Substance-induced psychosis (SIP) is the name given to a psychosis that starts in the context of substance abuse but persists for days and weeks with no substance use. There is as yet little knowledge about the longitudinal course of this psychosis, which suggests that significant numbers go on to be diagnosed with a severe mental disorder (SMD). The objective of this study was to analyse the progression of SIP to SMD in our environment and the possible factors that may be involved in that conversion.
Material and MethodsWe used a retrospective cohort follow-up design. We reviewed all diagnoses of patients discharged from the psychiatric hospitalisation unit of the University Hospital of Basurto from January 2002 to December 2015 inclusively. In addition to sociodemographic and clinical data, information was collected on the consumption of cannabinoids, opioids, amphetamines, cocaine and alcohol. The data were analysed using descriptive analysis, Kaplan-Meier survival curves and Cox regression.
ResultsOf the 116 patients, 78.4% were male, had an average age of 33.0 (SD = 8.9) years and 44.0% were single; 31.0% had a psychiatric family history; the most commonly used substance was cannabis (60.3%), followed by cocaine (40.5%). The cumulative risk of diagnostic conversion to an SMD in 16 years was 41.6% (95%CI: 32.2–52.2) over a mean 36.43 months.
ConclusionsIn interventions in episodes of SIP we must bear in mind that a significant proportion will progress to an SMD in the first three years.
Se denominan Psicosis inducida por sustancias (PIS) a las psicosis que empiezan en el contexto del uso de una sustancia pero persisten días y semanas en ausencia del uso continuado de la misma. Los conocimientos sobre el curso longitudinal de estas Psicosis son aún escasos y sugieren que un porcentaje importante son más adelante diagnosticadas de trastorno mental grave (TMG). El objetivo de este estudio es analizar la evolución de las PIS a TMG en nuestro medio y los posibles factores que puedan estar implicados en esa conversión.
Material y MétodosUtilizamos un diseño retrospectivo de seguimiento de una cohorte. Se revisaron todos los diagnósticos de los pacientes dados de alta en la unidad de hospitalización de psiquiatría del Hospital Universitario de Basurto desde enero de 2002 hasta diciembre de 2015 inclusive. Además de datos sociodemográficos y clínicos se recogió información sobre el consumo de cannabinoides, opioides, anfetaminas, cocaína y alcohol. Los datos se analizaron mediante estadística descriptiva, curvas de supervivencia Kaplan-Meier y regresión de Cox.
ResultadosDe los 116 pacientes incluidos el 78,4% fueron hombres, tenían una edad media de 33,0(DE = 8,9) años y el 44,0% estaban solteros; un 31,0% tenía antecedentes familiares psiquiátricos; la sustancia más consumida fue cannabis (60,3%), seguido por cocaína (40,5%). El riesgo acumulado de conversión diagnóstica a TMG en 16 años fue del 41,6% (IC95%:32,2 a 52,2) en un tiempo medio de 36,43 meses.
ConclusionesEn las intervenciones en episodios de PIS debemos tener presente que una proporción importante evolucionará a TMG en los tres primeros años.
Psychosis is broadly defined as a heterogeneous clinical picture that severely impacts the functioning of individuals with impaired judgement of reality and with psychotic symptoms, primarily delusions and hallucinations, and others, such as formal thought and affective disorders1. The vulnerability/stress model posits that certain individuals are vulnerable or predisposed to psychosis, and that some environmental factors may influence this predisposition triggering the disorder or, conversely, play a protective role2. Substance use can trigger psychotic symptoms in different contexts, including acute intoxication, withdrawal, delirium due to intoxication or withdrawal, affective disorders with substance-induced psychotic symptoms, and substance-induced psychosis (SIP) or toxic psychosis. SIP has been conceptualised as a condition where psychosis begins in the context of substance use, but persists for days to weeks in the absence of continued substance use3. The list of potentially inducing substances is extensive4. There is evidence that cannabis, amphetamines, and alcohol can also cause persistent psychotic symptoms5. SIP is a common presentation in hospitals and emergency departments, but there is little research on its treatment and longitudinal course6. It is difficult to differentiate between patients with SIP or psychosis secondary to substance use and those whose symptoms are due to a primary psychotic disorder or a severe mental disorder5. The concept of severe mental disorder (SMD) encompasses a heterogeneous group of mental illnesses that generally includes schizophrenia and schizophrenia spectrum disorders (SSD), affective disorders with psychotic symptoms, and some severe personality disorders. The concept of severe mental disorder (SMD) encompasses a heterogeneous group of mental illnesses that generally includes schizophrenia and schizophrenia spectrum disorders (SSD), affective disorders with psychotic symptoms, and some severe personality disorders. Their defining feature is that they generate mental disturbances of prolonged duration that entail relevant disability and social dysfunction, which will require prolonged care by social and health care resources and psychiatric care networks. The incidence and prevalence in the general population are unclear, as are the risk factors associated with SIP that induce a permanent mental disorder7. The guidelines for the early treatment of SIP are less clear than those for the early treatment of primary psychosis8,9.
