El manejo de la enfermedad aórtica que involucra el arco sigue siendo un gran desafío. El acercamiento a la patología del arco aórtico es complejo debido a la presencia de los vasos supra-aórticos. La patología clásica incluye disección, aneurismas, hematoma intramural y la úlcera penetrante. La indicación convencional de la cirugía sigue estando relacionada con el tamaño aórtico, la velocidad de crecimiento y los síntomas. Hay dos líneas de tratamiento bien definidas. La primera es la cirugía clásica abierta y uso de injertos de Dacron, con circulación extracorpórea (CE), pinzamiento aórtico y paro circulatorio en hipotermia profunda (PCHP). Este procedimiento sigue siendo considerado el tratamiento estándar, particularmente en pacientes jóvenes de bajo riesgo y aquellos con trastorno del tejido conectivo. Es evidente, particularmente cuando se trata de una disección aórtica, que una resección y reparación más extensas podrían ofrecer un mejor resultado a largo plazo con menor necesidad de otros procedimientos en el futuro. La combinación de técnicas quirúrgicas clásicas con tecnología endovascular ha ganado un rol fundamental y ha cambiado el paradigma del tratamiento quirúrgico aislado clásico y lo ha movido hacia un procedimiento combinado con el uso de un stent cubierto autoexpandible para prevenir el colapso del lumen verdadero y favorecer la trombosis y oclusión del lumen falso. Este procedimiento se conoce como “trompa de elefante congelada”. Esta segunda línea de tratamiento es esencialmente crítica para aquellos pacientes con más comorbilidades y mayor riesgo quirúrgico. Los procedimientos híbridos con una combinación de des-ramificado quirúrgico y TEVAR percutáneo, evitando la CE, el pinzamiento aórtico y el PCHP, tienen resultados similares a los de la cirugía estándar. Actualmente existen muchas experiencias con el tratamiento endovascular total con combinación de diferentes técnicas y dispositivos percutáneos. Claramente, este sigue siendo un proceso en curso que debe demostrar resultados comparables a corto, mediano y largo plazo.
The management of aortic disease involving the arch remains a formidable effort. The approach to aortic arch pathology is very challenging due to the presence of supra-aortic vessels. Classic pathology includes dissection, aneurisms, intramural hematoma, and penetrating ulcers. Conventional indication of surgery remains related to aortic size, growth rate and symptoms. There are two-well defined lines of treatment. The first one still remains the gold standard, particularly in young low risk patients and those with connective tissue disorder and involves open surgery grafts with extracorporeal circulation (EC), aortic cross clamp, and deep hypothermic circulatory arrest (DHCA). It is evident however, particularly when treating aortic dissection, that a more extensive resection and repair could offer better long-term results with less need of follow-up procedures. Combination of classic surgical techniques with endovascular technology have taken a role and led to a paradigm shift from classical isolated surgical treatment to a combination with self-expanding covered stent graft to prevent true lumen collapse and favor false lumen thrombosis and occlusion. This procedure is known as a frozen elephant trunk. This second line of treatment is essentially critical to those patients with more comorbidities and higher surgical risk. Hybrid procedures with a combination of surgical debranching and percutaneous thoracic endovascular aortic repair (TEVAR), avoiding EC, aortic cross-clamp and DHCA, have similar results when compared to standard surgery. Currently there are many experiences with total endovascular treatment combined with different percutaneous techniques and devices. Clearly this is still an ongoing process with emerging data that will need to be compared with open surgical outcomes in the short, medium, and long term.
The management of aortic disease involving the arch remains a formidable challenge. Aortic surgery has undergone major changes in the past half a century since the early series of aortic arch surgeries in the 1970s1. The approach to aortic arch pa- thology is very challenging due to the presence of supra-aor- tic vessels (with many anatomical variances), arch angulation, high blood flow, and the pulsatile nature of the proximal aorta, among other factors2. Conventional open surgery using Da- cron grafts requires a median sternotomy/thoracotomy, and cardiopulmonary bypass3. Moreover, aortic cross-clamping is associated with many neurological complications and also may predispose to retrograde aortic dissection4. Classic methods to reduce neurological complications such as deep hypothermic circulatory arrest (DHCA) and antegrade cerebral perfusion (ACP) have decreased neurological complications. Nevertheless, open surgical repair continues to be associated with significant mor- bidity and mortality5. Comorbid conditions and postoperative complications risk are most frequently defined using Society of Thoracic Surgeons (STS) definitions (www.sts.org) or the Europe- an System for Cardiac Operative Risk Evaluation II (EuroSCORE. http://euroscore.org/calc.html) to identify those “high-risk pa- tients” that are not good candidates for open surgical repair6.
In this article, classic aortic arch surgery approaches will be described along with different surgical techniques for neuro protection. We will also explore some novel hybrid and totally endovascular approaches for those patients who, based on their preoperative surgical risk, should not undergo a major open procedure. Large, open aortic arch repair series suggest that high-risk patients may be better managed by using a hybrid or totally endovascular approach7. Hybrid techniques have had promising results with good short-to midterm outcomes. Nev- ertheless, long-term follow-up studies are required to demon- strate whether hybrid strategies remain durable, including the patency of supra-aortic bypass grafts. Total endovascular repair for the management of aortic arch pathologies have also been described. Some preliminary reports are promising but the safe- ty of this technique is not known and high rates of complica- tions, including stroke have been initially reported8.
2Indications for Aortic Arch InterventionA range of aortic arch pathologies should be treated with open or endoluminal techniques including complicated aortic dissec- tions, aortic arch aneurysms, penetrating aortic ulcers and in- tramural hematomas (progressive or symptomatic). Intervention is indicated for elective repair of an isolated arch aneurysm at cm in size and annual growth rate of 5mm per year9,10.
