This study aimed to evaluate the frequency of the different alleles of the VNTR IL1RN gene (86bp RP, intron 2), in a Portuguese Caucasian population with implant-supported overdentures.
MethodsFifty-eight Caucasians rehabilitated with implant overdentures for at least 6 months, were randomly recruited from the Removable Prostheses Forms used in Faculty of Dental Medicine, University of Porto. After clinical examination, DNA was obtained through oral mucosa swab. PCR was used to identify the IL1RN gene alleles. The frequency estimates and the corresponding 95% CI for alleles and genotypes of the IL1RN gene were calculated using the exact binomial test.
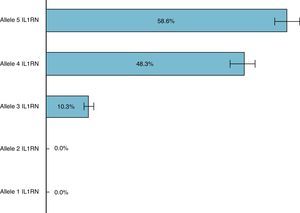
ResultsThe population included 44 females and 14 males. Only 2 subjects were smokers; 6 subjects had diabetes and 5 had chronic gastritis. The most frequent allele of IL1RN gene was allele 5, with an estimated frequency of 58.6%, followed by the allele 4 with a frequency of 48.3%. The alleles 1 and 2 were not detected. The most frequent genotype was allele 5/allele 5, followed by the allele 4/allele 4.
ConclusionsAllele 5 was the most frequent IL1RN allele and allele 5/allele 5 was the most frequent genotype. Further studies are required in order to understand the role of alleles 5 and 4, as well as of the allelic combinations of the IL1RN gene polymorphism, on the development of biological complications with dental implants.
Este estudo teve como objetivo avaliar a frequência dos diferentes alelos do gene IL1RN VNTR (86bp, intrão 2), numa população Caucasiana Portuguesa com sobredentaduras implanto-suportadas.
MétodosCinquenta-e-oito caucasianos reabilitados com sobredentaduras implantares por pelo menos 6 meses, foram recrutados aleatoriamente dos formulários de Prótese Removível da Faculdade de Medicina Dentária da Universidade do Porto. Após exame clínico, o ADN foi obtido por esfregaço da mucosa oral. A técnica PCR foi utilizada para identificar os alelos do gene IL1RN. A estimativa da frequência e respetivos IC a 95% dos alelos e genótipos IL1RN foram calculados usando o teste binomial exato.
ResultadosA população incluiu 44 mulheres e 14 homens. Apenas e indivíduos eram fumadores; 6 indivíduos apresentavam diabetes e 5 apresentavam gasrite crónica. O alelo 5 foi o alelo mais frequente do gene IL1RN, com uma frequência estimada de 58,6%, seguido do alelo 4 com uma frequência de 48,3%. Os alelos 1 e 2 não foram detetados. O genótipo mais frequente foi alelo 5/alelo 5, seguido de alelo 4/alelo 4.
ConclusõesO alelo 5 foi o alelo mais frequente do gene IL1RN e alelo 5/alelo 5 foi o genótipo mais frequente. São necessários mais estudos para se compreender o papel dos alelos 5 e 4, bem como das combinações de alelos do polimorfismo do gene IL1RN, no desenvolvimento de complicações biológicas com implantes dentários.