Several authors have studied the instability over time of psychiatric diagnoses10. In the diagnosis of SIP, some studies show that 25 %–50 % of patients receive a diagnosis of SMD in follow-ups over 3–20 years5,10–20. This may be due to the evolution of the disease itself, to new information about its onset or course, or to unreliable diagnostic assessment21,22. Sociodemographic (age, gender, family history) and clinical (length of stay, symptoms, type of substance) risk factors have been investigated that explain the diagnostic instability of SIP5,6,15,19,23–25.
It is important to distinguish SIP from primary psychotic disorder as they require different therapeutic approaches23. Early intervention in psychosis is a model of care that promotes recovery from psychosis through early detection and the most effective treatments from the onset of psychotic symptoms26. A diagnosis shapes the expectations of the patient, of their family, and of the treating clinicians themselves27. Diagnosing an episode of SIP as primary psychosis may lead to stigmatisation, longer use of antipsychotic medication and adverse social, educational, and vocational effects28,29. If episodes of SIP are early signs of SMD, a better understanding of SIP would enable earlier and more appropriate interventions for that SMD5.
Our working hypothesis is that many patients who are initially diagnosed with SIP progress to subsequently receive a diagnosis of SMD. The aim of this study is to determine the proportion of patients who, after being admitted to our hospital unit and diagnosed at discharge with a first episode of SIP, are later diagnosed with SMD and how long it takes for this change in diagnosis. We also analysed the influence of other factors related to this diagnostic conversion such as age, gender, family psychiatric history, substance use, duration of admission, and treatment received.
MethodsA retrospective follow-up study of a cohort based on the information collected from the clinical records of the Hospital Universitario Basurto (HUB). Authorisation was obtained from the HUB’s Ethics Committee prior to the start of the study. All patients diagnosed with SIP or toxic psychosis in the discharge report following an admission to the Acute Unit of the Psychiatry Service of the HUB between 1 January 2002 and 31 December 2015 were included. This is a 42-bed unit, and is the referral unit for patients residing in Bilbao, with a population of approximately 325,000 users, and about 800 patients are admitted each year. A single researcher oversaw the review of the histories and collecting the information through detailed reading. Diagnoses were made by urinalysis and routine clinical interviews in the inpatient unit.
Patients over 18 years of age with a diagnosis F10–F19 (with third character 5) according to the International Classification of Diseases, tenth revision (ICD-10), were included. Reasons for exclusion were a previous history of psychosis and the presence of a diagnoses in Axis I other than substance-induced disorders. Cases were followed up until the first diagnosis of SMD at discharge from a new admission, the main variable, or until the cut-off date established at 31 March 2018. For the purposes of this study, diagnoses included in ICD-10 codes F20–F29 (schizophrenia and SSD), F30–F31 (manic episode and bipolar affective disorder [BDD]), F32.3 (major depressive episode with psychotic symptoms) and F33.3 (recurrent depressive disorder, severe current episode with psychotic symptoms) are considered SMD. Severe personality disorder diagnoses as the only diagnosis were excluded.