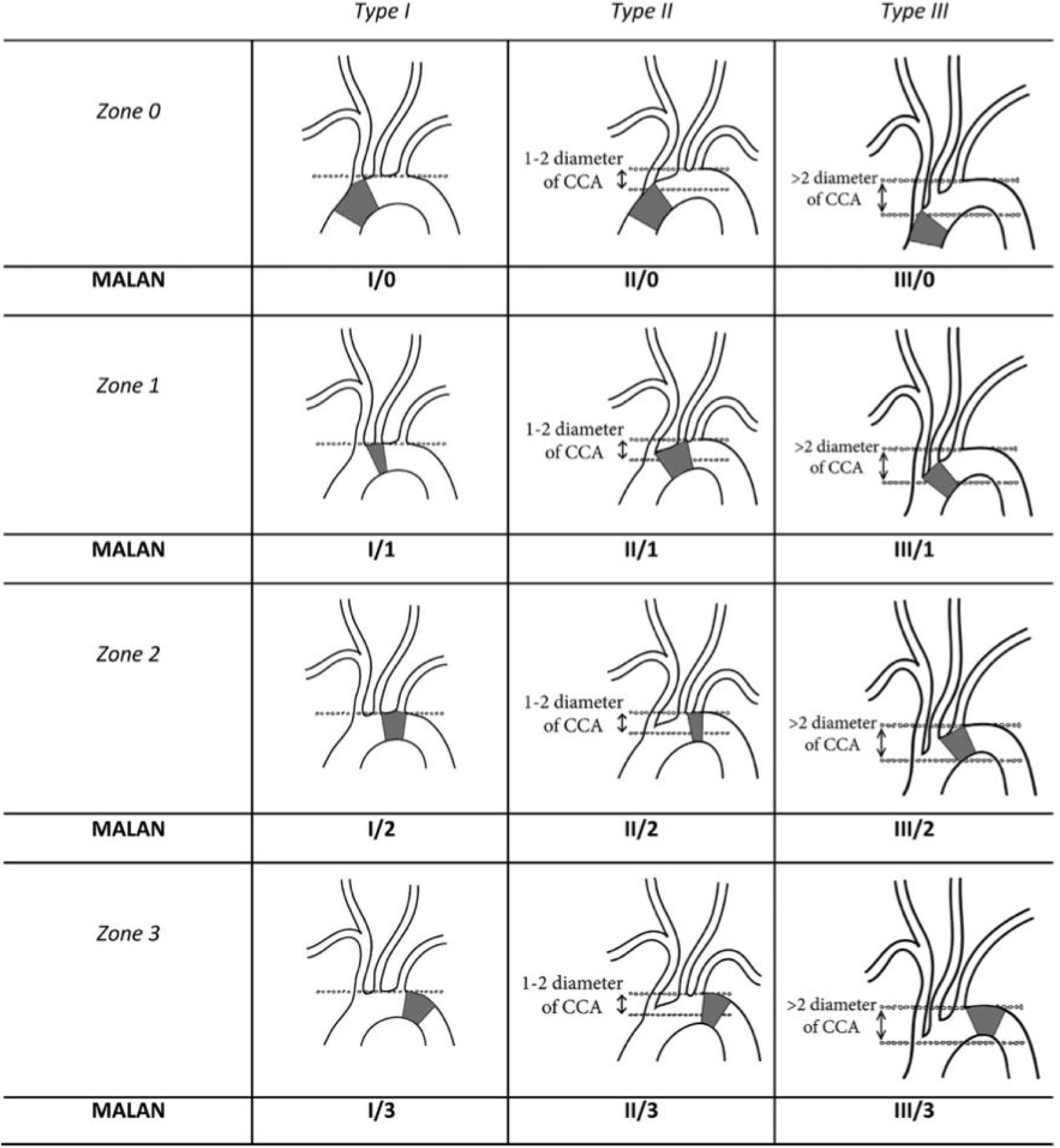
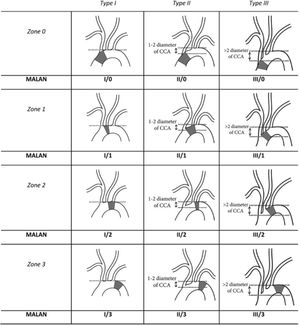
3Anatomical ConsiderationsDelving into the therapeutic options for aortic arch treatment as well as into the development of new prostheses for open, endovascular or hybrid aortic arch repair requires deep understanding of aortic arch anatomy. Most of the currently available stent grafts dedicated to aortic arch repair can be used as a stand-alone endograft or in com- bination with surgical debranching or via custom-made programs (mostly driven by physician modification). Since our knowledge of most common aortic arch anatomies is quite limited11, endovas- cular anatomy needs to be defined. Thoracic endovascular aortic repair (TEVAR) requires healthy proximal and distal landing zones of adequate diameter (<40mm) and length (>20mm) and a viable il- io-femoral or infrarenal aortic access route12. The angulation of the arch is also an important variable to be considered, since we know that a steep aortic arch angulation is highly predictive of endograft failure, and it might even represent a contraindication to TEVAR13. The initial endovascular anatomy was described by Ishimaru's ana- tomical work13,14, who described aortic arch zones as “landing zones” for endovascular treatment; nevertheless, it became obvious that, there were other important variables like the one we mentioned earlier, related to the aortic arch angulation, which are considered a critical condition for a successful deployment. A more comprehensive description then gave birth to a newly proposed modified arch landing areas nomenclature (MALAN), which comprises the proximal landing zones according to the Ishimaru aortic arch map and types of arches according to the aortic arch classification using the common carotid artery (CCA) as a reference to determine aortic arch angula- tion15 (Fig. 1).
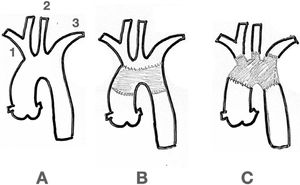
4Surgical Options4.1Open SurgeryAs mentioned above, the aortic arch is the connecting segment between the ascending and the descending thoracic aorta. Usually, it is affected along with them, either with the presence of aneurysms, complex arch dissections, penetrating ulcers, intramural hematomas or simply with atheromatous disease. Isolated artic arch disease is a rare condition. When a type A dis- section occurs, more than 20% of all patients also have the arch dissected, with or without a tear16. When the patient also has a connective tissue disorder, total arch replacement should be considered17. The supra-aortic vessels can be either reimplant- ed as an island or bypassed individually (Fig. 2).
