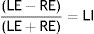Dichotic listening is one of the most common techniques used to determine the hemispheric lateralisation of language, using pairs of stimuli that are presented simultaneously, one in each ear to induce auditory competition between the two ears. Right-ear advantage for the perception of words is considered the most important indicator of left hemispheric lateralisation of language. Greater variability in hemispheric lateralisation has been found in patients with temporal lobe epilepsy due to mechanisms of brain plasticity.
ObjectiveConfirm right-ear advantage for the perception of word pairs using the dichotic listening technique in a group of right-handed patients with temporal lobe epilepsy.
MethodsA 60 word-pair dichotic listening technique was used, controlling the appearance, duration and ending of each pair of stimuli. Twenty-seven (27) right-handed patients with temporal lobe epilepsy were studied, obtaining their laterality index based on the number of words perceived in each ear.
ResultsRight-ear advantage with a significant difference (p<0.0001) was observed between both ears. According to the laterality index, 78% of the patients had left hemispheric lateralisation of language.
ConclusionThe presence of right-ear advantage for the perception of word pairs is a constant pattern that suggests hemispheric lateralisation of language in patients with epilepsy. Considering its scope and limitations, dichotic listening can be used to screen preoperative patients with temporal lobe epilepsy. There are no reports on the Mexican population on this subject.
La escucha dicótica (ED) es una de las técnicas más utilizada para la determinación de la lateralización hemisférica (LH) del lenguaje. Para ello se utilizan pares de estímulos que son presentados simultáneamente, uno en cada oído para inducir competencia. La ventaja del oído derecho (VOD) en la percepción de las palabras es considerada el indicador más importante de LH izquierda del lenguaje. Se ha encontrado mayor variabilidad en la lateralización en pacientes con epilepsia del lóbulo temporal (ELT) debido a mecanismos de plasticidad cerebral.
ObjetivoConfirmar la VOD en la percepción de pares de palabras mediante la técnica de ED en un grupo de pacientes diestros con ELT.
MetodologíaSe utilizó la técnica de ED compuesta de 60 pares de palabras controlando la aparición, duración y finalización de cada par de estímulos. Se estudiaron 27 pacientes diestros con ELT, en quienes se obtuvo el índice de lateralidad (IL) con base en la cantidad de palabras percibidas en cada oído.
ResultadosSe observó VOD con una diferencia significativa (p<0.0001) entre ambos oídos. De acuerdo al IL, el 78% de los pacientes presentaron lateralización hemisférica izquierda para el lenguaje.
ConclusiónLa presencia de VOD en la percepción de pares de palabras es un patrón constante que sugiere la LH del lenguaje en pacientes con epilepsia. Considerando sus alcances y limitaciones puede utilizarse como escrutinio en pacientes con ELT prequirúrgicos. Debido a que no hay reportes al respecto en nuestro país, consideramos que este trabajo servirá para plantear nuevos estudios que en su metodología consideren algunas características de nuestra población.