The following substances were considered: cannabinoids, opiates, amphetamines, cocaine, and alcohol. When the substance suspected of inducing the episode was not specified in the discharge report, we considered substances that tested positive in urine tests. We took into account whether there was use of a single substance (single drug use) or more substances (polydrug use). We also collected information on age (in years) at the time of the episode, gender, marital status, family history of mental illness other than substance use, and employment status. For the SIP event, the duration in days of hospital stay, the concomitant presence of other pathologies, and the treatment prescribed at discharge were recorded. The dose of antipsychotic prescribed in the event (if any) was matched with the equivalent dose of chlorpromazine, divided into three groups: low dose (less than 200 mg/day), medium dose (between 200 mg and 500 mg/day), and high dose (more than 500 mg/day).
Statistical analysisThe baseline characteristics of the sample are described as mean and standard deviation (median and interquartile range, if appropriate) for quantitative variables, and as frequency and percentages for categorical variables. Pearson's chi-square test (χ2) (or Fisher's exact test, if necessary) was used for between-group comparison of categorical variables, and continuous variables were compared using Student's t-test (or Mann-Whitney rank test if a non-parametric test was necessary). As not all the patients had the same length of follow-up, in addition to the crude percentages of the presence of SMD, we calculated incidence rates in the form of SMD episodes per person-year. Kaplan-Meier survival analysis for censored data was used to assess survival time and the cumulative probability of conversion to SMD from a first admission for SIP. In addition to the Kaplan-Meier method, the cumulative probability was calculated using the Nelson-Aalen method. Survival curves according to the different strata for the variables gender (male), days of index admission stay (>7 days), and age >30 years were compared using the log-rank method. Cox regression (after testing for proportional hazard assumption) was then performed to estimate the differential risk of triggering an SMD between the different substances and adjusted for variables that in the univariate analyses would have been significant or close to significance. Due to the limited sample size of this study no imputation techniques for missing data were used. The level of statistical significance was set at p < .05, and IBM SPSS v23.0 and Stata v14.2 were used for the statistical analyses30,31.
ResultsDuring the study period, 11,263 discharges were recorded in the Psychiatry Service, of which 116 were diagnosed for the first time with SIP. They were predominantly male (78.4%), with a mean age (standard deviation [SD]) of 33.0 (8.9) years; 44.0% were single; 31.0% had a psychiatric family history. Sociodemographic and SIP episode variables are summarised in Table 1. The mean follow-up time (onset of SMD or end of follow-up) was 84.8 (61.5) months (range 1–194). The most commonly used substance was cannabis (60.3%), followed by cocaine (40.5%). Only 51.7% of the patients used a single substance, cannabis being the most frequent (53.3%). The average length of stay was 11.6 (7.7) days. At discharge, 91.4% were treated with an antipsychotic, in 37.7% of cases as the only treatment; in more than two thirds (69.8%) a mid-range dose (between 200 and 500 mg chlorpromazine or equivalent) was administered.
Socio-demographic and baseline variables of the patients diagnosed with substance-induced psychosis participating in the study.
| Variable | n = 116 | Empty Cell |
|---|---|---|
| Age at diagnosis in years, mean (SD) | 33 | (8.9) |
| Gender, n (%) | ||
| Male | 91 | (78.4) |
| Female | 25 | (21.6) |
| Marital status, n (%) | ||
| Single | 51 | (44.0) |
| Married | 16 | (13.8) |
| Divorced | 20 | (17.2) |
| Not specified | 29 | (25.0) |
| Employment status, n (%) | ||
| Employed | 36 | (31.0) |
| Unemployed | 38 | (32.8) |
| Unable to work | 4 | (3.4) |
| Not specified | 38 | (32.8) |
| Family history of mental illness, n (%) | 36 | (31.0) |
| Days of admission for initial episode, mean (SD) | 11,6 | (7.7) |
| Suspected substance in SIP episodea | ||
| Cannabis [in single use] | 70 32 | 60.3% [27.6%] |
| Cocaine | 47 9 | 40.5% [7.8%] |
| Amphetamines | 37 13 | 31.9% [11.2%] |
| Alcohol | 27 4 | 23.3% [3.4%] |
| Opioids | 14 2 | 12.1% [1.7%] |
SD: Standard Deviation; SIP: Initial episode of substance-induced psychosis.
