To evaluate the effect of 2 different protocols of intra-articular hyaluronic acid (HA, hylan G-F20) to articular cartilage regeneration in acute full-thickness chondral defects.
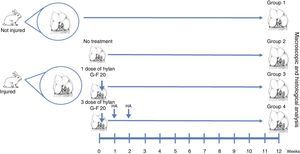
Materials and methodsFull-thickness chondral defects of 3mm×6mm were performed into the lateral femoral condyles of New Zealand rabbits, treated with a single or three doses of HA. The animals were sacrificed at 12 weeks and the regenerated tissue was evaluated by direct observation and histology with the ICRS scale.
ResultsMacroscopically, in both groups treated with HA the defects were filled with irregular tissue with areas similar to hyaline cartilage and others in which depressed areas with exposed subchondral bone were observed. Histological analysis showed in both groups treated with HA a hyaline-like cartilage compared to control group. However, the score of the International Cartilage Repair Society (ICRS) scale did not show differences between the groups treated with HA.
ConclusionThe use of single dose or 3 doses of AH in acute chondral lesions has a limited and similar benefit in articular cartilage regeneration.
Evaluar el efecto de 2 protocolos diferentes de ácido hialurónico (AH) intraarticular (hylan G-F20) sobre la regeneración del cartílago articular en lesiones condrales agudas de espesor completo.
Material y métodoSe realizaron lesiones condrales de espesor completo de 3×6mm en los cóndilos femorales de conejos New Zealand, tratados con una dosis única o tres dosis de AH. Los animales fueron sacrificaron a las 12 semanas y el tejido regenerado fue evaluado mediante observación directa e histología con la escala ICRS.
ResultadosMacroscópicamente, en ambos grupos tratados con AH los defectos se rellenaron con tejido irregular, con zonas similares al cartílago hialino y otras en las que se observaron áreas deprimidas con exposición de hueso subcondral. El análisis histológico demostró en ambos grupos tratados con AH un tejido similar al cartílago hialino comparado con el grupo control. Sin embargo, la puntuación de la escala Internacional Cartilage Repair Society (ICRS) no mostró diferencias entre los grupos tratados con AH.
ConclusiónEl uso de dosis única o 3 dosis de AH en lesiones condrales agudas tiene un beneficio limitado y similar en la regeneración del cartílago articular.
Chondral knee injuries are common among athletes, especially those who practice contact sports.1 We know that these lesions have a very limited potential for repair, including small lesions which, if untreated, may predispose toward joint degeneration with functional disability and high treatment costs.2 Multiple treatment options have been proposed for the management of joint cartilage lesions, including subchondral drilling, microfractures, osteochondral autografts and autologous chondrocyte implantation, with poor results in some cases.3,4 New therapeutic options to prevent the development of osteoarthritis after suffering focal chondral lesions have also been recommended, including symptomatic slow-acting drugs for osteoarthritis (SYSADOAS), such as glucosamine and chondroitin sulfate, and intraarticular injections, like hyaluronic acid (HA), with a joint protection effect.5–8 HA is a high molecular weight mucopolysaccharide (5–7×106Da) with the viscoelastic properties of synovial fluid, which is essential to normal joint function. Thus, HA has a mechanical and healing effect on the cells of the cartilage and synovial membrane. In patients with osteoarthritis, the concentration of HA in the synovial fluid of the knee is reduced by 2–3 times and its molecular weight also decreases to minimum levels, 2×105Da.8,9
Intraarticular injections of HA have been shown to relieve pain and improve joint function in patients with knee osteoarthritis in the short and medium term.10,11 Experimental studies have shown that HA decreases the expression of metalloproteinases (MMP) and IL-1 in the synovium and prevents changes in the proteoglycans (PG) of the cellular matrix, reducing synovial inflammation and increasing PG content.12–14 Kaplan et al.15 conducted a study of acute cartilage lesions in sheep and showed that 3 doses of HA improved histological parameters and PG content of the joint cartilage.