The dichotic listening (DL) technique consists of the simultaneous presentation of two different verbal stimuli (one in each ear).1 Two syllables (e.g., “pa-ba”) or two words are generally used to induce competition between the two auditory pathways. This competition comes to an end when the perception of one ear has an advantage over the other. DL can therefore determine which ear perceives a greater amount of stimuli.2 Right-ear advantage (REA) in DL is a common phenomenon in most people and reflects the hemispheric lateralisation (HL) of language, also known as language dominance. It consists of the ability of one of the two cerebral hemispheres to process verbal or written linguistic signs in terms of comprehension and expression.3–5
There are different versions of the DL test,5–8 and in the most common ones the subject's task consists of identifying and repeating aloud the stimuli presented. All versions aim at determining ear advantage under the premise that only under DL conditions (competition stimuli) will each of the stimuli presented reach the contralateral hemisphere to be (initially) processed in each temporal lobe. There are hemispheric differences in the processing of stimuli, with the left hemisphere generally taking advantage, because the information that reaches the right hemisphere must travel longer to reach the hemisphere in charge of stimulus processing.2
Because the basis of DL is the competition between the two ears, most authors have used very similar stimuli that differ only in the phonemes used to induce greater competition, thus obtaining a significant difference in the perception of stimuli between the ears.9–11
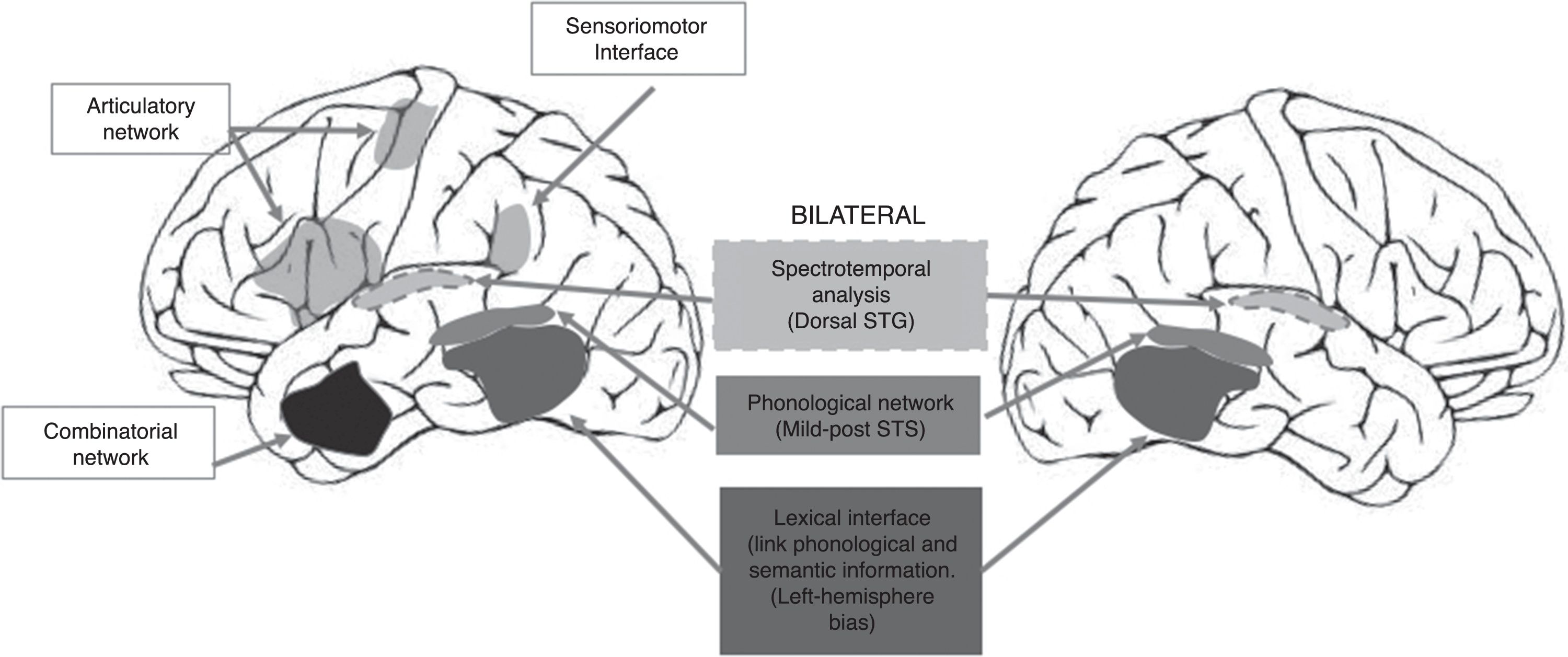
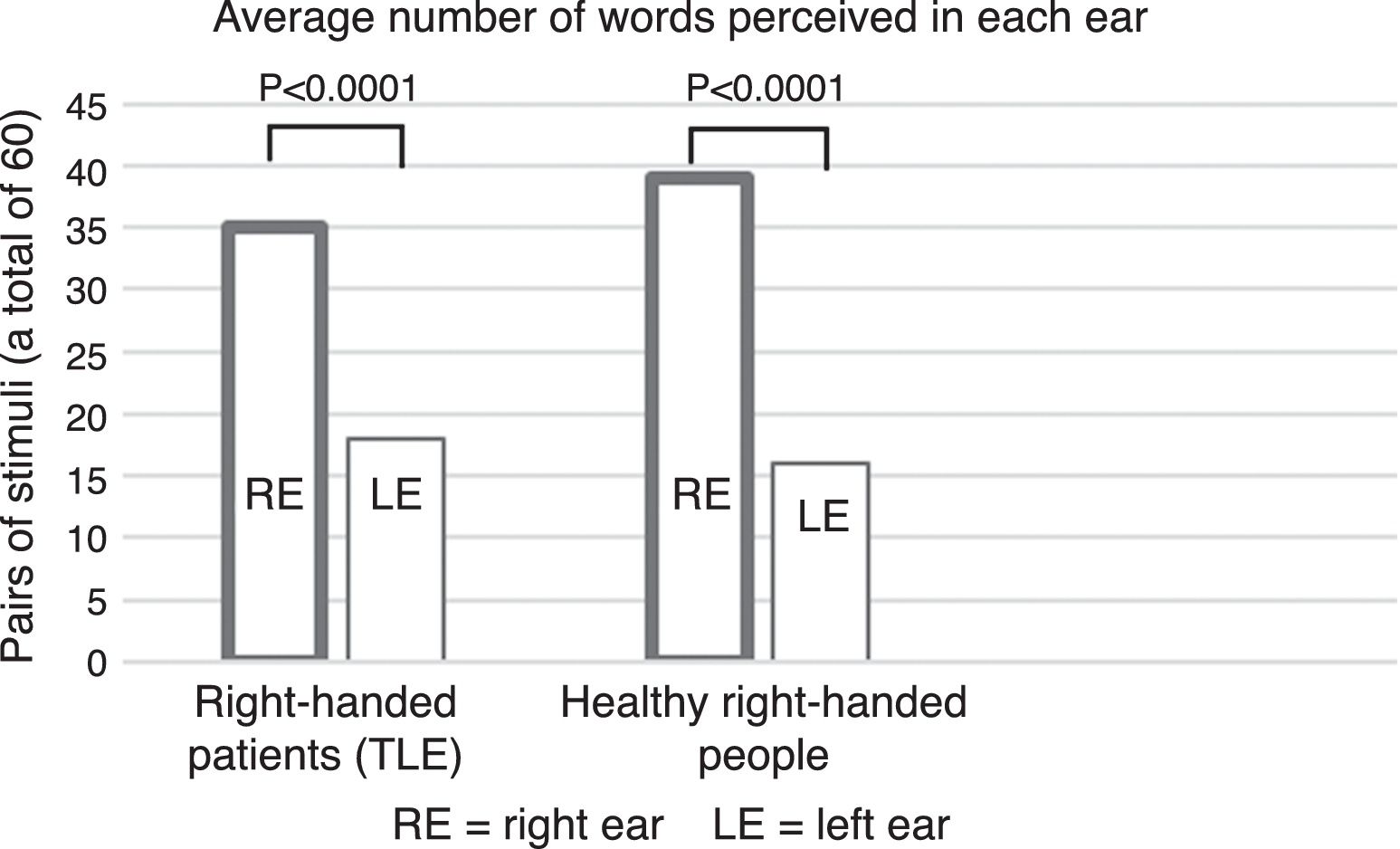
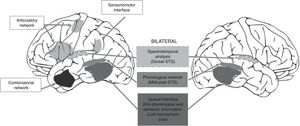
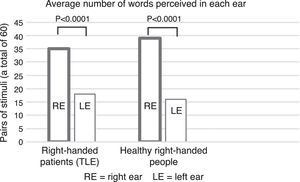
The use of this type of auditory stimuli pairs effectively renders the perception of phonological differences more difficult (increased competition). Current reports, however, consider that consonant-vowel stimuli are processed by the brain bilaterally12 because they correspond to early stages of phonological processing (see Fig. 1), thus reducing the possibility of finding a clear advantage by one ear. This point is highly relevant because the use of tests focused on this type of stimulus (consonant-vowel) has shown that the DL technique is not a reliable method for determining the HL of language.10 By contrast, a study carried out by our working group in 53 healthy right-handed subjects, using word pairs in which a significant difference (p<0.0001) was found between the right and left ears (unpublished article, Fig. 2), showed that the difference in methodology for selecting stimuli can greatly influence the presence of an auditory advantage.