All percentages are on the total patients (n = 116).
There were 42 conversions (36.2%) from a diagnosis of SIP to SMD, with a mean time of conversion of 36.4 (34.8) months (median: 25.3 months). No significant differences in conversion time were found between the different substances. Most of the conversions to SMD (69.0%) took place during the first three years of follow-up. At the time of diagnosis of SMD 50.0% were not using substances. Schizophrenia and SSD were the most frequent diagnoses (85.7%). The higher probability of diagnostic conversion between the group with a family history (44%) and those without (32.5%) was not significant. Nor did the variables age, gender, marital or employment status, substance used, single/polydrug use, antipsychotic dose at discharge, or length of stay show significant variations, although in the latter case there was a significant difference between stays of less than 8 days (25.0% of SMD) and those of longer duration (42.1%). These variables are summarised in Table 2.
Description of the episodes of SMD and comparison with stable patients.
| Empty Cell | SMD (n = 42) | No episode (n = 74) | RR (95% CI) | p |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Female | 8 (32.0) | 17 (68.0) | ref | .621 |
| Male | 34 (37.4) | 57 (62.6) | 1.05 (.87–1.27) | |
| Family history, n (%) | ||||
| No | 26 (32.5) | 54 (67.5) | ref | .216 |
| Yes | 16 (44.4) | 20 (55.6) | 1.41 (.82–2.41) | |
| Age at onset, mean (SD) | ||||
| Under 31 years | 16 (32.7) | 33 (67.3) | ref | .496 |
| 31 years or under | 26 (38.8) | 41 (61.2) | 1.12 (.82–1.53) | |
| Duration of admission for SIP, mean (SD) | ||||
| 7 days or less | 10 (25.0) | 30 (75.0) | ref | .068 |
| 8 days or more | 32 (42.1) | 44 (57.9) | 1.28 (.99–1.65) | |
| Initial referral, n (%) | ||||
| Drug addiction service | 9 (30.0) | 21 (70.0) | ref | .408 |
| Mental health centre | 23 (42.6) | 31 (57.4) | 1.21 (.88–1.65) | |
| Other | 10 (31.3) | 22 (68.8) | 1.03 (.61–1.73) | |
| Chlorpromazine equivalent dose in SIP, n (%)a | ||||
| Mean/low | 28 (33.3) | 56 (66.7) | ref | .291 |
| High | 10 (45.5) | 12 (54.5) | 1.49 (.71–3.12) | |
| Final diagnosis SMD episode, n (%) | ||||
| Schizophrenia and SSD spectrum | 36 (85.7) | n.a. | ||
| Affective disorder | 6 (14.3) | n.a. | ||
| Mean time to diagnosis, months, mean (SD) | 36.4 (34.8) | n.a. | ||
CI: Confidence Interval; n.a.: not applicable; RR: Relative Risk; SD: Standard Deviation; SIP: Substance-induced psychosis; SMD: Severe mental disorder.
Because the length of follow-up of patients with a first episode of SIP varies greatly (range: .8–193.9 months), time was also considered a modulating factor in the analysis. The overall incidence rate of the onset of an SMD in patients previously admitted for SIP is estimated at 5.1 cases of SMD per 100 patient-years. Table 3 shows that single use of cannabis use (in addition to single use of alcohol) appears to have higher incidence rates than poly-substance use (6.6 versus 4.6 cases of SMD per 100 patient-years), although the limited sample size precludes drawing conclusions in this regard. Table 3 also shows that cannabis users have a lower mean age than users of other substances or those with poly-substance use.