Oral rehabilitation with dental implants has become increasingly relevant, given the evolution and diversity of the existing implant systems and their accessibility to the population. In general, dental implant rehabilitation can provide predictable and lasting results in the replacement of missing teeth and preservation of the adjacent natural tooth structures.1–5
Oral implants have become a widespread practice and their popularity will probably increase in the next few years.6 Consequently, cases of peri-implant complications and implant failure tend to increase.7 The clinical observation of repetitive unsuccessful dental implants in certain individuals has raised some questions related to host susceptibility to dental implant failure.8 Several risk factors for implant failure were pointed out: hypertension, cardiovascular diseases, diabetes, autoimmune disorders, osteoporosis, bisphosphonate therapy, radiotherapy, history of periodontitis, smoking, implant site bone quality and genetic traits.9 In fact, in a specific group of individuals, some specific host characteristics, which may disturb the osseointegration process, were suggested to be influenced by genetic factors.8,10,11 Moreover, the number, identity and role of regulatory factors that can determine and maintain the success of osseointegration process are still a mystery.9
Nowadays, genetics research is concerned with studying DNA sequence variations (polymorphisms) and potentially associating them with increased risks for developing a particular condition or disease by a restricted population. In fact, these DNA variations may condition the gene transcription rate, the messenger RNA stability, or even the amount and activity of the produced protein.12
In the last years, several studies have pointed out the impact of certain interleukin-1 (IL1) gene single nucleotide polymorphisms (SNP) in the host response and in the development of peri-implant biological complications.6,8,13–21
More recently, some authors have suggested the existence of a possible relationship between an 86bp (base pairs) repeat polymorphism (a variable number tandem repeat, VNTR) in intron 2 of the interleukin-1 receptor antagonist gene (IL1RN) and the development of biological implant complications, such as implant loss and peri-implantitis.11,22,23
Interleukin-1 (IL1) is a potent proinflammatory mediator mainly released by monocytes, macrophages, and dendritic cells, against microbial pathogens. This action is induced by lipopolysaccharides from gram-negative bacteria, present in the peri-implant sulcus. The interleukin-1 protein has two distinct structural forms encoded in two different genes – alpha in IL1A and beta in IL1B – located in the same chromosome 2 (2q13–q21).24
The interleukin-1 receptor antagonist (IL1RN) is a cytokine that binds to IL1 receptors, inhibiting their connection with IL1A and IL1B. Thus, the IL1RN molecule competes with IL1A and IL1B in target cells and acts as a negative regulator, with an anti-inflammatory effect. The IL1RN gene is also located in chromosome 2 (2q14.2).25
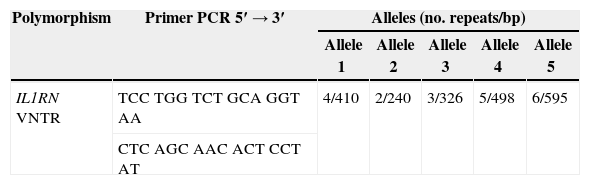
The reported VNTR of the IL1RN gene (intron 2) includes a variation of 2–6 repeats (RP), which results in 5 different alleles: 4 RP for allele 1, 2 RP for allele 2, 3 RP for allele 3, 5 RP for allele 4 and 6 RP for allele 5. Allele 1 was reported as the most common of these alleles, followed by allele 2. The remaining alleles were considered less frequent. Most individuals were found to be homozygous for allele 1 IL1RN or heterozygous for allele 1 and allele 2 IL1RN. Allele 2 IL1RN is characteristically found in homozygosity in less than 10% of the general population.26
Allele 2 IL1RN has been associated with a reduced production of the IL1RN protein and with inflammatory process increase.27,28 Additionally, an association of this allele with an increased in vitro IL1B production was reported.29
In this regard, many studies concerning the presence of the VNTR IL1RN gene and the individual susceptibility to autoimmune diseases, chronic inflammatory diseases, cancer, and bacterial and virus infections were conducted.30 Among the studied conditions, the following should be highlighted: lupus erythematosus,31 multiple sclerosis,32 inflammatory bowel disease,33 gastric cancer,34 tuberculosis35 and Epstein–Barr virus infection.36
Although there are few studies in the literature regarding the VNTR IL1RN gene and biological implant complications,11,22,23 these point out an association between allele 2 of the IL1RN gene and bone loss or peri-implantitis.
This study aimed to evaluate the frequency of the different alleles of the VNTR IL1RN gene polymorphism (86bp RP, intron 2), in a Portuguese Caucasian population rehabilitated with dental implant overdentures.
MethodsThe study was outlined according to the established legal norms (Helsinki Declaration; Additional Protocol to the Convention on Human Rights and Biomedicine–Strasbourg 2005; Law No. 12/2005), and the research protocol was approved by the Ethical Committee of the Faculty of Dental Medicine of the University of Porto (Portugal). All included patients gave their informed consent to participate in the present study.
Fifty-eight Portuguese Caucasian unrelated individuals, rehabilitated with implant-supported overdentures during at least 6 months (inclusion criteria), were randomly selected from the Removable Prostheses Forms used in the Masters and Specialization in Oral Rehabilitation of the Faculty of Dental Medicine, University of Porto, during the period between September 2012 and September 2014. The exclusion criteria were changes or loss of the implant rehabilitation, and pregnant, postpartum or breastfeed women.