Our hypothesis was that the use of HA increases hyaline cartilage regeneration in focal chondral lesions and that 3 injections of HA increase tissue regeneration. Therefore, the aim of our study was to evaluate joint cartilage regeneration in acute, full-thickness, chondral lesions in an animal model through 2 different treatment protocols with high molecular weight HA.
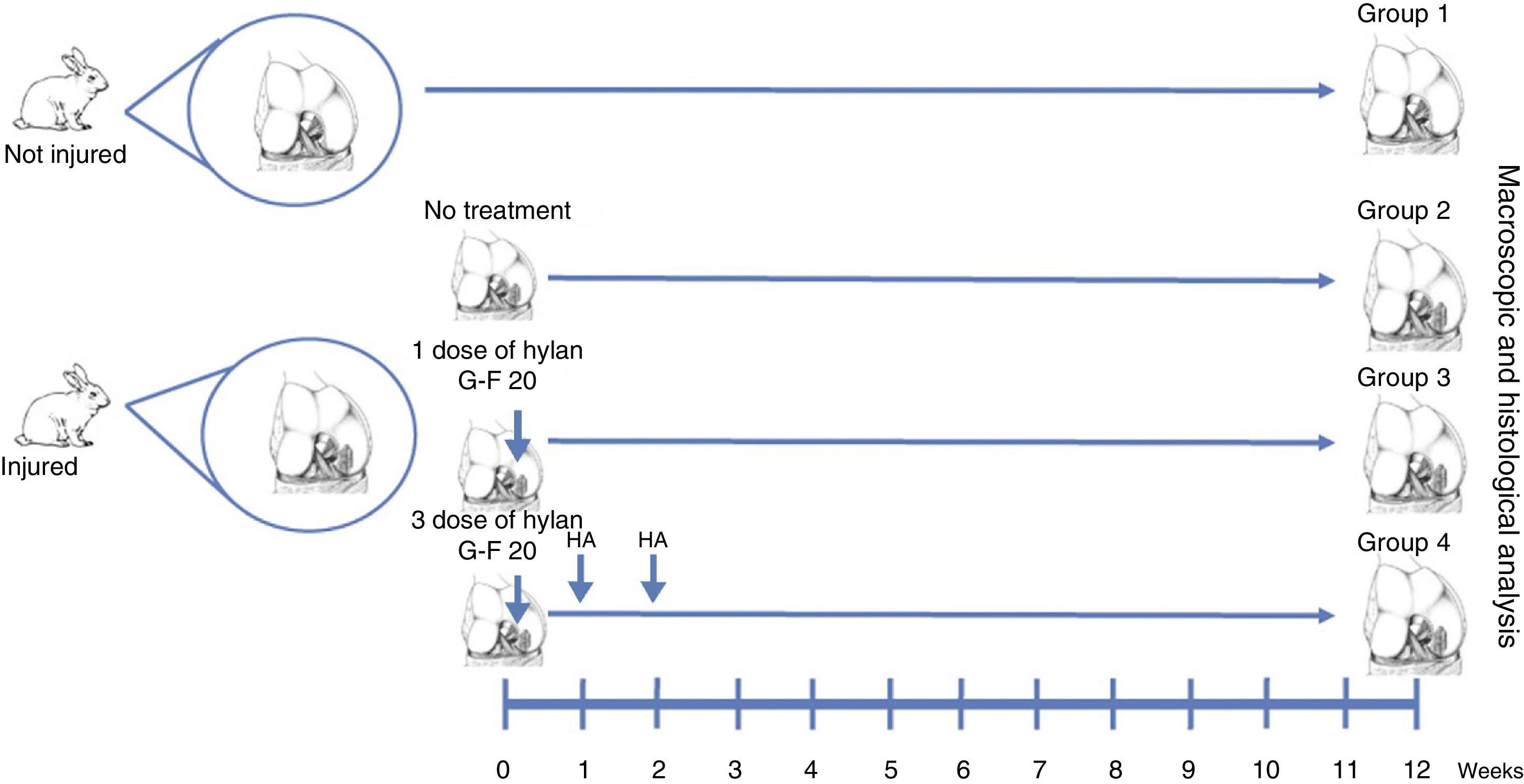
Materials and methodsWe created full thickness chondral lesions in the femoral condyles (n=30) of 15 New Zealand rabbits following the protocol depicted in Fig. 1. All the animals were 3-month-old males weighing between 2.5 and 3.5kg.
