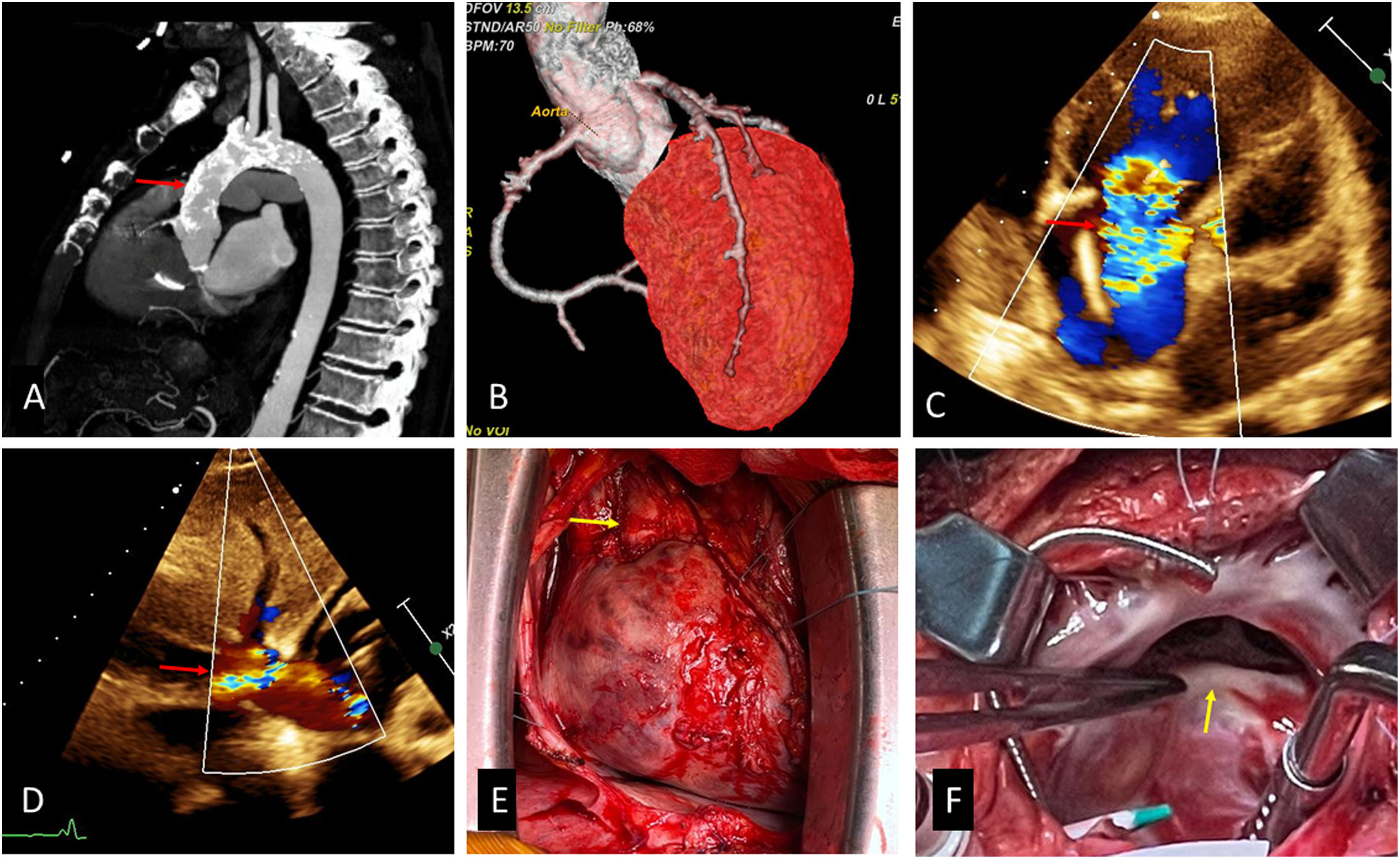
When he was admitted in our hospital reported NYHA III dyspnoea despite treatment with furosemide 120mg and spironolactone 50mg daily. Physical examination showed +++/+++ oedema in the lower limbs, III/VI Systolic murmur at tricuspid focus, decreased vesicular murmur at the bases of both hemithorax. Examination of the abdomen showed the edge of the liver 5cm below the costal margin and shifting dullness, however transaminase and bilirubin levels were normal. Transthoracic echocardiography (TTE) (Fig. 1C and D) showed massive TR (annulus diameter: 32mm, 19mm/m2) and mild aortic regurgitation, left ventricular ejection fraction was normal (60%), right ventricular outflow tract fractional shortening was 48%. Transesophageal echocardiography (TEE) confirmed a torrential primary TR due to restriction of movement of the anterior and posterior leaflets and interference of the pacemaker lead with movement of the septal.
(A) Porcelain aorta, (B) coronary angiotomography, (C) TTE, modified subcostal view, showing massive TR (arrow), (D) reversal flow in inferior vena cava (arrow), (E) heart and cardiac pedicle (arrow), we can observe severe fibrosis an adhesion, (F) tricuspid valve, the anterior and posterior leaflets were thickened and fibrotic.
Computed tomography (Fig. 1A, B) revealed a severe calcification involving ascending, arch and descending aorta (porcelain aorta). Coronary angiotomography showed coronary calcifications without severe lesions.