The sample size was calculated based on a statistical estimate, with a 95% confidence interval and an associated error of 6.1%. The minimum number of individuals was determined according to the reported frequency of the most studied interleukin-1 gene polymorphisms in European Caucasian populations8,37,38 and the incidence rate of 6% for biological complications in implant rehabilitations.10,39–46
For each patient, clinical history collection concerning general and oral health, a clinical examination involving implant, occlusal and prosthetic evaluation, and a biological sample collection were carried out. The latter was performed using sterile and decontaminated DNA buccal swabs (Fig. 1), provided by the laboratory responsible for processing the samples (CGC Genetics/Genetic Centre Clinic SA, Portugal). The DNA extraction was performed using the QIAsymphonyDNA Mini Kit in the QIAsymphony equipment (QIAGEN®, Germany).
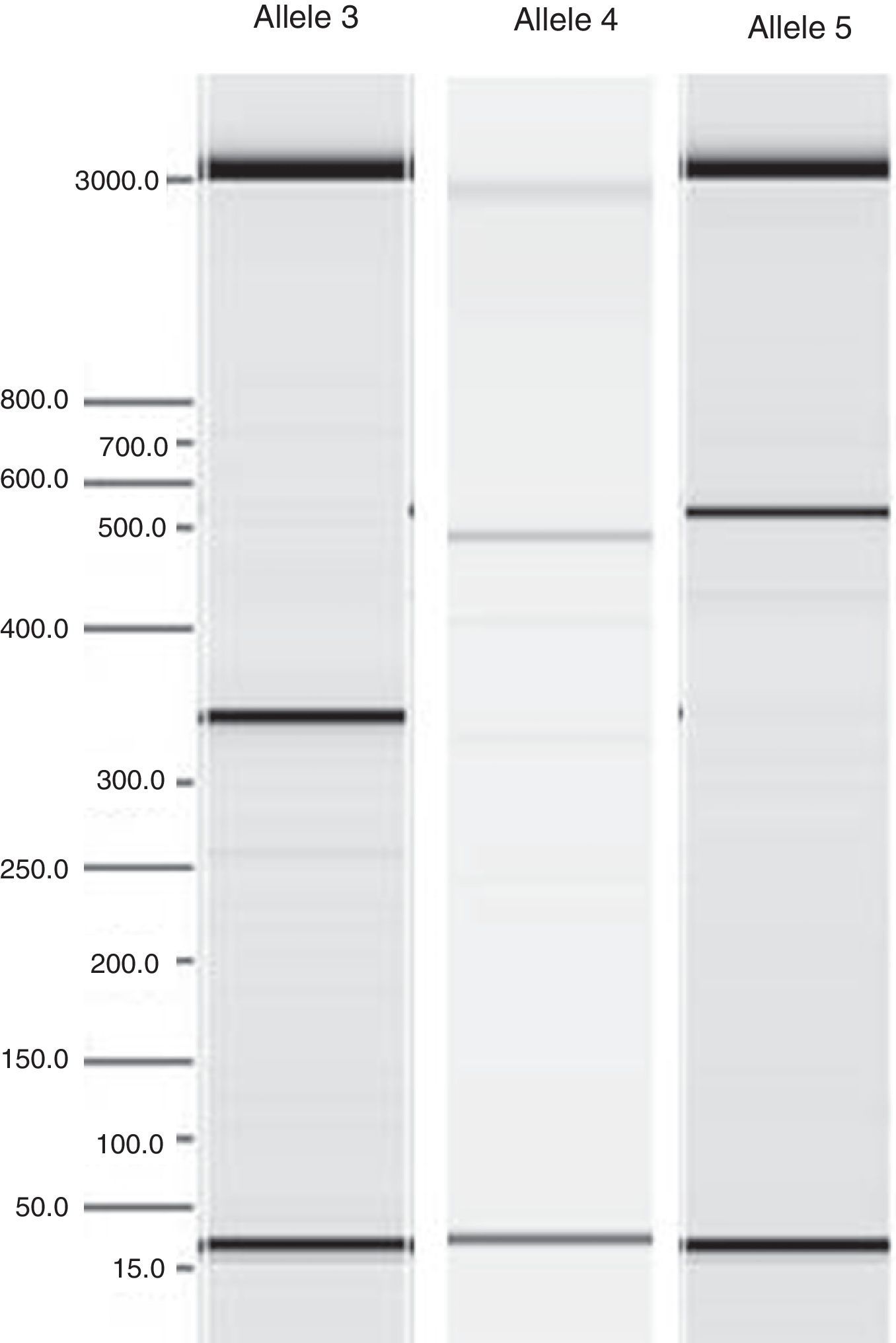
After the DNA extraction, its amplification was accomplished, and the five alleles of the IL1RN gene were identified by Polymerase Chain Reaction (PCR). The primer used and the classification of IL1RN alleles are presented in Table 1. Verification and quantification of PCR products (Fig. 2) were performed in agarose gel using the QIAxcel equipment (QIAGEN®, Germany).