Aortic arch surgery is one of the most technically demanding procedures in cardiovascular surgery. Becoming an aortic sur- geon requires a thorough knowledge of all the standard cardiac surgical techniques related to perfusion and myocardial protec- tion in addition to special expertise on brain, spinal cord, and lower body preservation accompanied, of course, with a rigor- ous surgical technique. During the last two decades several new ways to approach brain and end organ protection have been proven to be safe. Planning with regards to cerebral protection and cannulation strategy are essential when it comes to open aortic arch surgery. A bloodless surgical field is mandatory and so is protecting the brain and the rest of the organs.
4.1.1Brain protection, monitoring, cannulation and surgical considerations1.- Brain protection: cerebral perfusion demand is crucial. Complications arising from inadequate cerebral protection re- main considerable and often represent the limiting factor for good outcomes. There are surgical techniques that can reduce metabolic demands through deep hypothermic circulatory ar- rest (DHCA), antegrade cerebral perfusion (ACP) or retrograde cerebral perfusion (RCP).
DHCA has remained the cornerstone of effective brain protec- tion strategies and is one of the most widely practiced tech- niques today18. By lowering brain temperatures, intracellular metabolism is reduced, thus prolonging the duration of “safe” circulatory arrest time. Clinically, the duration of DHCA has been associated with the incidence of temporary neurologic deficit. It is recommended that DHCA be limited to less than 30–40min, with prolonged durations resulting in poorer short- and long-term outcomes19. The extent of hypothermia during hy- pothermic circulatory arrest (HCA) (nasopharyngeal tempera- ture), as established by the International Aortic Arch Surgery Study Group (IAASSG)20: Profound hypothermia ≤14°C; Deep hypothermia 14.1–20°C; Moderate hypothermia 20.1–28°C; Mild hypothermia 28.1–34°C.
ACP must be planned beforehand if the DHCA is anticipated to be greater than 30min, ACP is typically used as an extra pres- ervation technique added to the DHCA, by directly supplying warm or mildly cold blood to the brain via the axillary or su- pra-aortic vessels. While DHCA is the preferred method among American surgeons, ACP approach is preferred by most Europe- an and Japanese centers, representing up to 90% of surveyed institutions21. Since most of the time the perfusion of the brain is initiated unilaterally (most likely right-side through the axil- lary artery or innominate trunk), anatomically, this unphysiolog- ic perfusion pattern relies on the integrity of the circle of Willis and secondary extra-cranial collateral networks. Despite scien- tific evidence favoring bilateral ACP, clinical evidence this far has not demonstrated a clear superiority22.
RCP was first described by Ueda and colleagues in 199023, RCP relies on supplying cerebral blood flow through the superior vena cava (SVC) and the associated venous system. In theory RCP provides metabolic substrates, maintains brain hypother- mia, and flushes out embolic debris. Numerous animal and in vivo models have challenged the role of RCP, which explains why in recent decades it is only used in select centers24. Although enough scientific studies on RCP exist, there is little clinical ev- idence to conclusively demonstrate RCP's superior clinical out- comes. Nevertheless, RCP has been shown to offer similar ce- rebral protection to ACP25. It seems that the utility of RCP rests on its ability to provide localized topical cooling of parenchymal tissue as well as its potential for embolic washout.
2.- Brain monitoring:Transcranial Doppler (TCD) ultrasonogra- phy can be used to detect and quantify cerebral micro emboli during surgery (open or endovascular). Ultrasound probes are placed bilaterally on the temple, overlying the middle cerebral vessels. Emboli cause an increase in the reflected ultrasound, causing high-intensity transient signals (HITS). These HITS are the footprints of micro emboli26.
Another method is the Cerebral Perfusion Index: regional cerebral oxygen saturation is monitored noninvasively using a near-in- frared spectroscopy (NIRO or NIRS) which is a representation of brain activity and degree of ischemia. NIRS is simple and non-invasive. It provides real time and continuous monitoring during the entire procedure. A bedside monitor of easy access and reading is connected to bilateral frontal sticky pads with an interior sensor that captures instant cerebral oxygen saturation. This device should be a standard of care and routinely applied in all cases27. More than a single instant value, the fluctuations and the trend in sensor levels can alert surgeons to either main- tain or modify their brain protection approach to avoid potential risks28. Electroencephalography, and jugular venous oxygen sat- urations are other available monitoring methods.
3.- Cannulation: In early series, the femoral artery was used as a cannulation site by Griepp and colleagues29. Over the ensu- ing years femoral cannulation has decreased in use and inter- est since the risks of retrograde embolization have proven to be higher, especially in those patients with a diseased descending aorta. This is particularly important in aortic dissections since intimal flap behavior becomes totally unpredictable after fem- oral retrograde flow is established. Malperfusion of one or more organs can also be increased because of this unpredictable shifting of the intimal flap30. Any type of cannulation allowing forward flow and perfusion (axillary artery or the innominate trunk) is deemed to be favorable in overcoming the limitations of femoral artery cannulation. Lately, central cannulation (R ax- ilary artery or innominate trunk) has become the preferred op- tion in many centers because it reduces the probability of embolic strokes, decreases the disruption of atheroma or calcified plaques, minimizes the risk of malperfusion in dissection cases, and could provide an easy method to rapidly establish ACP if required.