Crude rates of conversion to SMD according to the type of substance use (single drug use) or poly-substance use at the time of the SIP episode (n = 116).
| Substance | Patients, n | Male, n (%) | Female, n (%) | Age, years, mean (SD) | Conversions, n | Years of follow-upa | Crude rate (95% CI)b |
|---|---|---|---|---|---|---|---|
| Alcohol | 4 | 4 (100) | 0 | 45.5 (6.0) | 2 | 23.13 | 8.64 (2.16–34.57) |
| Amphetamines | 13 | 5 (38.5) | 8 (61.5) | 32.5 (6.9) | 4 | 73.51 | 5.44 (2.04–14.50) |
| Cannabis | 32 | 28 (87.5) | 4 (12.5) | 29.9 (9.8) | 14 | 212.25 | 6.60 (3.91–11.13) |
| Cocaine | 9 | 6 (66.7) | 3 (33.3) | 36.4 (9.8) | 1 | 54.96 | 1.82 (.26–12.92) |
| Opioids | 2 | 2 (100) | 0 | 32 (0) | 1 | 20.64 | 4.84 (.68–34.39) |
| Polydrug use | 56 | 46 (82.1) | 10 (17.9) | 33.5 (8.0) | 20 | 435.25 | 4.60 (2.96–7.12) |
CI: confidence interval; SD: standard deviation; SIP: substance induced psychosis.
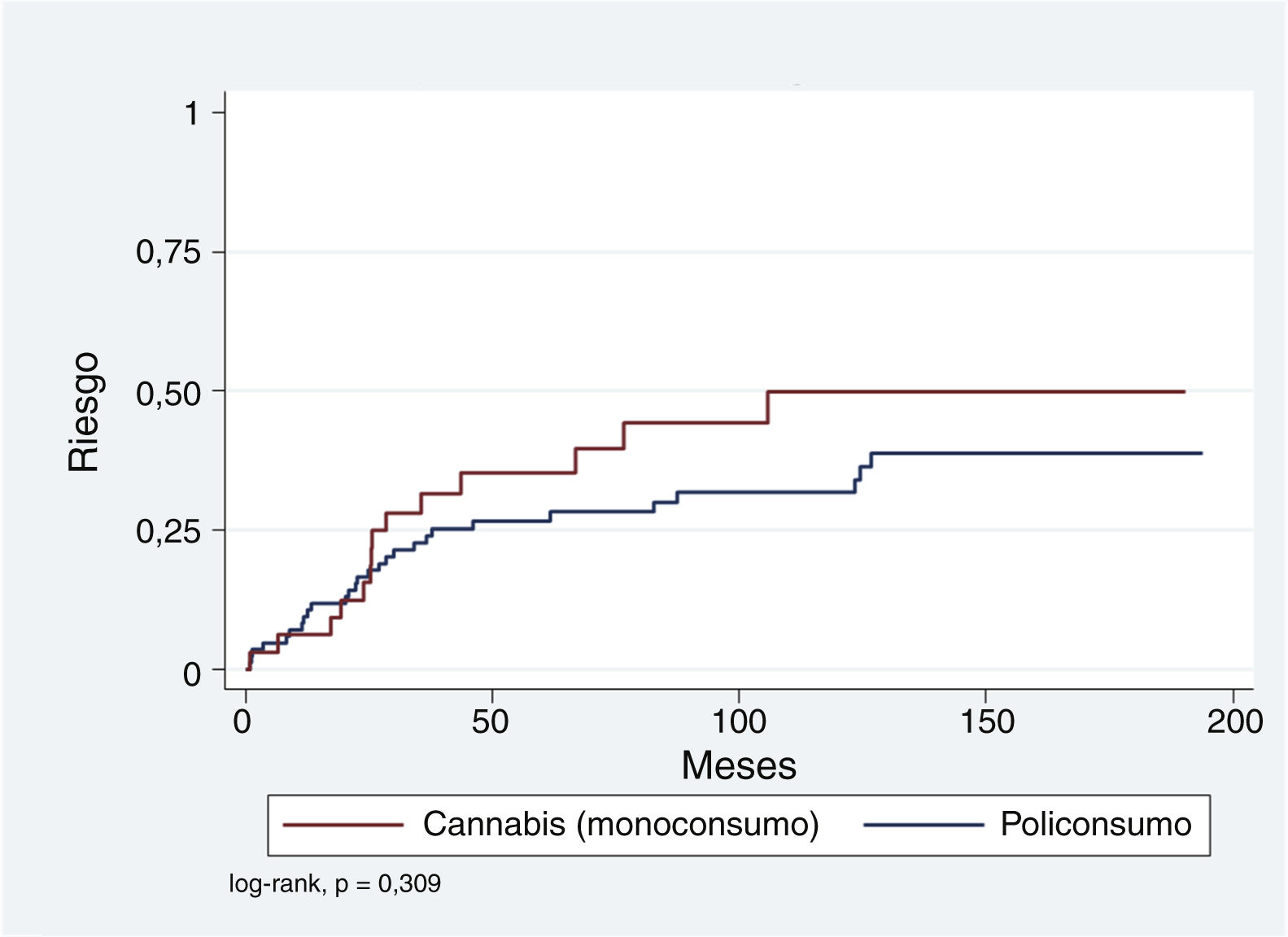
First, we calculated the cumulative risk of developing a SMD event in patients with a previous episode of SIP, according to the substance associated with the SIP in single- or polydrug use (Table 4). The estimated cumulative risks for a maximum follow-up of 16.1 years using the Kaplan-Meier (KM) method for single use of cannabis is 49.9% (standard error [SE]: 10.1). Polydrug use has a lower cumulative risk of 38.5% (SE: 6.9). As shown in Table 4, the Nelson-Aalen method of calculation (more appropriate with censored data) yields even higher risks, although KM results are also retained to facilitate comparison with other published studies.
Cumulative risks according to the type of substance use (single or poly substance use (n = 116).
| Substance | Patients, n | Conversions, n | Gross % | Cumulative risk, % (SE)a | Cumulative risk, % (SE)b |
|---|---|---|---|---|---|
| Alcohol | 4 | 2 | 50.0% | 50.0 (25.0) | 58.3 (41.7) |
| Amphetamines | 13 | 4 | 30.8% | 48.7 (22.3) | 58.5 (36.4) |
| Cannabis | 32 | 14 | 43.8% | 49.9 (10.1) | 67.1 (19.4) |
| Cocaine | 9 | 1 | 11.1% | 11.1 (10.5) | 11.1 (11.1) |
| Opioids | 2 | 1 | 50.0% | ||
