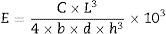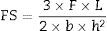To evaluate the influence of the surface used for manipulation (paper block and glass slab) on the mechanical properties (flexural strength and elastic modulus) and ion release (F−) of two resin modified glass ionomer cements (RMGIC).
MethodsTwo RMGICs: Vitro Fil LC (DFL) and Riva Light (SDI) were used. The materials were manipulated on two different surfaces: block of paper and glass slab. A 3-point-bending test (Instron 3342) was used to obtain the modulus of elasticity (GPa) and flexural strength (MPa). To measure fluoride release (ppm), cylindrical test specimens were fabricated. The data were submitted to two-way ANOVA and Holm–Sidak test for the contrast among means (α=0.05).
ResultsThe surface used for manipulation had no influence on the flexural strength and modulus of elasticity of the RMGICs (p>0.05). Manipulation on the glass plate considerably reduced fluoride release for all the RMGICs tested (p<0.001).
ConclusionsAccording to the results of this study, RMGICs must not be manipulated on glass slabs, because there was a reduction in fluoride release.
Avaliar a influência da superfície usada para manipulação (bloco de papel e placa de vidro) nas propriedades mecânicas (resistência à flexão e módulo de elasticidade) e liberação iónica (F-) de 2 cimentos de ionômero de vidro modificados por resina (CIVMR).
MétodosDois CIVMR, Vitro Fil LC (DFL) e Riva Light (SDI), foram utilizados. Os materiais foram manipulados em 2 superfícies diferentes: bloco de papel e placa de vidro. Um teste de flexão de 3 pontos (Instron 3342) foi realizado para obter o módulo de elasticidade (GPa) e resistência flexural (MPa). Foram confeccionados corpos de prova cilíndricos para medir a libertação de fluoreto (ppm). Os dados foram submetidos aos testes ANOVA two-way e Holm-Sidak para contraste entre as médias (α=0,05).
ResultadosA superfície utilizada para manipulação não teve influência na resistência flexural e módulo de elasticidade dos CIVMR (p>0,05). A manipulação na placa de vidro reduziu consideravelmente a liberação de fluoreto para todos os CIVMR testados (p<0,001).
ConclusãoDe acordo com os resultados deste estudo, os CIVMR não devem ser manipulados em placas de vidro, devido a redução dos valores de liberação de flúor.
For the development of resin-modified glass-ionomer cement (RMGIC), the acid-based reaction was maintained, but in order to eliminate inconvenient aspects of the material initially proposed by some authors,1 a second setting process initiated by light was added. Hydrophobic and hydrophilic resin monomers such as HEMA, UDMA and Bis-GMA, of around 18–20% (wt), were also introduced into the process. This change brought about various benefits: (1) The working time diminished and was now controlled by the operator; (2) the sensitivity to humidity was reduced, because the material began to set after polymerization, guaranteeing protection against imbibition and syneresis; (3) the mechanical properties of the material were improved; (4) Esthetics gained significant advancement, due to greater smoothness and addition of pigments.2–5
RMGICs are very similar to conventional ionomer cements regarding the most available commercial presentation (powder and liquid) and must be agglutinated in a similar manner. Therefore, the manner of manipulation is crucial for determining good performance of the material, because of the possibility of generating insufficient mechanical properties and fluoride release. In this procedure, the correct proportion including drop counting for the liquid and measuring the powder must also be considered.6–10
The literature presents innumerable studies that evaluate the influence of manipulation variables, such as: powder:liquid ratio,6,8,11 poly(acrylic acid) concentration,12 tartaric acid concentration13 and form of presentation (encapsulated vs. hand-mixed)14,15,16 on the mechanical properties and fluoride release of RMGICs.
One question about the manipulation of RMGIC is, however, still not clear; that is, with regard to the surface on which this material must be prepared. Text books that are the source of teaching in the education of future professionals are vague and at times contradictory. Some books indicated the block of paper,17 others, the glass slab,18 or cooled glass slab with the purpose of increasing the working time,19,20 and there are authors that have taken up no position about the topic.21–23 Use of the paper block that is sometimes provided by the manufacturers is justified by a possible reaction of fluoride ions with the silica present in glass slabs.
