In general, COVID-19 vaccines are safe and effective, but minor adverse effects are common. However, adverse effects have not been measured in several countries including Greece.
ObjectiveTo estimate the prevalence of adverse effects after the first COVID-19 booster dose, and to identify possible risk factors.
Material and methodsWe conducted a cross-sectional study with a convenience sample in Greece during November 2022. We measured several adverse effects after the booster dose, such as fatigue, headaches, fever, chills, nausea, etc. We considered gender, age, chronic disease, self-assessment of health status, COVID-19 diagnóstico, and self-assessment of COVID-19 course as possible predictors of adverse effects.
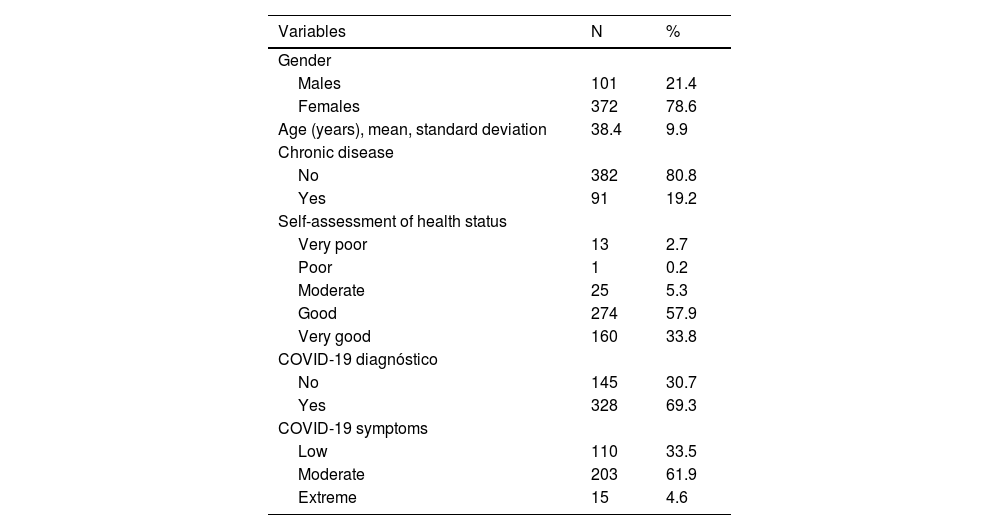
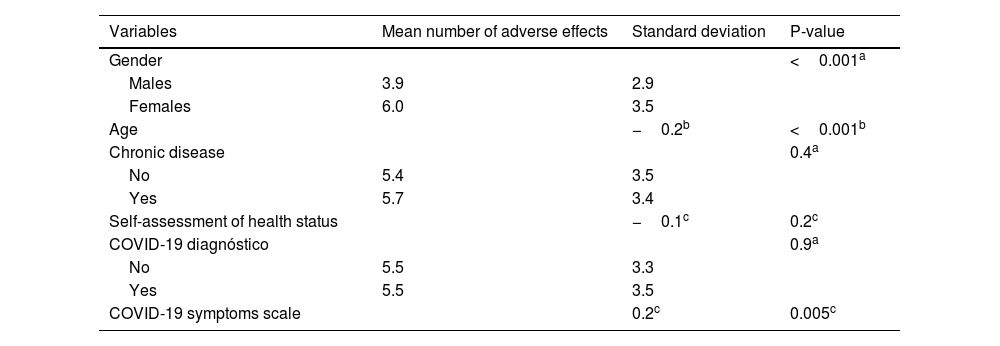
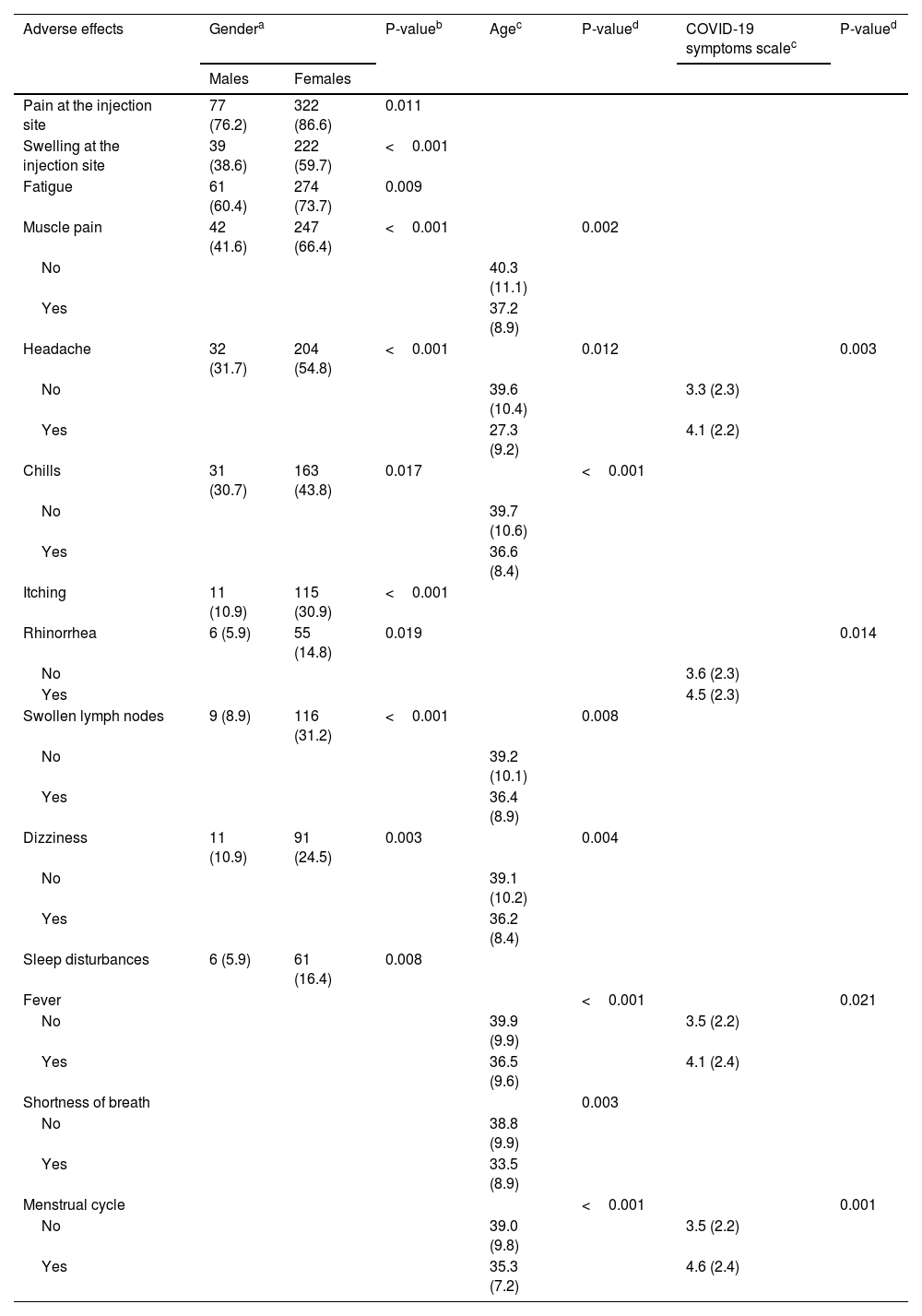
ResultsIn our sample, 96% developed at least one adverse effect. Half of the participants (50.2%) developed one to five adverse effects, 35.9% developed six to ten adverse effects, and 9.5% developed 11 to 16 adverse effects. Mean number of adverse effects was 5.5. The most frequent adverse effects were pain at the injection site (84.3%), fatigue (70.8%), muscle pain (61%), swelling at the injection site (55.2%), headache (49.8%), fever (42.9%), and chills (41%). Females developed more adverse effects than males (p < 0.001). The prevalence of adverse effects of COVID-19 vaccines was statistically significant and positively associated with the severity of COVID-19 among COVID-recovered individuals (p < 0.05). Moreover, younger age was associated with increased adverse effects (p < 0.001).
ConclusionsAlmost all participants in our study developed minor adverse effects after the booster dose. Female gender, COVID-19 patients with worse clinical course, and younger individuals experienced more often adverse effects.