The animals were kept in separate 40cm×40cm×60cm cages under constant temperature and humidity conditions, with a light-dark cycle of 12/12h, ad libitum feed and ability to roam freely, without restraints.
The research protocol was approved by the Ethics Committee of the German Clinical Medicine School-Development University. All procedures were performed under aseptic conditions, using intramuscular anesthesia with ketamine (35mg/kg), xylazine (5mg/kg) and acepromazine (1mg/kg). We also used enrofloxacin (10mg/kg) and tramadol (4mg/kg) preoperatively and for 2 days after surgery.
Surgical techniqueThe cartilage defects were full thickness, 3mm×6mm, created in load areas of the lateral femoral condyles of each knee, via a longitudinal parapatellar arthrotomy.16 The chondral defects were created with a 3mm osteotome up to the calcified layer, which was removed with a curette and taking care not to damage the subchondral bone. The condyles were divided into 4 groups. In group 1, no defect was created and no treatment was applied (normal group, n=12). In group 2, a chondral defect was created, but no treatment was applied (injured untreated group, n=6). In group 3, the chondral defect was created and the animals received a single dose of HA (Hylan G-F 20®, 3.5mg/0.5ml) at the end of the procedure (single dose of Hylan G-F 20 group, n=6). In group 4, the chondral defect was created and the animals received 3 weekly doses of HA (Hylan G-F 20® (3 doses of Hylan G-F 20 group, n=6).
No complications were recorded and no animals had to be sacrificed before the end of the study. The rabbits were sacrificed at 12 weeks through an intravenous overdose of pentobarbital, and the condyles were resected for study.
Macroscopic analysisAll condyles (n=30) were observed directly, immediately after being obtained. We recorded characteristics such as tissue color, appearance of the joint surface and the presence or absence of subchondral bone exposure.
Histological analysisThe condyles (n=30) were fixed in formalin, decalcified in EDTA and then embedded in paraffin. We obtained 10μm thick sagittal sections of the tissue. The sections were stained with hematoxylin/eosin (H/E) and blindly analyzed by a trained anatomical pathologist, using the International Cartilage Repair Society (ICRS) scale to assess cartilage repair.17 This scale consists of 6 categories and the score is based on the most representative feature present in each sample.
Statistical analysisThe unit of analysis used in this study was each lateral femoral condyle (n=30). The score in the ICRS scale (median) was analyzed using the Mann–Whitney U test, and values of p<.05 were considered statistically significant.