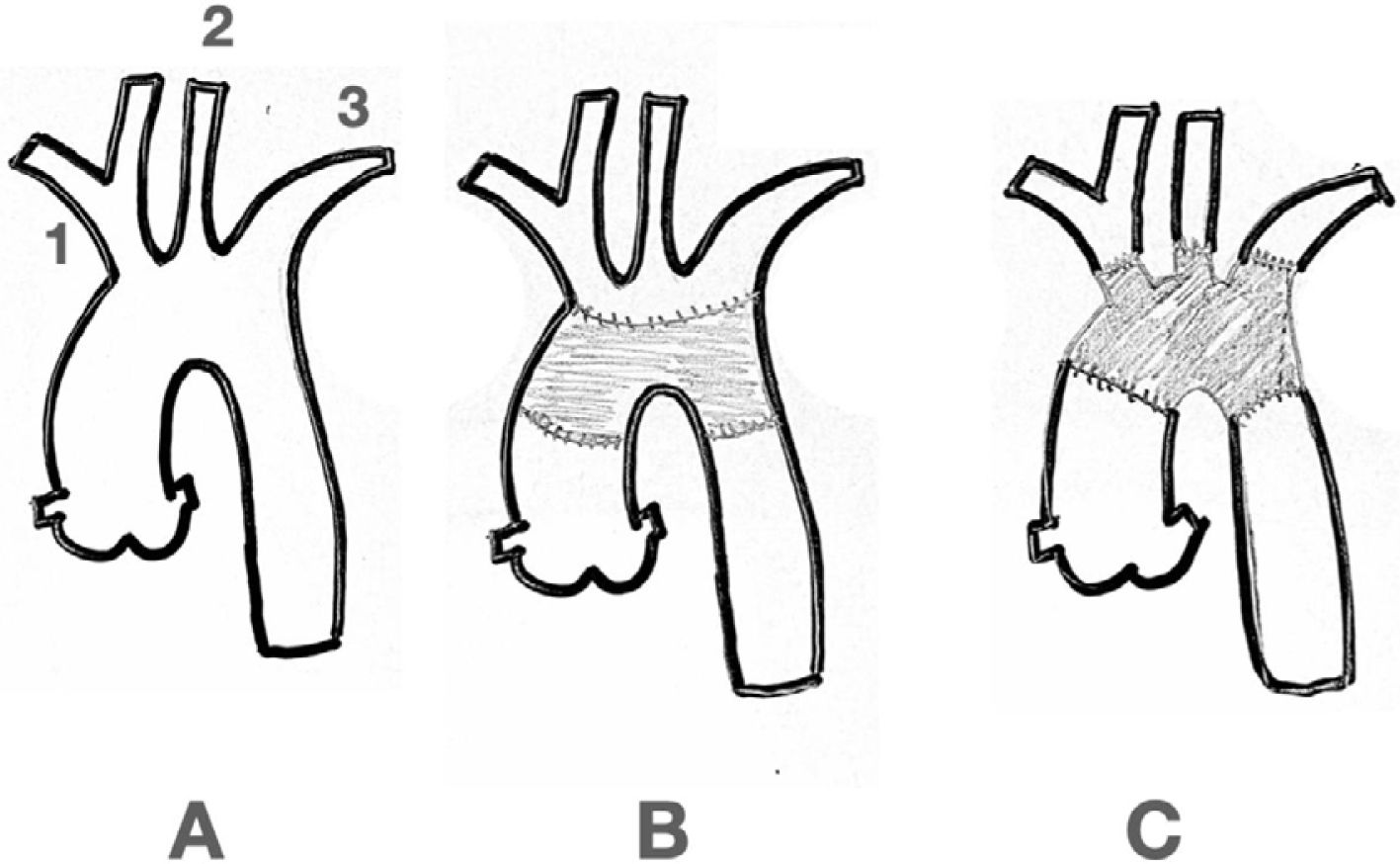
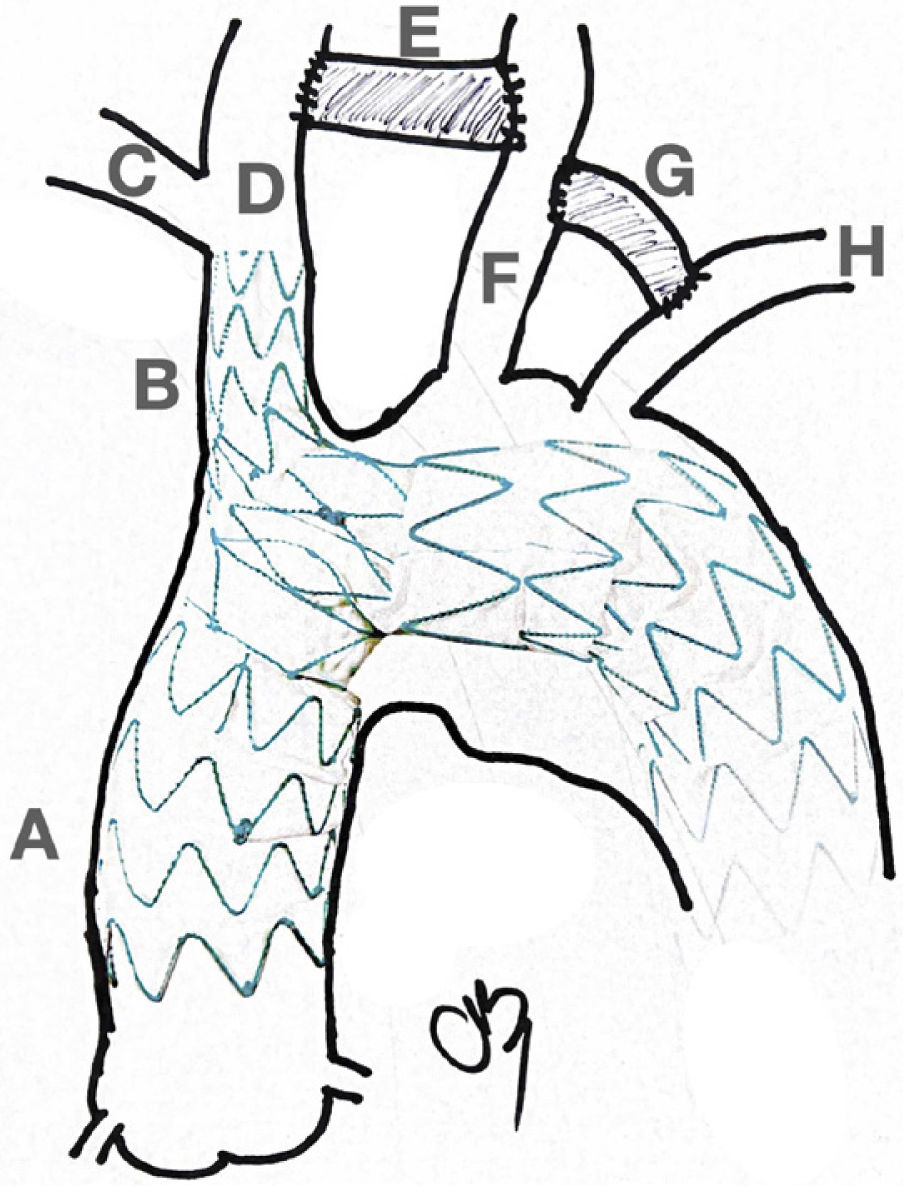
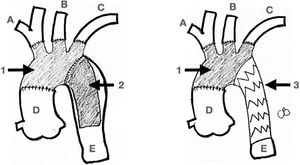
4.- Surgical Considerations: For Type A (Debakey I) aortic dis- section treatment, the traditional model adopts a conservative approach, with resection of the intimal tear within the dissected segment of the aorta. The main purpose of this emergency op- eration has always been to bring a patient alive back from the operating room. Most of the times this is accomplished by re- placing just the ascending aorta and with an occasional exten- sion to the hemiarch. However, with notorious improvements in surgical techniques and the availability of new devices, the first approach is now deemed to be more extensive with regards to aortic resection and reconstruction. It has been recognized that a residual patent false lumen in the arch and descending tho- racic aorta is present in up to 60% of patients with up to 30% of patients requiring reoperations32,33. Considering the long-term complications and the availability of endovascular stent-graft devices, numerous centers have started to advocate for a more extensive initial repair, typically involving a procedure called the frozen elephant trunk (FET). The introduction of the elephant trunk (ET) concept and technique by Borst and colleagues in 1983 promoted the combination of open aortic arch replace- ment with a soft distal Dacron graft hanging down in the true lumen of the descending thoracic aorta for a staged surgical re- pair of a distal residual aneurysm or dissection34. The concept of “frozen” is related to the use of a self-expanding covered stent graft instead of a “floppy” Dacron. This is considered a shift in the treatment paradigm from traditional open repair to the in- novative use of combined open and endovascular techniques (Fig. 3).
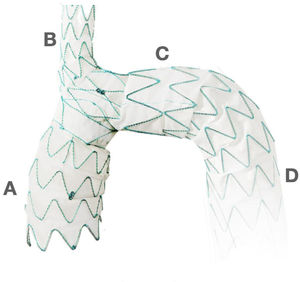