| Polydrug use | 56 | 20 | 35.7% | 38.5 (6.9) | 48.1 (11.2) |
| Polydrug use/Otherc | 84 | 28 | 33.3% | 38.8 (6.2) | 48.7 (10.0) |
| Global | 116 | 42 | 36.2% | 41,6 (5.2) | 53.5 (8.9) |
CI: Confidence Interval; SE: Standard Error.
The log-rank method of the KM survival curves was also used to analyse the possible influence of the variables gender, age (over and under 30 years), length of hospital stay (greater or less than 7 days), and single drug use of cannabis on the presentation of a SMD over time. In none of these comparisons was the log-rank result statistically significant.
Because the representation of SIP episodes associated exclusively with alcohol, amphetamines, cocaine, or opioids is relatively low compared to single drug use of cannabis and polydrug use, it was decided to group those with polydrug use and compare them with the single drug use of cannabis. Fig. 1 shows that, although the cumulative risk of conversion to SMD is higher for the single drug use of cannabis compared to the rest, this difference is not significant (log-rank p = .309). This relationship was explored using Cox regression and adjusted for variables that have been found to be relevant in other studies: age <30 years, male gender, and SIP admission >7 days. As shown in Table 5, single drug use of cannabis and a hospital stay >7 days appear to be associated with an increased risk (hazard ratio [HR]: 1.54 and 1.69, respectively), while younger age at the time of SIP appears to have a protective effect (HR: .64). However, neither of these associations is statistically significant.
Cox regression results for conversion to SMD; influence of different variables.
| Empty Cell | HR | SE | z | p > z | 95% CI | Empty Cell |
|---|---|---|---|---|---|---|
| Cannabis single use | 1.5369 | .5383 | 1.23 | .220 | .7735 | 3.0534 |
| Sex, male | 1.0558 | .4207 | .14 | .892 | .4835 | 2.3056 |
| >7 days of admission | 1.6911 | .6135 | 1.45 | .148 | .8305 | 3.4434 |
| Age ≤ 30 years | .6385 | .2153 | −1.33 | .183 | .3297 | 1.2366 |
CI: Confidence Interval; HR: Hazard Ratio; SE: Standard Error; z: z-statistic.