The collected data were analyzed with IBM® SPSS® Statistics 22.0 and with the R 3.1.1. software (R Core Team 2014).
The frequency estimates and the corresponding 95% CI (confidence interval) for alleles and genotypes of the IL1RN gene were calculated using the exact binomial test.
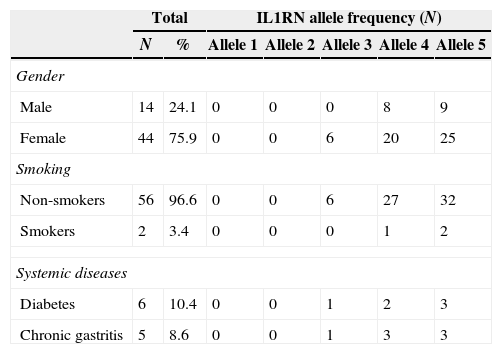
ResultsThe patients’ ages ranged between 50 and 86 years old (mean 68.8±8.3) and none presented genetic disease or family genetic disease. The sample characterization and allelic distribution for IL1RN gene polymorphism is presented in Table 2. Our population included 75.9% female and 24.1% male. Only 2 subjects were smokers and few patients had systemic diseases (6 with diabetes and 5 with chronic gastritis).
Sample characterization and allelic distribution for IL1RN gene polymorphism.
| Total | IL1RN allele frequency (N) | ||||||
|---|---|---|---|---|---|---|---|
| N | % | Allele 1 | Allele 2 | Allele 3 | Allele 4 | Allele 5 | |
| Gender | |||||||
| Male | 14 | 24.1 | 0 | 0 | 0 | 8 | 9 |
| Female | 44 | 75.9 | 0 | 0 | 6 | 20 | 25 |
| Smoking | |||||||
| Non-smokers | 56 | 96.6 | 0 | 0 | 6 | 27 | 32 |
| Smokers | 2 | 3.4 | 0 | 0 | 0 | 1 | 2 |
| Systemic diseases | |||||||
| Diabetes | 6 | 10.4 | 0 | 0 | 1 | 2 | 3 |
| Chronic gastritis | 5 | 8.6 | 0 | 0 | 1 | 3 | 3 |
N: number of subjects in the sample; %: percentage of subjects in the sample.
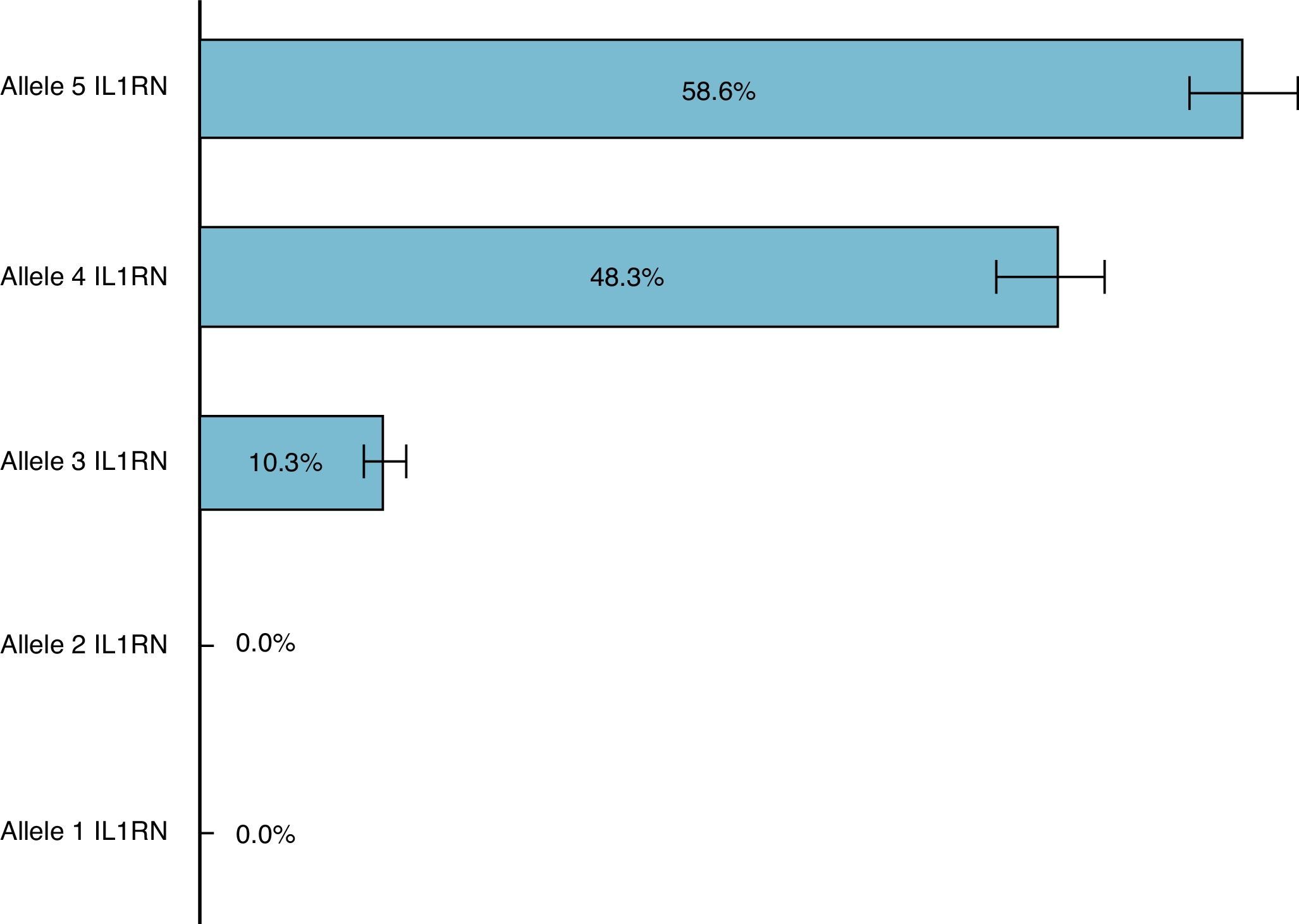