Elephant Trunk. The classic Elephant Trunk procedure (left picture) is the combination of the classic open aortic arch replacement with a Dacron graft (1) and a free-floating extension of the arch prosthesis into the descending aortic true lumen (2). The Frozen Elephant Trunk (right picture) version is with the forward endovascular deployment of a self-expandable covered stent. (3) in the descending thoracic aortic true lumen. A: Innominate trunk B: Common carotid artery C: Left subclavian artery D: Ascending aorta E: Descending thoracic aorta.
By exerting radial pressure inside of the true aortic lumen, it promotes false lumen thrombosis. This procedure could obviate the need for second-stage operations to repair the downstream aorta. With promising initial results, many devices combining sewn-in-graft and self-expanding stent graft appeared in the market including, among others, the E-vita Open Plus (Jotec GmBH, Hechingen, Germany), Thoraflex Hybrid (Vascutek, In- chinnan, Scotland, UK), Cronus (MicroPort, Shanghai, China), Chavan-Haverich (Curative GmbH, Dresden, Germany), and the J Graft (Japan Lifeline, Tokyo, Japan)35. Some of these devices are currently going through the FDA approval process for com- mercial use within the United States, hence restricting their use mostly to Europe and Asia.
Some promising mid-term results with the use of FET have been reported. A 10-year follow-up have showed a decent morbid- ity and mortality and high rate of false lumen thrombosis/oc- clusion. There are still some concerns of spinal cord ischemia related to the extent of the aortic coverage36. When it comes to comparison of conventional hemiarch and total arch replace- ment with FET, the latter is technically more demanding and is related with longer periods of circulatory arrests. The cardiovas- cular surgery community demands more extensive and longer follow-up to determine the long-term benefits of FET.