Of patients diagnosed with a first episode of SIP during a hospital admission, 36.2% were diagnosed with an SMD in a subsequent admission to the same units, primarily schizophrenia and SSD (85.6%). This percentage and the cumulative risk of diagnostic conversion, estimated at 41.6% (95% CI: 32.2–52.2), are similar to those observed in other studies5,12–15,19,32, but higher than those observed in a recent Scottish study33 that only considers the diagnosis of schizophrenia. It contrasts with studies that observe greater consistency over time for a diagnosis of SIP34,35, albeit with very small samples and a follow-up of only 2 years in the latter. The fact that we only included diagnostic changes occurring in patients re-admitted to the same unit where they had previously been given a first diagnosis of schizophrenia is a strength in the validity of the diagnoses, but prevents us from considering cases in which there was a diagnostic conversion without requiring a new admission or when the admission was to a different unit.
Most diagnostic conversions occur in the first few years5,12,14,19,33. In our study an average of 36.43 months elapsed. In the study by Starzer et al.19 half of the cases that were diagnosed with schizophrenia also occurred within 3.1 years after the diagnosis of SIP and conversion to BAD at 4.4 years. The sample of patients diagnosed with BAD (6/42) does not allow us to consider this difference. In the Finnish study5 most of the conversions to SSD occurred during the first three years of follow-up, especially in SIP attributed to cannabinoids. SIP due to opioids, cocaine, or amphetamines showed a more linear pattern of diagnostic change over time. Consistent with these data, in our study 69% of the conversions occurred during the first three years, and 78.6% of the conversions had already occurred by the end of the fourth year. No differences between substances were observed.
The most frequent SMD diagnosis was schizophrenia and SSD, with 85.7% of patients, similar to other studies5,12–14,19,23,33. Schizophrenia accounts for 44.6% of all conversions to SSD. This raises questions about the underlying seriousness of the diagnosis of SIP, which should not be treated as a transient condition associated only with substance use. Diagnostic tools such as the Psychiatric Research Interview for Substance and Mental Disorders (PRISM) specifically designed to differentiate between primary and substance-induced mental disorders could help in this task36.
The study of substances associated with SIP episodes as a variable shows polydrug use in almost half of the cases. The most frequent single substance use is cannabis, with a cumulative risk of conversion to SMD of 49.9% (10.1) over 16.1 years of follow-up. No differences are observed between polydrug use and use of cannabis or other substances, as shown in Table 3. The available literature13,15,19,23,25,37 shows similar results. A study25 reviewing the diagnostic stability of acute psychoses after 5 years’ evolution in more than 24,000 patients, including cannabis and amphetamine-induced psychosis, found a 46% change of diagnosis to schizophrenia, where the odds ratio (OR) for cannabis is 1.2 (95% CI: 1.01–1.24) and for amphetamines .81 (95% CI: .67–.97), thus concluding that amphetamine-attributed SIP had a better prognosis than cannabis-attributed SIP.
Our results do not support the hypotheses of other studies18,23 which indicate that patients with a family history of mental illness are more likely to have a change in diagnosis, suggesting a greater genetic vulnerability to developing SMD. Neither is this confirmed by other studies5,11,32,33.
We observed a non-significant difference between the conversion rate of patients with SIP with admissions >7 days (42.1%) compared to those admitted for 7 days or less (25%). This variable is of interest to us because previous studies5,33 relate the risk of conversion to longer stays, however, we must bear in mind that the length of a hospital stay does not depend solely on the persistence of psychotic symptoms, because other factors, including insight or social difficulties may have a significant influence.
Another way in which we considered whether the severity of symptoms of the first episode could be related to the likelihood of diagnostic conversion was by looking at the dose of antipsychotic received, assuming that the greater the severity, the higher the doses required. We were not able to find statistically significant differences, like the other study38 that uses this strategy, also considering that patients with SIP may be more behaviourally disruptive and therefore require higher doses of medication.