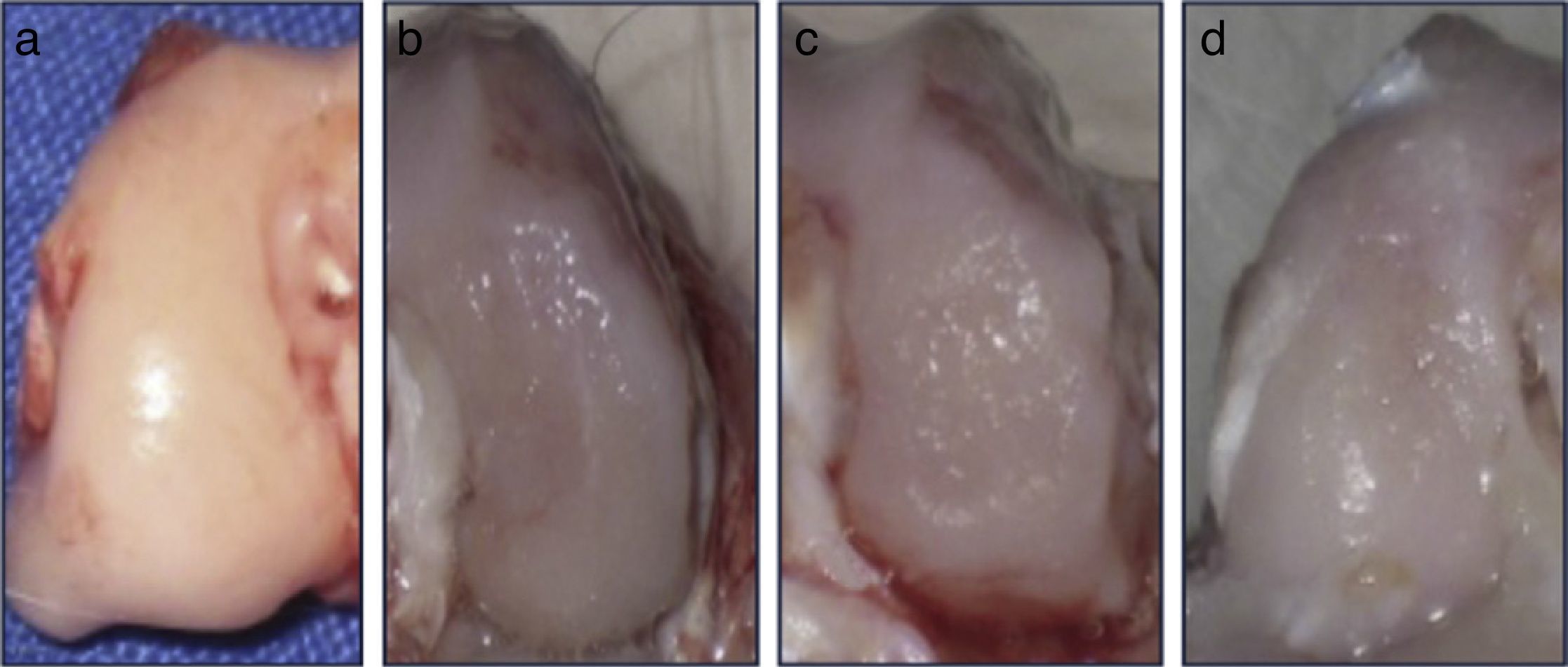
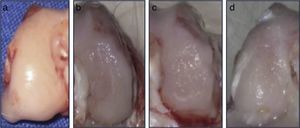
ResultsMacroscopic analysisGroup 1 (normal group) presented a soft, shiny and semitransparent tissue. At 12 weeks after surgery, the femoral condyles in group 2 (injured untreated group) presented a very fine and irregular tissue, with a rough appearance and a well-defined area of injury. In group 3 (single dose of HA group), the tissue was thin, with a hyaline appearance but with depressed areas and exposure of subchondral bone. In group 4 (3 doses of HA group), the newly formed tissue was slightly irregular and thin, similar to that observed in group 3 (Fig. 2). In groups undergoing surgery, although the defects were clearly differentiated from the surrounding cartilage, no signs of joint degeneration were observed.