Therefore, the aim of this study was too evaluate the influence of the surface used for manipulation (paper block and glass slab) on the mechanical properties (flexural strength and elastic modulus) and ion release (F−) of two resin modified glass ionomer cements (RMGIC). The null hypotheses to be tested were as follows: (i) the manipulation surface has no influence on the flexural strength and modulus of elasticity of the materials evaluated; (ii) the manipulation surface does not change the fluoride ion release of RMGICs.
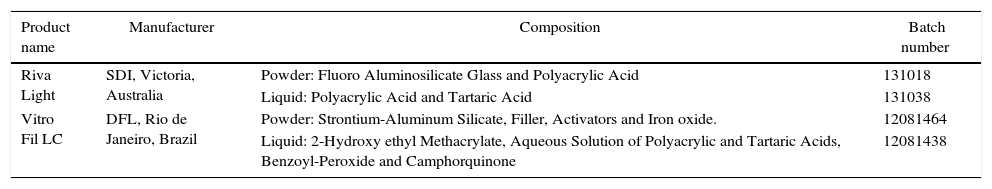
Material and methodsFor this study, two commercial resin-modified glass-ionomer cements were used: Riva Light (SDI, Victoria, Australia) and Vitro Fil (DFL, Rio de Janeiro, Brazil). The composition of the materials used is shown in Table 1.
Resin-modified GI restoratives investigated including the manufacturers’ details, batch number and powder/liquid composition.a
| Product name | Manufacturer | Composition | Batch number |
|---|---|---|---|
| Riva Light | SDI, Victoria, Australia | Powder: Fluoro Aluminosilicate Glass and Polyacrylic Acid | 131018 |
| Liquid: Polyacrylic Acid and Tartaric Acid | 131038 | ||
| Vitro Fil LC | DFL, Rio de Janeiro, Brazil | Powder: Strontium-Aluminum Silicate, Filler, Activators and Iron oxide. | 12081464 |
| Liquid: 2-Hydroxy ethyl Methacrylate, Aqueous Solution of Polyacrylic and Tartaric Acids, Benzoyl-Peroxide and Camphorquinone | 12081438 |
The surfaces used for manipulation in this study were: block of paper or glass slab. Agglutination was performed by a single operator using a plastic spatula (Maquira, Maringá, Paraná, Brazil). The powder:liquid ratio used was in accordance with the instructions of each manufacturer. Thus, a powder spoon was divided into two equal parts, with the first part being agglutinated for 10–15s, and the remaining portion for the same length of time. Total time of mixture did not exceed 25–30s.
To standardize the fabrication of test specimens, a two-piece stainless steel matrix with the following internal dimensions of 10mm length, 2mm width and 2mm height was used. After placement of the material in the mold, the surface of the restorative materials was covered with a polyester strip and a glass slab under pressure to expel excess material from the mold. After this, light activation was performed for 20s with an Optilux 501 appliance (600mw/cm2, Kerr, Orange, CA, USA). On conclusion of light activation, the specimen was removed from the matrix, excesses removed with a scalpel, and stored in distilled water in hermetically sealed receptacles (without the passage of light) at 37°C for 24h.
Twelve repetitions were performed for each of the experimental groups, totalizing 48 test specimens. After storage the specimens were subjected to a three-point flexural test to measure the flexural strength (FS) and elastic modulus (E), at a crosshead speed of 1.0mm/min with a 6mm span on a universal testing machine (Instron Model 3342, Instron Corp., Canton, MA, USA). Prior to the test, the dimensions of each specimen were recorded with Bluehill 2 software (Instron Corp., Canton, MA, USA), which calculated the elastic modulus (GPa) and flexural strength (MPa) values, according to the dimensions and tension. Based on the linear portion of the load vs. displacement curve, flexural modulus was calculated according to Eq. (1):
where E is the flexural modulus (GPa), C is the load recorded (N), L is the span between the supports (mm), b is the width of the specimen (mm), h is the height of the specimen (mm) and d is the deflection (mm) corresponding to C.Flexural strength was calculated according to Eq. (2):
where is the flexural strength (MPa), F is the maximum load recorded before the specimen fractured (N), L is the span between the supports (mm), b is the width of the specimen (mm), and h is the height of the specimen (mm).To fabricate the test specimens for the purpose of fluoride release analysis, a cylindrical, screw-top, stainless steel matrix with the following dimensions was used: 2mm high, 4mm in diameter (area=0.5cm2). Three specimens were fabricated for each experimental condition (n=3).