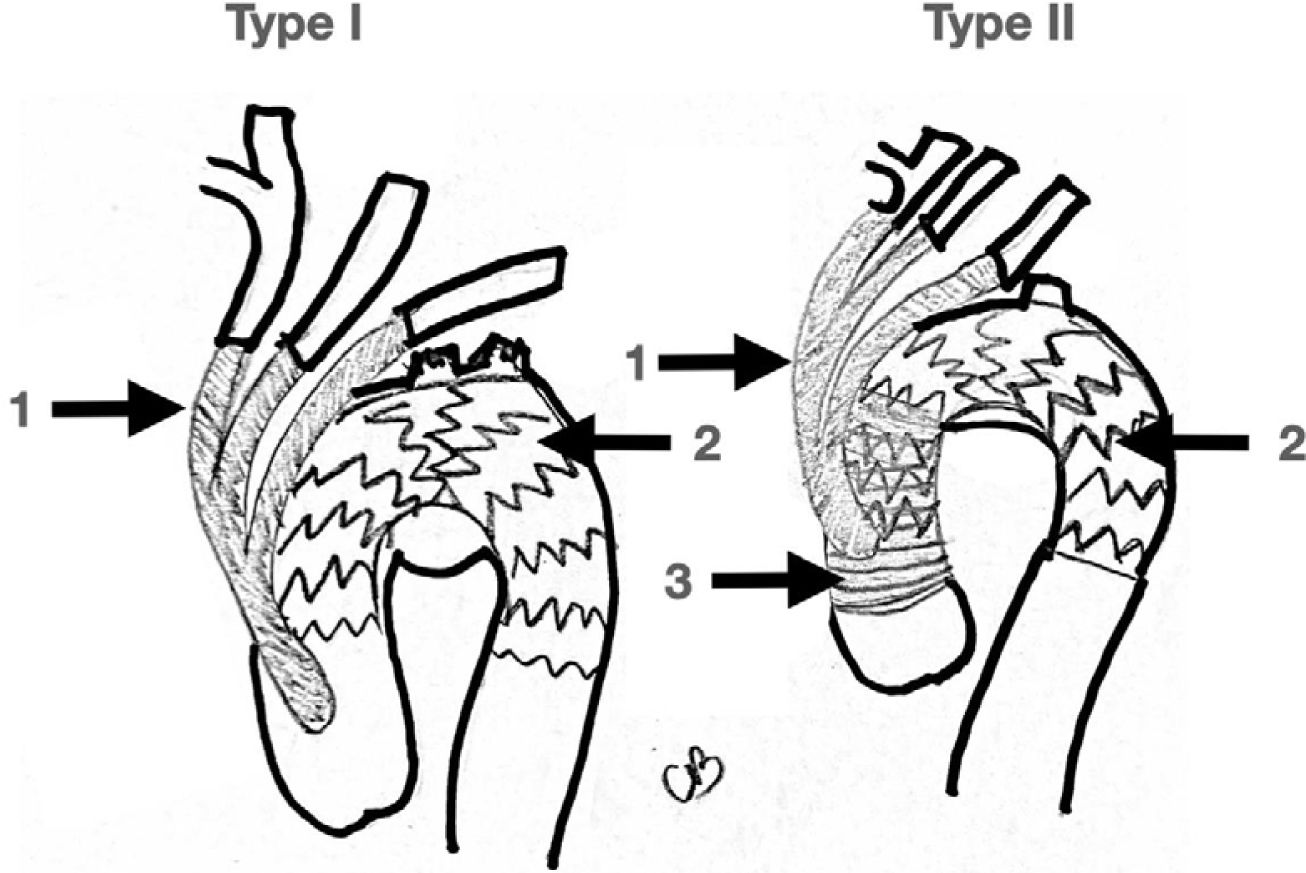
4.2Hybrid Approach: DebranchingRelatively early in the development of endovascular surgery a variety of open/endovascular hybrid solutions were designed to address difficult problems. In 1998, Buth published the first hy- brid repair case for the treatment of an aortic arch aneurysm37. Bavaria has proposed a classification for hybrid arch repair38:
Type I: Supra-aortic vessel debranching without ascending aorta or aortic arch reconstruction followed by TEVAR.
Type II: Supra-aortic vessel debranching+TEVAR with ascending aortic reconstruction.
Type III: Supra-aortic vessel debranching+ TEVAR with ascending aorta and aortic arch reconstruction with an elephant trunk (ei- ther soft or frozen).
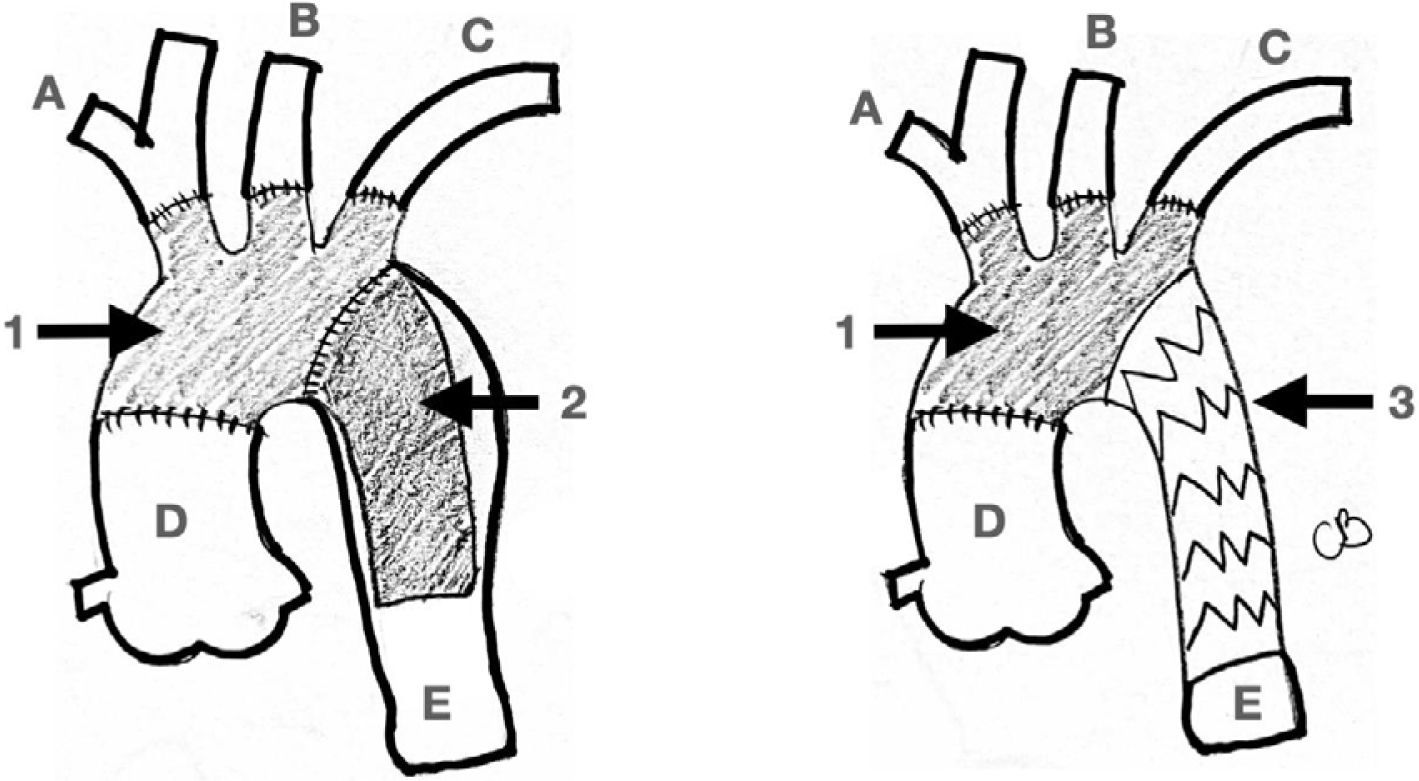
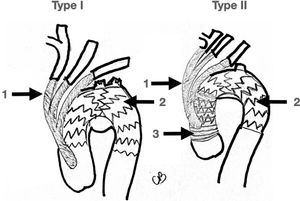
Real hybrid approaches are type I and II since both approaches avoid clamping of the aortic arch distal to the innominate trunk and avoid DHCA. On the other hand, type III always requires aor- tic arch clamping and circulatory arrest, and so it was described earlier here as open aortic arch replacement (Fig. 4).