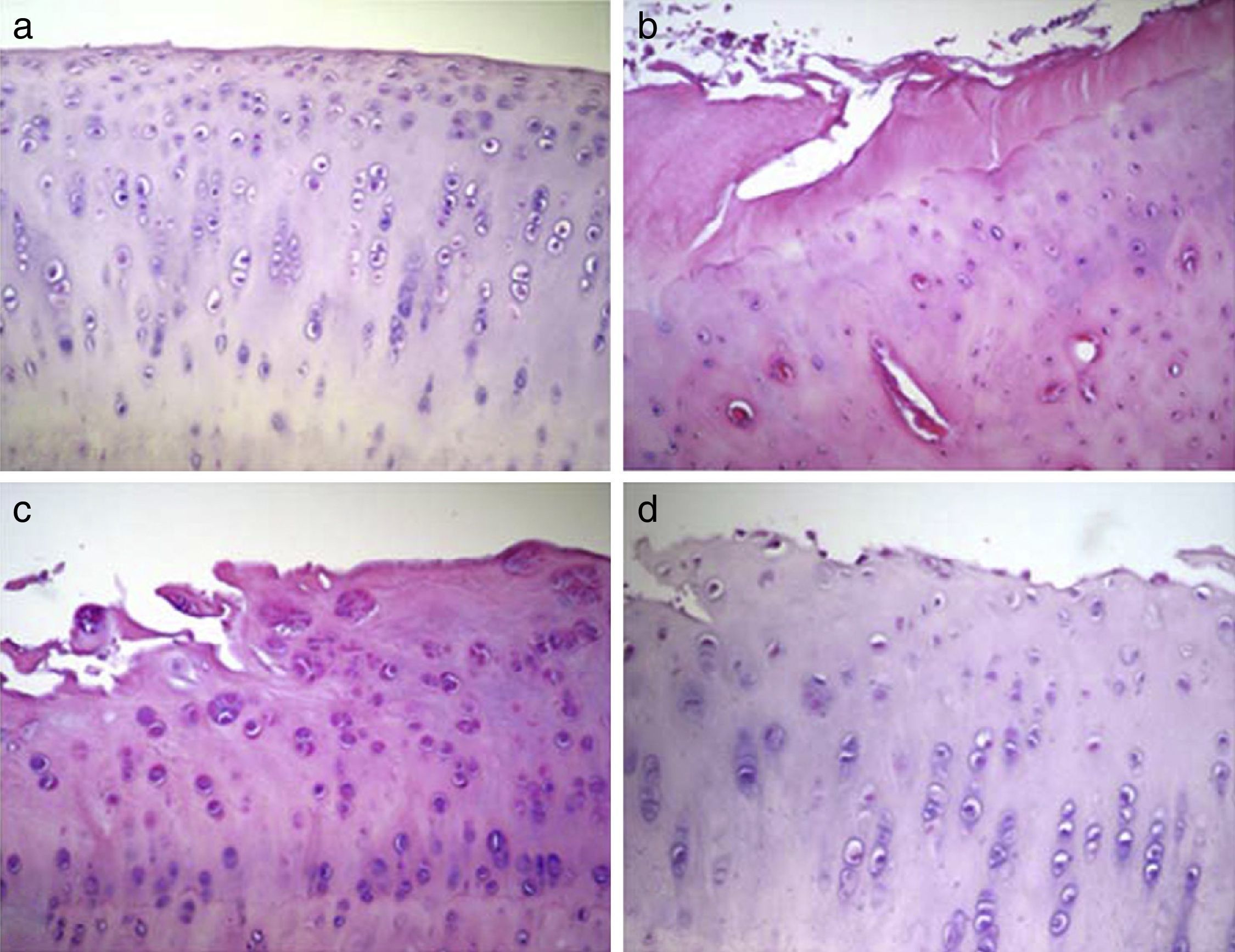
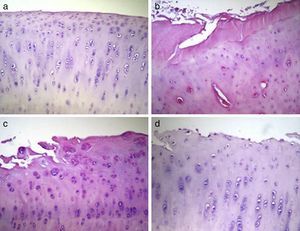
Histological analysisThe condyles in group 1 presented a regular joint surface, with a hyaline matrix and chondrocytes arranged in columns. The majority of condyles in group 2 presented an irregular surface, with a fibrocartilage matrix and unevenly distributed chondrocytes. In group 3, most samples presented an irregular surface, with a predominantly hyaline matrix but with a cellular distribution in columns and areas of clusters. Lastly, the condyles in group 4 showed irregular surfaces and considerable variability in cell distribution among samples, with some samples presenting an uneven distribution and others an arrangement in columns and clusters (Fig. 3).