After manipulating the material, the matrix was completely filled and on its surface, a polyester strip and glass slide (1mm thick) were placed using smooth pressure on the matrix to standardize filling the cavity with the material. The specimen was polymerized for 20s and excesses were removed with a scalpel. Afterwards the specimens were stored in 2ml deionized water at 37°C, in closed receptacles.
The quantity of fluoride (ppm) was measured by using a fluoride ion electrode (Quimis, Model Q400ISE, Diadema, SP, Brazil) coupled to a digital pH/F− analyzer appliance (Quimis, Model Q838-F− Diadema, SP, Brazil), previously calibrated with a series of standard solutions with the following concentrations of fluoride: 1.0; 2.0; 4.0; 8.0, and 16ppm of fluoride. To analyze the fluoride released, the stored solutions were also buffered with TISAB II in the ratio of 1:1, at the time of readout, using 300μml of the sample to 300μml of TISAB II. The readouts of solutions were made in triplicate; after each readout, the electrode was washed in deionized water, and dried. Readouts were taken at the time intervals of 24h, 7 and 14 days. By measuring fluoride in parts per million (ppm) in a known volume of water, it was possible to calculate the total amount of fluoride released from the specimens. After each reading, the total fluoride released in micrograms was calculated by multiplying the parts per million (1ppm=1μg/mL) by the water sample volume (2ml). The total fluoride was then divided by the area of the sample disk (0.5cm2) to obtain the fluoride release in micrograms per square centimeter.24,25
After confirmation of the normality of distribution by the Shapiro–Wilk test (p>0.05), the data of the flexural strength and elastic modulus were analyzed by two-way ANOVA (materials vs. manipulation surface) and the data of fluoride release was analyzed by two-way ANOVA at each storage period. The Holm–Sidak honestly significance difference (HSD) test was used to compare the individual data. The level of significance was set at α=0.05.