4.3Total Endovascular Treatment Options and Future DirectionsUntil today, open surgery remains the gold standard specially for those young and fit patients, as well as for those with con- nective tissue disorders. As we mentioned earlier, regular open aortic hemi or total arch repair require cardiopulmonary bypass, DHCA and ACP or RCP, which increases the overall morbidity and mortality. The duration of the surgical procedure and the intensive care unit length of stay are significantly less in those patients with endovascular repair compared to open surgical re- pair39. Of notice is that comparable perioperative mortality and stroke rates have been reported between the endovascular arch repair and open arch repair, despite the older age and a higher comorbid profile of patients with an endovascular approach40. Increasing life expectancy has led to a greater elderly popula- tion, with many associated comorbidities and increased frailty.
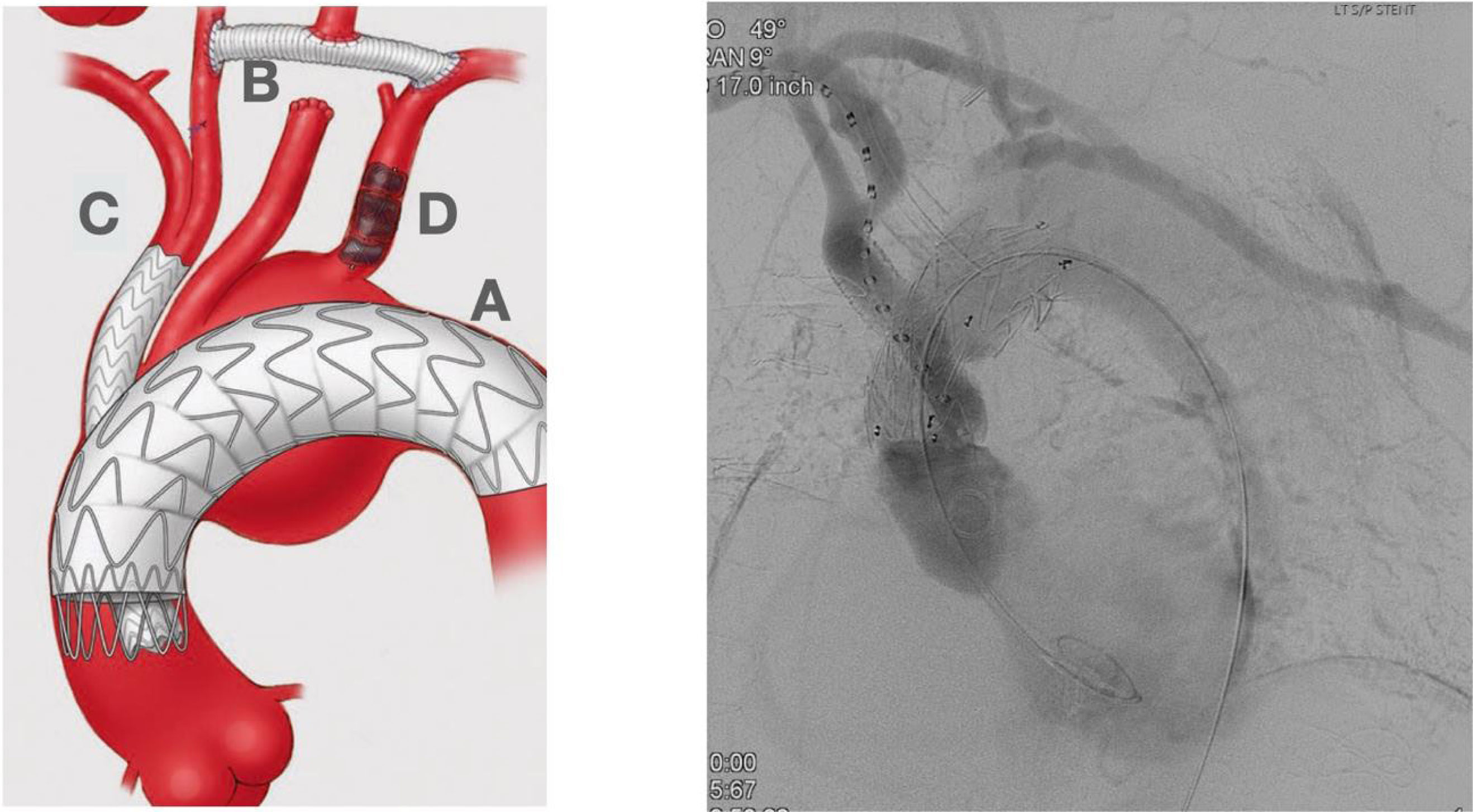
Traditional open aortic arch repair is often too risky, and the pa- tients were denied of an opportunity for a surgical repair. Total endovascular repair arises as an incredible opportunity for this subset of patients to be treated. Total endoluminal strategies in- volve a wide variety of custom-made devices with fenestration, scallop, inner branched endo-grafts, parallel (chimney) endog- rafts, etc. Fig. 5 the first and only CE-approved off-the-shelf branched endovascular aortic arch system.