Dual-stream model of speech processing.12 It should be noted that the early cortical stages of perception are bilateral (recognition of spoken words). Hemispheric processing differences are observed when a lexical (temporal lobe) interface and a sensorimotor (temporo-parieto-occipital) interface are required to connect to previous systems of the dominant hemisphere in order to repeat the word (adapted from Hickok, 2009).
Moreover, determining the HL of language in patients with epilepsy is an essential aspect, especially in those who present with TLE refractory to pharmacological treatment14–17; because surgery is a likely option for these patients, HL of language is a factor considered in the extent of brain tissue resection.
There is evidence of a higher frequency of TLE patients with an atypical brain organisation for language since the presence of irritative activity promotes the presence of brain reorganisation. For example, Weber et al. (2006) found reduced lateralisation of language in patients with epilepsy originating in the hippocampus (and in early childhood), compared with other patient groups with epilepsy of a different origin (lateral and/or frontal temporal lobe).18 For this reason, looking for an improvement in the existing methods for determining the HL of language is a very important subject within the field of epilepsy surgery.
The Wada test (WT) is considered the gold standard for determining HL, with reports that 96% of right-handed epileptics and up to 70% of left-handed epileptics have a left lateralisation of language.1,5,16,19,20 Functional magnetic resonance imaging (fMRI) is a relatively new technique that is much easier to use and up to three times cheaper than the WT.21 fMRI has been compared to the WT in multiple studies, and has been found to have high sensitivity and specificity16,21–24 that ranges from 80% to 95%15 and up to 91% in patients with epilepsy.17
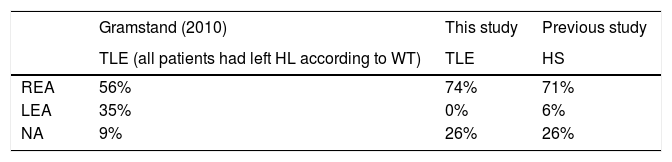
In turn, the comparison between fMRI and DL in terms of HL of language has shown a concordance between 74% and 88%.6,24,25 However, as mentioned above, there are reports on DL that recommend exercising caution in the use of this technique in patients with epilepsy.10,13 For instance, Gramstand et al. (2010),36 who compared the WT using a consonant-vowel version of DL in 46 patients with epilepsy, found three patients (of the total sample) with right lateralisation (according to the WT), two of whom showed no advantage in either ear, while only one had left-ear advantage (LEA) as expected. Of the 43 patients who were classified with left HL, 56% had REA, 35% LEA and 9% had no advantage in either ear, i.e., in almost half of the patients the DL results for determining HL of language failed. The authors mention that due to an early dysfunction of the left hemisphere, there was a decrease in REA, which is not necessarily synonymous with atypical HL of language. Therefore, because DL is a technique that may be sensitive to other unknown factors, it should not be considered as a strong indicator of HL of language in patients with epilepsy.13
This study aims at confirming REA for the perception of word pairs using the DL technique in a group of right-handed patients with TLE.
MethodType of studyThis is an analytical, observational, cross-sectional, prospective and prolective study.
ParticipantsA group of 27 patients with TLE from the Epilepsy Clinic of Hospital General de México was included. Hand dominance was determined according to the Edinburgh Handedness Inventory.26 All participants were aged 21–50 years (see Table 1).
None of the participants had auditory difficulties per the clinical interview and neurological examination. Subjects who, at the end of the test, reported having paid attention to only one of the ears were excluded, since attention directed to a certain auditory canal greatly influences stimulus perception.