In this Portuguese Caucasian population with implant-supported overdentures, the most frequent allele of the IL1RN gene was allele 5, with an estimated frequency of 58.6% and a CI between 44.9% and 71.4%. The second most frequent allele of the IL1RN gene was allele 4, presenting an estimated frequency of 48.3% (CI between 33.9% and 61.8%), followed by allele 3 with an estimated frequency of 10.3% (CI between 3.9% and 21.2%). It should be noted that alleles 1 and 2 of the IL1RN gene were not detected in the present study (Fig. 3).
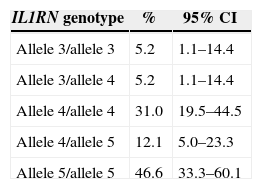
Table 3 shows the genotype frequencies (allelic combinations) of the IL1RN gene polymorphism found in the study sample. From the 15 total possible genotype combinations of the VNTR IL1RN gene, five allelic combinations were identified: allele 3/allele 3, allele 3/allele 4, allele 4/allele 4, allele 4/allele 5 and allele 5/allele 5. The remaining genotype combinations, from the 15 possible, were not detected in the studied sample (allele 1/allele 1, allele 1/allele 2, allele 1/allele 3, allele 1/allele 4, allele 1/allele 5, allele 2/allele 2, allele 2/allele 3, allele 2/allele 4, allele 2/allele 5 and allele 3/allele 5).
Genotype distribution for IL1RN gene polymorphism in the sample (N=58).
| IL1RN genotype | % | 95% CI |
|---|---|---|
| Allele 3/allele 3 | 5.2 | 1.1–14.4 |
| Allele 3/allele 4 | 5.2 | 1.1–14.4 |
| Allele 4/allele 4 | 31.0 | 19.5–44.5 |
| Allele 4/allele 5 | 12.1 | 5.0–23.3 |
| Allele 5/allele 5 | 46.6 | 33.3–60.1 |
%: estimated frequency, CI: confidence interval at 95%.