The role of the age of onset of the first episode of SIP in the subsequent development of a primary disorder has been discussed. In our sample we see no relationship between age of onset and risk of conversion to SMD. A study that includes patients from the age of16,18 unlike those aged 18 years in our study, and another study5 in which the sample had a mean age 10 years younger than ours (23.4 versus 33 years) reported a higher risk for young patients. Presenting SIP under the age of 30 years has also been described33 as a risk factor for progression to schizophrenia and that people with cannabis-induced psychosis who subsequently developed schizophrenia were younger than those who underwent diagnostic conversions after psychosis induced by other substances (p < .0001 for alcohol, p = .005 for opioids, p = .018 for stimulants, and p = .007 for polydrug use). In our study, we did not collect data on time of substance use until the development of the SIP episode, amount of use, or age of starting use, which seems more relevant than the age of diagnosis of the episode itself39,40.
Over the last decade, efforts have been made to explain the role of gender in addictive disease and in the development of psychotic symptoms as there is still a significant lack of knowledge, largely because there is a deficiency of data that only multidisciplinary and integrated research can provide41,42. A deeper understanding of the differences will enable the design of more specific treatments42,43. Although our study shows no differences when gender is considered a variable, other authors have observed higher conversion rates in men than in women12,33. Part of the difficulty in analysing gender is also because in studies such as this one, the percentage of men is generally much higher than that of women, and this means overestimating the influence of this variable in favour of the male gender. A recent study44 conducted in our country with 141 patients with a first psychotic episode (31.25% women) reported that 58.9% had some problematic substance use. Significant gender differences were found in age at onset, age at programme entry, marital status, cohabitation, and differences in substance use and frequency of use. However, gender was not a predictor of re-admission.
LimitationsThis study aimed to take a pragmatic approach to determine the clinical reality, so that the information obtained will help in making specific decisions, and is based on data extracted retrospectively from clinical records, which is a relevant limitation when interpreting the results. The clinicians making the diagnoses varied; to minimise this variation we used the strategy of considering only diagnoses made in readmissions to the same psychiatric unit, we also assumed the consequence of not having information on patients with episodes of SIP that did not require admission and losing the follow-up of diagnostic conversions of patients who did not need to be readmitted, who went to another hospital, or who had moved home. The quality of the information is at the level of care and data on previous time of substance use, age of onset of use, frequency, and intensity of use have not been detailed. No structured or semi-structured diagnostic interviews or clinical scales were used. We did not unequivocally assign the cause of the SIP to a specific substance, because the multiple use of substances and the way in which the information was collected in the clinical history prevents this. Every attempt was made to obtain all necessary information on patients lost to follow-up, but in some cases, this was not possible due to the retrospective nature of the study. The small sample size of our study, compared to others5,12,33, may have prevented us from reaching statistical significance in the influence observed in those studies of certain variables (age, hospital stay, sex) on the onset of SMD. In any case, our study sample was what was available at that time, as there was no sample selection bias, and it allows the main variables to be studied with guarantees.
Another limitation is that there was no comparison group without a previous SIP episode.
ConclusionsThe main conclusion of this study is that in real clinical situations more than 35% of patients who suffer a substance-induced psychotic episode, diagnosed as SIP, will progress towards persistence of symptoms that will lead to a diagnostic change to one of the categories included in the group of SMD. This will occur within 3 years of the first diagnosis in most cases. This information flags up the lack of knowledge about the longitudinal course of SIP and corroborates at the national level information provided by foreign studies. It makes it clear that this is a group of patients who need to be considered from the outset as potential SMD patients and early and proactive therapeutic interventions need to be developed far removed from the expectation of spontaneous resolution of symptoms. It also seems clear that the first few years after a diagnosis of SIP are crucial. Unfortunately, we have not been able to clarify the characteristics of SIP episodes that make a poor outcome more likely so as to be able to focus interventions. We believe that these particular variables are an interesting area for future research.
FundingThe 2018 Internal Call for Research Grants of the Hospital Universitario Basurto made it possible to draft this article. However, the project received no type of funding.
Conflict of interestsThe authors have no conflict of interests to declare.
We would like to thank Itxaso Muñoz, Iñigo Zubiaguirre, and the Hospital Universitario Basurto for their collaboration and support.