Determination of hand dominanceHand dominance was determined using the Edinburgh Handedness Inventory, which is part of a neuropsychological battery26 and consists of 10 standardised questions aimed at investigating hand dominance when performing different activities. It can thus establish the degree of hand dominance by assigning a score from 1 to 5 based on each activity being investigated (sum of the ten items), with No 1=very dominant right hand; 2=dominant right hand; 3=no dominance; 4=dominant left hand; and 5=very dominant left hand. Thus, a sum is obtained indicating the degree of hand dominance. Ten (10) points for a totally right-handed person, 30 for an ambidextrous person and 50 for a totally left-handed person.
Dichotic listeningIn this study, a version of the Spanish DL test was used consisting of 30 pairs of stimuli recorded using a programme found on the market, which controlled the appearance, duration and simultaneous end of each pair of verbal stimuli with precision. These pairs were presented to each subject twice via noise-cancelling headphones, with the second stimuli being presented in inverted form (a total of 60 stimuli for each subject).
On the answer sheet, number one was assigned to the word that was repeated first and number two to the second word, making it possible to determine the amount of stimulus perceived first in each ear.
The stimuli consisted of 60 word pairs, and the words were taken from Diccionario del Español Usual en México [Dictionary of Everyday Mexican Spanish] by Lara et al. (2003).27 The words did not differ in terms of the number of syllables or the amount of letters.
The DL technique lasts approximately 8min and is part of the routine neuropsychological evaluation that all patients undergo at the Epilepsy Clinic of Hospital General de México.
Task and general procedureParticipants performed the task alone in an office while seated comfortably at a desk. After being asked about any hearing problems and undergoing a quick auditory examination by means of neurological manoeuvres, each participant carefully put on the headphones and was asked (with the statement: “This activity consists of listening to something and repeating it. Please repeat what you hear. You will hear each item twice before you moving on to the next item. If you hear two things at the same time, please repeat first the one you understood.”) to repeat aloud what they could hear. No further information was provided to prevent predisposition to the perception of the two stimuli and to observe spontaneously the strategies adopted by the subjects to complete the task adequately.
Subjects who said they switched their attention each time (a different ear each time) as a strategy to repeat the words were excluded.
At the end of the test, routine questions were asked to obtain additional clinical information on the performance of the activity, such as: On which side do you think you heard better? Did you hear words at the same time?, etc.
The total number of words perceived in the right ear (RE) and the left ear (LE) were obtained for qualification purposes. The total number of words perceived in each ear was converted into percentages, obtaining three percentages: LE percentage, RE percentage and a third percentage corresponding to the number of stimuli eliminated. Together these three percentages amount to 100%, corresponding to the 60 word pairs.
The Laterality Index (LI) was determined with the number of words perceived in each ear using the following formula28:
Thus, according to the score obtained, each subject was classified into one of the following categories28:
Score
- 1.
From +0.50 to +100 Marked Left Laterality (MLL).
- 2.
From +0.25 to +0.49 Slight Left Laterality (SLL).
- 3.
From +0.24 to −0.24 Bilateral (B).
- 4.
From −0.25 to −0.49 Slight Right Laterality (SRL).
- 5.
From −0.50 to −100 Marked Right Laterality (MRL).
Continuous endpoints are shown as the mean and standard deviation, while categorical endpoints as a percentage. A Student's t-test was used to compare the mean values of words perceived in the right and left ears for related samples. The LI obtained for each subject was used to classify them into one of the five categories in a descriptive way. Data were captured and analysed using the SPSS (Statistical Package for the Social Sciences) software for Windows, version 15 in Spanish.
ResultsThe results of the comparison between RE and LE were significant, similar to the group of healthy, right-handed subjects mentioned above (p<0.0001) (Fig. 2). In other words, REA was observed.