The most frequently found genotypes for the IL1RN gene polymorphism, in a Portuguese Caucasian population with implant-supported overdentures, were allele 5/allele 5, followed by allele 4/allele 4. Together, the allelic combinations allele 5/allele 5 and allele 4/allele 4 account for more than 75% of the sample.
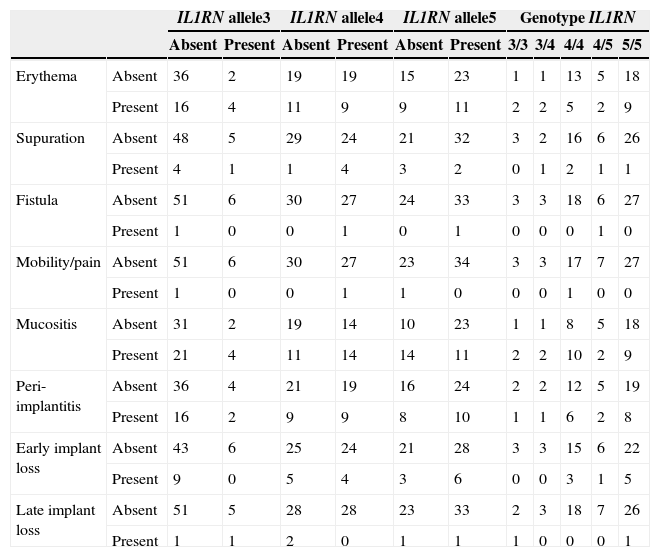
Table 4 summarizes the biological complications and the IL1RN allele and genotype distributions. It is noted a tendency toward an association between mucositis and peri-implantitis and the allelic combination 4/4 and 5/5.
Biological complications and IL1RN gene polymorphism.
| IL1RN allele3 | IL1RN allele4 | IL1RN allele5 | Genotype IL1RN | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Absent | Present | Absent | Present | Absent | Present | 3/3 | 3/4 | 4/4 | 4/5 | 5/5 | ||
| Erythema | Absent | 36 | 2 | 19 | 19 | 15 | 23 | 1 | 1 | 13 | 5 | 18 |
| Present | 16 | 4 | 11 | 9 | 9 | 11 | 2 | 2 | 5 | 2 | 9 | |
| Supuration | Absent | 48 | 5 | 29 | 24 | 21 | 32 | 3 | 2 | 16 | 6 | 26 |
| Present | 4 | 1 | 1 | 4 | 3 | 2 | 0 | 1 | 2 | 1 | 1 | |
| Fistula | Absent | 51 | 6 | 30 | 27 | 24 | 33 | 3 | 3 | 18 | 6 | 27 |
| Present | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | |
| Mobility/pain | Absent | 51 | 6 | 30 | 27 | 23 | 34 | 3 | 3 | 17 | 7 | 27 |
| Present | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | |
| Mucositis | Absent | 31 | 2 | 19 | 14 | 10 | 23 | 1 | 1 | 8 | 5 | 18 |
| Present | 21 | 4 | 11 | 14 | 14 | 11 | 2 | 2 | 10 | 2 | 9 | |
| Peri-implantitis | Absent | 36 | 4 | 21 | 19 | 16 | 24 | 2 | 2 | 12 | 5 | 19 |
| Present | 16 | 2 | 9 | 9 | 8 | 10 | 1 | 1 | 6 | 2 | 8 | |
| Early implant loss | Absent | 43 | 6 | 25 | 24 | 21 | 28 | 3 | 3 | 15 | 6 | 22 |
| Present | 9 | 0 | 5 | 4 | 3 | 6 | 0 | 0 | 3 | 1 | 5 | |
| Late implant loss | Absent | 51 | 5 | 28 | 28 | 23 | 33 | 2 | 3 | 18 | 7 | 26 |
| Present | 1 | 1 | 2 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | |
The interleukin-1 superfamily includes some of the most important cytokines involved in host-pathogen immune response. The IL1A and IL1B act as potent proinflammatory molecules and the IL1RN (antagonist of the receptor of IL1) has an anti-inflammatory role, avoiding the transmission of proinflammatory signals and preventing the immune response.