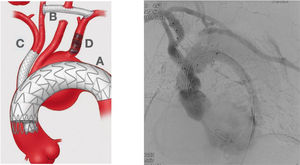
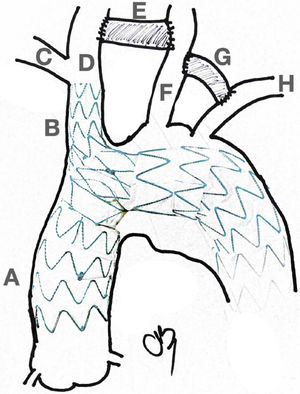
Hybrid Approach. Left picture shows an almost total endovascular aortic arch replacement. A: TEVAR. B: extra anatomic right carotid to left carotid to left subclavian bypass. C: chimney stent to the innominate trunk. D: left subclavian artery amplatz plug to avoid type II endoleak. Right picture shows the completion angiogram with patency of the chimney stent and flow through the extra-anatomical bypass.
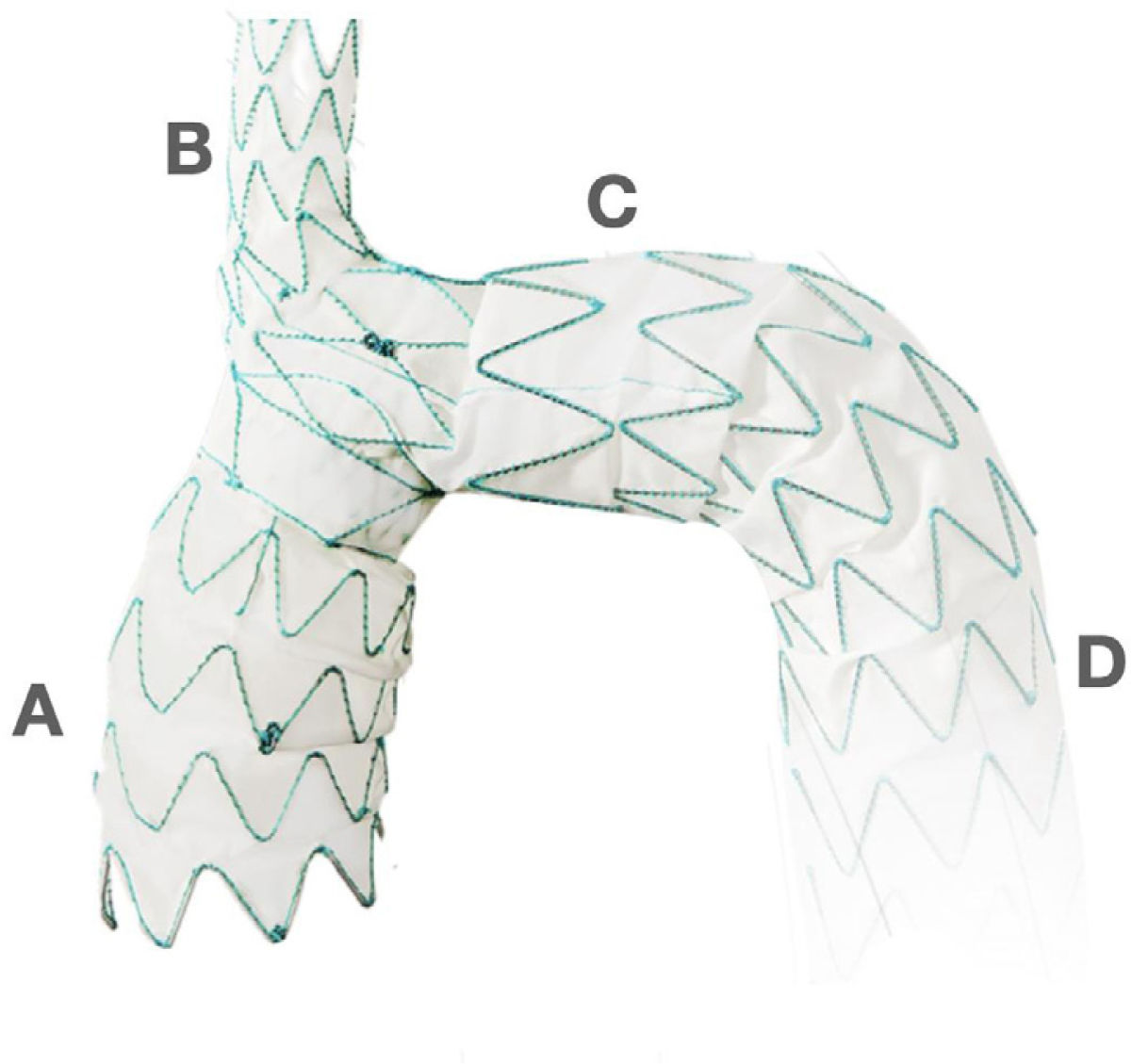
New technologies are becoming available for full or hybrid en- dovascular approach of the aortic arch. They are designed to overcome the specific challenges of the aortic arch anatomy in a wide range of aortic pathologies, including arch aneurysms, thoracic dissections, and penetrating atherosclerotic ulcers. One of them, worth mentioning, is an aortic arch stent graft with a single branch for the innominate trunk that has recently obtained Food and Drug Administration (FDA) approval for its clinical use as a part of the study called the Endospan TRIOMPHE trial in August 2020 (Fig. 6). This stent works in combination with an extra anatomic right to left cross cervical carotid-carotid bypass grafting and left carotid to left subclavian bypass graft- ing (Fig. 7).
The International Registry for Acute Dissections (IRAD), the In- ternational Aortic Arch Surgery Study Groups Multi-Institution- al Database, the upcoming ARCH Prospective Registry, and the newly formed Aortic Research Consortium in the United States
have all developed during the last decade showing a significant growing trend toward collaborative research. All these com- bined efforts will mingle together and enrich the new clinical data to overcome research limitations that have previously stalled advances in surgical practice.
Finally, surgical boundaries will continue to fade away. With in- creasing practice of minimally invasive cardiovascular surgery and the continued development of endovascular devices, open aortic arch surgery will continue to evolve and transform42.
Declaration of conflict of interestThe authors have no conflicts of interest to declare that are relevant to the content of this article.
Referencias no citadas