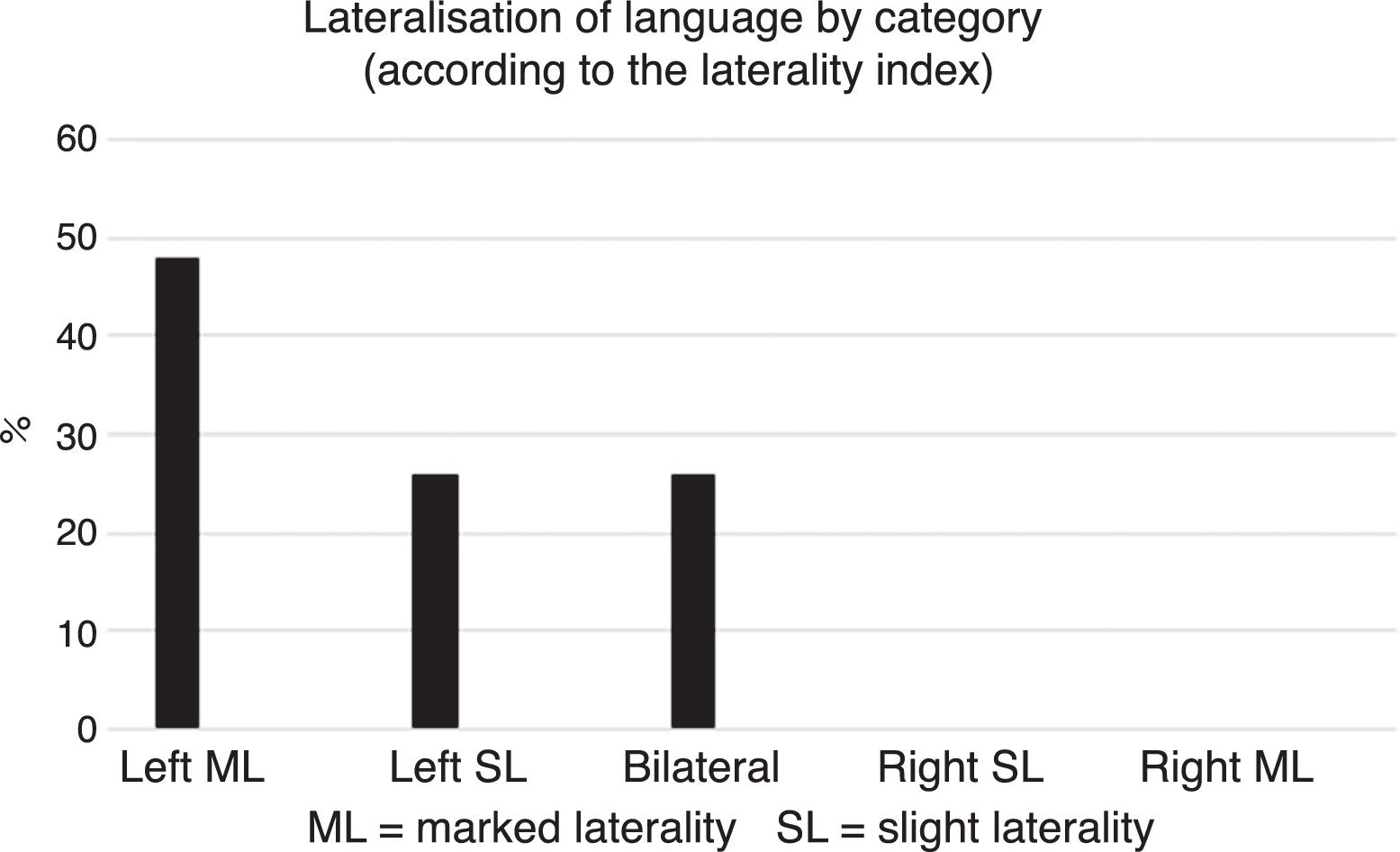
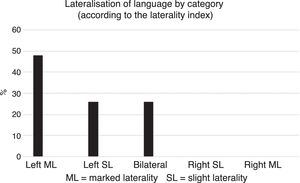
By contrast, according to the five categories established based on the LI obtained in each subject, most participants were classified with left HL (approximately 74%) (Fig. 3).