Bone is a tissue in continuous remodeling, through apposition and resorption, mediated by the local production of inflammatory cytokines, such as IL1B and IL1RN. IL1B contributes to bone resorption and IL1RN inhibits IL1B activity through competitive binding to the IL1B receptor, thus suppressing the IL1B induction of cytokine.6,8,21
The polymorphisms found in the IL1B (+3954) and IL1RN (intron 2) genes may account for variations in the IL1B and IL1RN proteins. Allelic variation in the IL1RN gene could be expected to have an impact on inflammatory processes and on the occurrence of biological complications, such as implant loss or peri-implantitis.11,23
The number of 86bp RP present in the intron 2 IL1RN gene may be of functional significance, as the sequence contains three potential protein-binding sites: an alpha-interferon silencer A, a beta-interferon silencer B, and an acute phase response element. The alpha-interferon silencer A acts as a repressor of viral response, when 4 RP (allele 1) are positioned between an enhancer and a promoter. The 2 RP (allele 2) were reported as inactive or very weakly active in the viral response. The beta-interferon silencer B and the acute phase response element are also contained within the viral response element, in the human beta-interferon gene response.26 Since individuals with different copy numbers of the 86bp repeat sequence in the IL1RN gene intron 2 have correspondingly different numbers of these protein-binding agents, these individuals should, hypothetically, exhibit different functional effects of the polymorphism.
In our study, alleles 5 and 4 were the most frequent, present in 58.6% and 48.3% of the sample, respectively. Also, there are not differences on the frequency of these two alleles, according to the gender (Table 2).
In the study of Bessler et al., allele1 was more prevalent in women and the allele2 was more frequent in men.12 This difference was also expressed by a higher incidence of the genotype combination allele1/allele1 in men compared with women. These results are different from ours probably due to the genetic characteristic, size and gender distribution. In that study12 319 healthy subjects had two Jewish origins (45.8% Ashkenazi of European descent, 48.9% Sepharadic) and the gender distribution was equilibrated (156 women, 163 men). In our study, all 58 patients were Caucasians, with more women than men, probably due to the selection method.
Our allele prevalence results are not similar to those presented in other investigations performed in populations rehabilitated with dental implants.11,22,23
In the study performed by Campos et al., the most frequent alleles were alleles 1 and 2. The frequency of allele 4 was 1.4% in a control group (Brazilian patients with successful implants) and 1.7% in a test group (Brazilian patients with failed implants).22 The difference between these results and the ones presented in our research probably reflect the miscegenation of the Brazilian population in comparison with the more homogeneous Portuguese population.
In the work of Laine et al., allele 2 showed a frequency of 34.8% in the case group (patients with peri-implantitis) and of 21.9% in the control group (patients without signs of peri-implant disease).23 That research was conducted to evaluate the role of allele 2 in peri-implantitis and does not mention the individual frequencies of the other four alleles of the IL1RN gene. Also, that study's design (case and control groups) and sample (Caucasian individuals from Northern Europe) were different from the ones presented in our study, which probably justifies the results of each study.
Regarding the results obtained by Montes et al., those cannot be compared to ours, because that study evaluated the frequency of an allele “X”, which included the presence of alleles 1, 3, 4 or 5, and of allele 2 in the IL1RN gene. Therefore, the most frequent allele was allele “X” in a control group (patients with no multiple implant losses), present in 75% cases and in 61% of the cases of a test group (patients with multiple dental implant losses).11
Considering the biological complications, our results show a tendency toward an association between mucositis and peri-implantitis and the allelic combination 4/4 and 5/5, which are different from the studies aforementioned11,22,23 probably due to the study design and the population characteristics.
The study limitations are the sample size and the differences in number of individuals based on gender.
ConclusionThe most frequent allele of the IL1RN gene polymorphism was allele 5, with an estimated frequency of 58.6% (CI 44.9–71.4%) in a Portuguese Caucasian population with implant-supported overdentures. Alleles 1 and 2 were not detected in the present study.
The estimated frequency of the allele 5/allele 5 combination for the VNTR (intron 2) of the IL1RN gene polymorphism was 46.6% (CI 33.3–60.1%) and for the allele 4/allele 4 combination was 31.0% (CI 19.5–44.5%).
Further studies are required in order to understand the role of alleles 5 and 4, as well as of the allelic combinations of the IL1RN gene polymorphism, on the development of biological complications with dental implants.
Conflicts of interestThe authors have no conflicts of interest to declare.
Ethical disclosureProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.