After an analysis in the heart team, surgical treatment was decided. The surgical plan included peripheral cannulation for cardiopulmonary bypass (CPB), tricuspid valve repair or replacement without aortic cross-clamping, and removal of endocardial pacemaker leads and implantation of a dual-chamber epicardial pacemaker.
Surgical findingsFibrous pericardium was adhered firmly to the entire epicardial surface, mainly in right atrium and cardiac pedicle (Fig. 1E). Ascending aorta had severe calcifications in all its walls. The right atrial wall was thickened and fibrotic. We found the anterior and posterior tricuspid leaflets thickened and retracted (Fig. 1F) and the ventricular pacemaker lead adhered to septal leaflet, these two mechanism caused TR.
Surgical techniqueWe performed the surgery by total median sternotomy. The rights femoral artery and vein and superior vena cava were cannulated to enter vacuum-assisted CPB without aortic cross clamping (beating heart) in normothermia. Superior and inferior vena cava were not snared due to severe pericardial adhesions. After a right longitudinal atriotomy, the endocardial leads were removed. The anterior leaflet and the anterior third of the posterior leaflet were detached from their annular attachment, then we performed leaflets augmentation with bovine pericardium using polypropylene 4/0 (the diameter of the patch was the distance between the anteroseptal commissure and to the end where the posterior veil was detached and its height was the greatest distance between the detached leaflet and the annulus), finally we placed a semi rigid annuloplasty ring (30mm) (Fig. 2A–C).
(A) Surgical sketch showing the anterior (AL) and posterior leaflets (PL) detached from the annulus (arrow). SL=septal leaflet. (B) Leaflets augmentation. (C) Surgical sketch, complete repair. (D) Postoperative TEE showing adequate coaptation surface (red arrow) and mild TR (white arrow).
The patient was disconnected from the mechanical ventilator 8h after surgery; however, he required inotropic support for 5 days due to right ventricular dysfunction. Postoperative TEE showed mild TR (Fig. 2D) at discharge (14 days after surgery). After 6 months follow up, patient is in NYHA functional class II and therapy included furosemide 40mg and spironolactone 25mg daily.
DiscussionLike in our patient, MRT improves cancer survival but increases the risk of developing radiation-induced HVD. The most important factor for developing HVD is the total dose delivered to the heart valves (HVD has been reported to occur in 81% of patients receiving more than 35Gy to the heart).1,2 The precise pathophysiological mechanisms of radiation-induced HVD are not fully understood, radiation causes damage to the interstitial cells of the valves and activation of the inflammatory cascade which releases pro-fibrotic cytokines, these mechanisms induce fibrosis and calcification of the valve apparatus.1,3,4 On the other hand, myocardial fibrosis and vascular involvement can contribute to ventricular remodelling and, consequently, further impair valve function.2
In the vasculature, ionizing radiation causes direct endothelial oxidative damage and induces the release of inflammatory cytokines, these mechanisms cause inflammation, cell death, atherosclerosis with parenchymal damage, and fibrosis. Coronary artery disease can occur within a year or two after doses above 30–35Gy. At lower doses, the latency period is much longer (more than a ten years).5 MRT increases the risk of presenting major cardiovascular events (myocardial infarction, stroke, etc.) and these patients are often younger than those with typical atherosclerotic.5
A retrospective analysis of 261 consecutive patients with prior MRT who underwent valvular operations found a higher operative mortality than predicted by STS risk of mortality (hazard ratio, 2.24 for primary surgeries). Aortic stenosis was the most common obstructive HVD (more than 50% of surgical indications) followed by mitral stenosis.6 TR is the most common right-sided valvular lesion (20.4%), but isolated involvement is rare.2
In our search we found only a case of surgical treatment for isolated TR and CHB after MRT. In this case surgeons performed a De Vega annuloplasty and limited pericardiectomy, and then placed a dual-chamber pacemaker.7 In our case, we decided leaflets augmentation plus ring annuloplasty because the anterior leaflet was severity retracted and the use of a pericardial patch to enlarge the leaflets effectively increases the surface of coaptation. Dreyfus et al. reported a similar technique using autologous pericardium to address severe tethering in functional TR and in 6–20 months follow-up available on five patients, no one had greater than trace TR.8 Choi et al. also report the enlargement of the anterior leaflet and part of the posterior leaflet as a safe technique for the treatment of severe tethering of the tricuspid valve, they used an elliptic pericardial patch before implantation of a rigid annuloplasty ring and suggest that failure to achieve complete leaflet coaptation with the saline test, even with the smallest No. 28 ring size, is an indication of leaflet augmentation.9
Although it is true that patients who undergo tricuspid valve replacement usually have long-standing valve disease and significant right ventricular dysfunction, it is well established that tricuspid valve replacement (biological or mechanical) has high mortality rates (>10%) and complications; that is why we decided not to replace the valve.10,11 We think tricuspid valve repair should always be the first treatment option for TR, and cardiac surgeons should acquire skills for complex tricuspid valve repair.
Ethical considerationsEthics Committee from the National Cardiovascular Institute (Certificate 29/2022, CEI-INCOR-ESSALUD) approved the publication of the case and written informed consent was obtained from the patient.
FundingThis work was supported by self-financing.
Conflict of interestsNone declared.