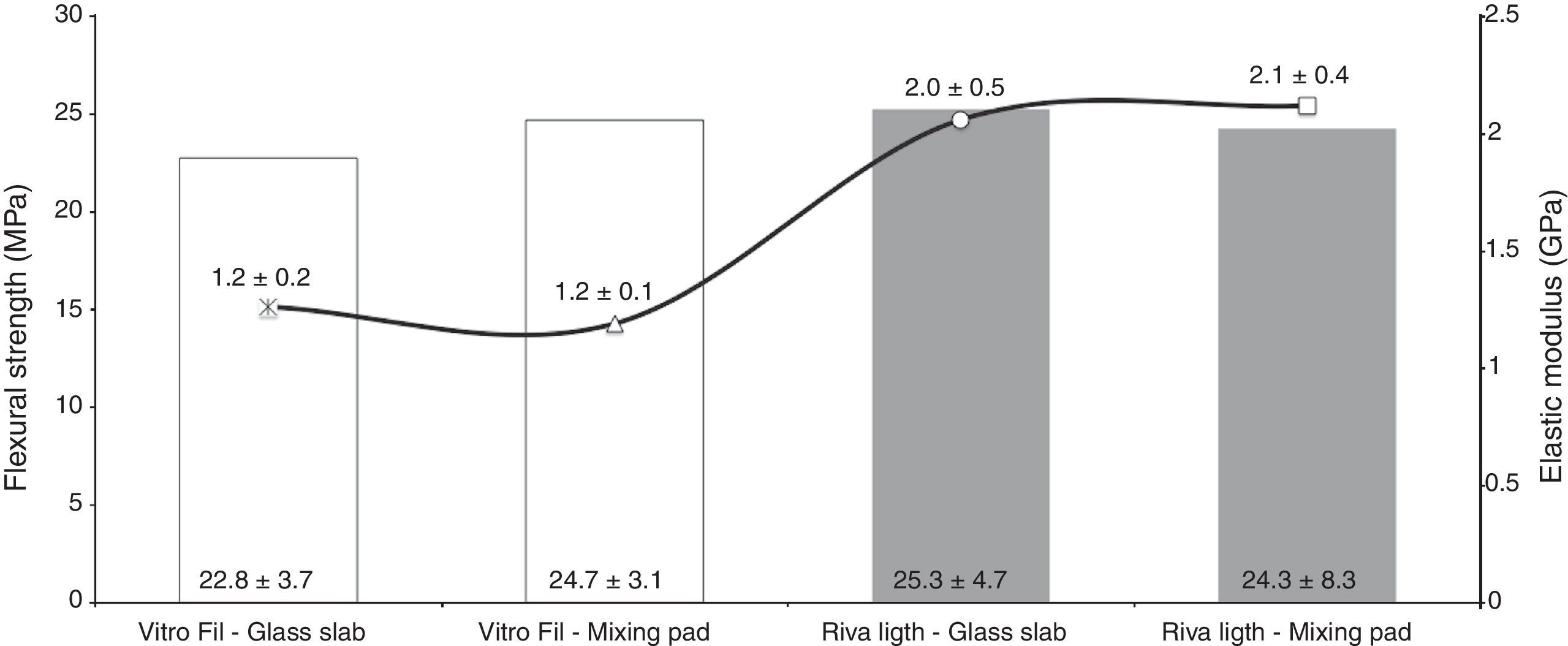
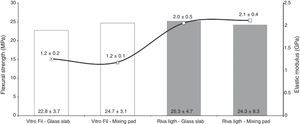
ResultsThe flexural strength data (MPa) and modulus of elasticity (GPa) are shown in Figure 1. ANOVA revealed that the interaction between factors was not significant between Material and Manipulation Surface (p>0.05). The surface used for manipulation did not change the flexural strength and modulus of elasticity of the RMGICs tested (p>0.05). There was also no difference in flexural strength for the factor Material (p>0.05). However, for the modulus of elasticity, statistical difference was found only between the RMGICs tested, in which the material Riva Light presented a higher value when compared with Vitro Fil (p<0.05).
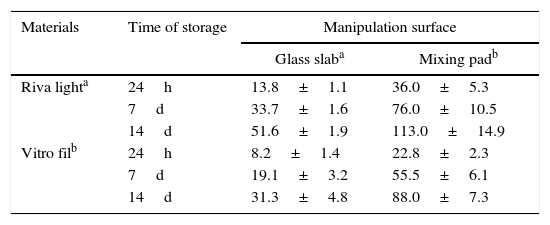
In Table 2, ion release (μgF−/cm2) of the RMGICs tested may be observed, considering the manipulation surface and storage periods. ANOVA revealed that the interaction between factors was not significant in all the time intervals evaluated (p>0.05): 24h, 7 and 14 days. However, statistical difference was observed between the manipulation surface and materials (p<0.05). Thus it was possible to observe difference in fluoride release by the RMGICs, in which Riva Light presented higher ion release values than Vitro Fil (p<0.05). Moreover, manipulation performed on a glass slab presented lower release when compared with manipulation on paper blocs, in all time intervals evaluated (p<0.05).
Mean value and standard deviation of the cumulative fluoride ion (F) released per specimen area (μg/cm2) of RMGIC manipulated on different surfaces in the 3 evaluation time intervals (24h, 7 days and 14 days).
| Materials | Time of storage | Manipulation surface | |
|---|---|---|---|
| Glass slaba | Mixing padb | ||
| Riva lighta | 24h | 13.8±1.1 | 36.0±5.3 |
| 7d | 33.7±1.6 | 76.0±10.5 | |
| 14d | 51.6±1.9 | 113.0±14.9 | |
| Vitro filb | 24h | 8.2±1.4 | 22.8±2.3 |
| 7d | 19.1±3.2 | 55.5±6.1 | |
| 14d | 31.3±4.8 | 88.0±7.3 | |
Different letters indicate significant differences among groups.