DiscussionAs in other studies,24,28 we also found REA with a significant difference between both ears in our study (Fig. 2). It should be noted that unlike other studies, we used pairs of words instead of syllables, which explains most of the REA results found, given that the use of words requires a larger brain network (in the hemisphere specialised for language) than the use of consonant-vowel syllables (Table 2). In this sense, our results are in line with Hickok's dual-stream model of speech processing, according to which the early stages of auditory perception of oral language (phonological aspects) are processed bilaterally—as with syllables—while word processing involves a lateralised brain network for the dominant hemisphere in charge of connecting semantic aspects with phonological and articulatory aspects.12
Comparison of the perception of stimuli in each ear between the TLE group of Gramstand et al. (2010) and this study. The group of healthy subjects (HS) is included as reference. NA=no advantage.
| Gramstand (2010) | This study | Previous study | |
|---|---|---|---|
| TLE (all patients had left HL according to WT) | TLE | HS | |
| REA | 56% | 74% | 71% |
| LEA | 35% | 0% | 6% |
| NA | 9% | 26% | 26% |
The use of pairs of syllables (consonant-vowel), as opposed to words, in DL can be an important factor that results in no significant differences between ears. Perhaps for this reason some authors do not recommend using this technique as a reliable method for determining HL of language,10 especially in patients with epilepsy, in whom this neurological condition may also lead to reduced REA in auditory perception,13,29,30 in addition to the bilateral processing of phonological aspects described by Hickok. This phenomenon was observed in this study, that is, even though REA was present in the TLE group, this advantage seems to be smaller than that observed in the group of healthy right-handed subjects.
Other factors that may influence the percentage of auditory perception may include the type of instructions given to subjects, the amount of control over the psycholinguistic and phonological endpoints for the stimuli, and the amount of control over very specific aspects in the digital version of the verbal stimuli.
Our finding of 74% of patients classified with left HL according to the LI is in line with the study of Springer et al. (1999),14 which used fMRI and reported 78% of TLE patients with left HL.
Future studies will analyse endpoints related to age at the onset of seizures and laterality of the main epileptic activity, since it appears to have an important impact on the hemispheric brain organisation for language,18 as well as other aspects such as the frequency of seizures and whether they are a result of an injury.
Finally, in Mexico there is a great need for the use of non-invasive, low-cost methods that help to determine the HL of language in preoperative patients with TLE. The DL technique with word pairs is an acceptable alternative; however, to our knowledge, there are no records of its use in Mexican patients with TLE.
ConclusionsAccording to the objective and results of this study, it was possible to determine that right-handed patients with TLE have REA for the perception of word pairs. We believe this may be due in part to the fact that the use of word pairs is associated with a greater participation of temporal regions that are primarily distributed and lateralised in the hemisphere specialised for language, compared with the use of pairs of syllables in other studies which involve bilateral hemispheric networks.
Currently, the use of DL in patients with epilepsy should be considered as a screening test (or approach) for brain organisation, especially when the difference in percentages between both ears is not significant (more than two thirds). Our study therefore provides evidence on the usefulness of using word pairs to find a greater difference in word perception between ears and to better characterise patients. Nevertheless, doubts remain about the patients reported in the literature who had LEA (thus suggesting right dominance), as the findings there are not in line with the WT or fMRI since left hemisphere dominance is present.
The technique's sensitivity may increase when patients are asked about their perception of the difference in appearance of the words used—in terms of volume and/or clarity of the stimuli, and the quantification of the words repeated.
Lastly, the findings of this study could serve as background information on the study of HL of language by means of the DL technique in Mexican patients with TLE, since no studies on this subject exist in this particular population. They may also lead to new studies, the methodology of which may consider the characteristics of the Mexican population (e.g., the type of words, level of education, language, etc.) in order to increase the sensitivity of this method.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article.
FundingWe received no funding for this study.
Conflict of interestThe authors declare that they have no conflict of interest.