En general, las vacunas COVID-19 son seguras y eficaces, pero son frecuentes los efectos adversos leves. Sin embargo, los efectos adversos no se han medido en varios países, entre ellos Grecia.
ObjetivoEstimar la prevalencia de efectos adversos tras la primera dosis de refuerzo de COVID-19 e identificar posibles factores de riesgo.
MétodosRealizamos un estudio transversal con una muestra de conveniencia en Grecia durante noviembre de 2022. Se midieron varios efectos adversos tras la dosis de refuerzo, fatiga, dolores de cabeza, fiebre, escalofríos, náuseas, etc. Consideramos el sexo, la edad, la enfermedad crónica, la autoevaluación del estado de salud, el diagnóstico de COVID-19 y la autoevaluación del curso de COVID-19 como posibles predictores de los efectos adversos.
ResultadosEn nuestra muestra, el 96% desarrolló al menos un efecto adverso. La mitad de los participantes (50,2%) desarrollaron de uno a cinco efectos adversos, el 35,9% desarrollaron de seis a diez efectos adversos, y el 9,5% desarrollaron de 11 a 16 efectos adversos. La media de efectos adversos fue de 5,5. Los efectos adversos más frecuentes fueron dolor en el punto de inyección (84,3%), fatiga (70,8%), dolor muscular (61%), hinchazón en el punto de inyección (55,2%), cefalea (49,8%), fiebre (42,9%) y escalofríos (41%). Las mujeres presentaron más efectos adversos que los hombres (p < 0,001). La prevalencia de los efectos adversos de las vacunas COVID-19 fue estadísticamente significativa y se asoció positivamente con la gravedad de COVID-19 entre los individuos recuperados de COVID (p < 0,05). Además, la menor edad se asoció con mayores efectos adversos (p < 0,001).
ConclusionesCasi todos los participantes en nuestro estudio desarrollaron efectos adversos menores tras la dosis de refuerzo. El sexo femenino, los pacientes de COVID-19 con peor evolución clínica y los individuos más jóvenes experimentaron efectos adversos con mayor frecuencia.