This study evaluated the effect of the surface (glass slab and paper blocks) on which two resin modified glass ionomer cements were manipulated, on the mechanical properties and ion release. The authors of this study were able to observe that the manipulation surface did not influence the flexural strength and modulus of elasticity values of the RMGICs evaluated. Therefore, the null hypothesis was accepted.
On the other hand, manipulation of resin modified glass ionomer cements underwent drastic reduction in the quantity of fluoride released when the glass slab was used. In this case, the second null hypothesis must be rejected.
Although the flexural strength results pointed out no differences between the two RMGICs evaluated, the Riva Light cement showed a higher modulus of elasticity in comparison with Vitro Fil. According to the manufacturers, the liquids of these materials are basically composed of aqueous mixtures of polyacrylic and tartaric acids with monomers and initiators, and are relatively similar between them. However, the composition of the powder of these products differs significantly. While Vitro Fil cement is composed of strontium-aluminum silicate, filler, activators and iron oxide in unidentified properties, Riva Light also presents the presence of polyacrylic acid and aluminum-silicate-fluoride in its powder.
Changes in the powder composition of RMGICs have been developed over the course of time, and may generate materials with different mechanical properties.12 For example, an increase in the concentration of polyacrylic acid leads to an increase in the modulus of elasticity of GIC.12 Therefore, the presence of polyacrylic acid in the liquid and also in the powder of Riva Light cement may be related to the higher modulus of elasticity value observed in this study.26
Fluoride plays a fundamental role in the composition of glass ionomers, and are responsible for the reduction in the melting point of the glasses,27,28 simplifying the manufacturing process and increasing the translucence of GIC after manipulation.29
With agglutination of the powder to liquid, ionization of the polyacrylic acid occurs; this dissolves the external layer of the powder particles and releases hydrogen, sodium, calcium, aluminum and fluoride ions. Therefore, fluoride ions remain inside the gel, or reacted with calcium (CaF), and this compound has no relationship with the mechanical properties of the material.26 For this reason, no statistically significant difference was found between the two manipulation surfaces.
Whereas, during manipulation of the RMGICs, fluoride ions released from their glass particles, and due to their high reactivity, they may bind with calcium or with the silica present in the superficial layer of the glass plate. Therefore, the fluoride is sequestrated from the “reservoir” of the silica gel matrix.29 This phenomenon may be the reason for the results of the present study, when the glass plate or paper block were used during manipulation of the RMGICs, since a significant reduction in the ion release values were observed when the glass slab was used with the two cements analyzed. However, further studies must be conducted to confirm this high affinity of these elements and a possible compound formed by them.
In the fluoride release analysis, the Riva Light cement presented higher values than those found in comparison with Vitro Fil. According to some authors,30 some of the characteristics of these materials may explain the different release rates. Materials with a higher proportion of fluoride-containing filler particles in the powder tend to release more F−. Therefore, as these materials have a more hydrophilic matrix, this facilitates the release of these ions in an aqueous medium. Fluoride release plays an important role in acid production of caries-related oral streptococci at acidic pH, decreasing the virulence of cariogenic biofilms and subsequent secondary caries formation.31,32
Therefore, the results of the present study open a pathway for the development of new in vitro and in situ researches, with the purpose of verifying whether the reduction in fluoride release found (when manipulation is performed on a glass slab) may affect the anticariogenic benefit of the material.
ConclusionsAccording to the limitations presented by this study, the authors are able to affirm that the fluoride release values of resin modified glass ionomer cements analyzed were reduced when they were manipulated on glass slabs. However, the values with reference to mechanical properties were not influenced by the manipulation surface.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
This study was supported by the Foundation for the Support of Scientific and Technological Research of Maranhão (FAPEMA – 01628/14 and 03730/13). Authors would like to express their gratitude to DFL and SDI for kindly donating the materials.