Histological analysis of cartilage 12 weeks after the intervention. The data shown represent 4 sections (20×) per animal. (a) Group 1 or normal (n=12); (b) Group 2 or injured untreated (n=6); (c) Group 3 or single dose of Hylan G-F 20 (n=6); (d) Group 4 or 3 doses of Hylan G-F 20 (n=6).
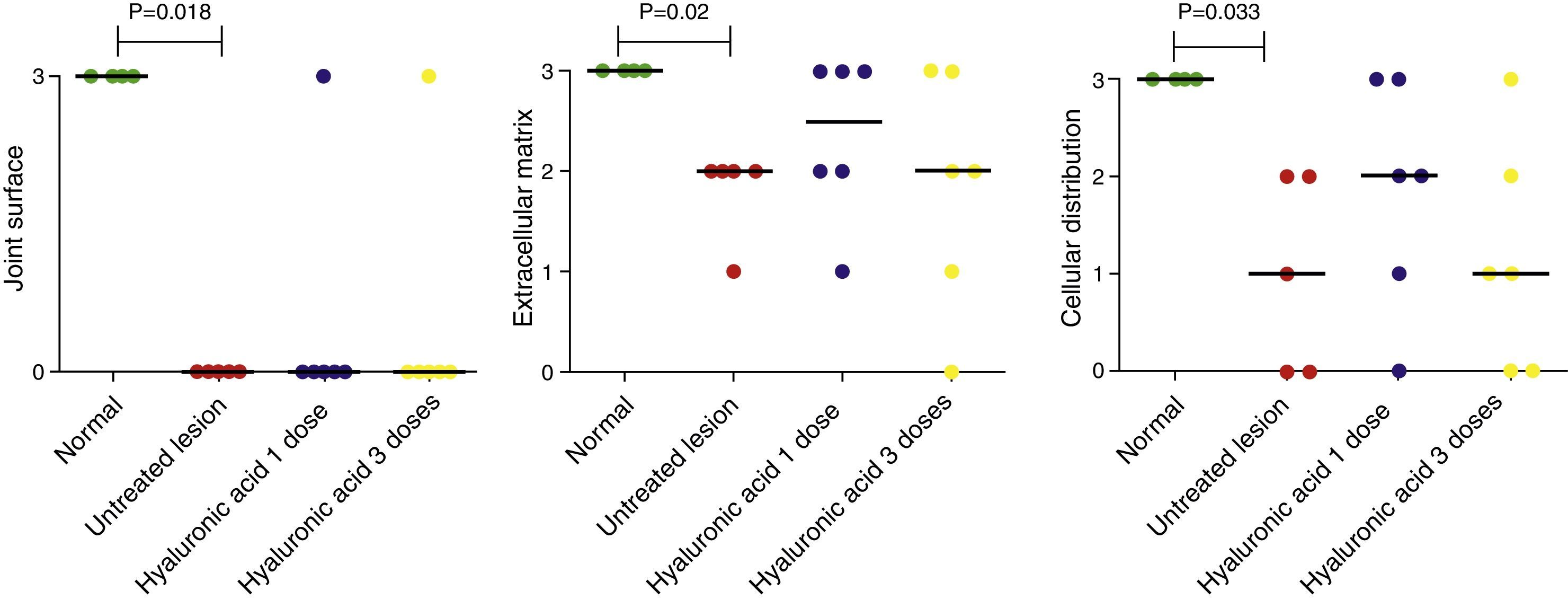
Quantitative analysis of histological data showed that cellular viability, subchondral bone involvement and cartilage mineralization did not change between the groups. However, characteristics like joint surface, extracellular matrix and cell distribution varied from one group to another (Fig. 4). The condyles in group 1 had the highest score (3 points), whilst samples from group 2 presented a significant reduction in the scores in all 3 categories (3–0, 3–2 and 3–1, respectively) (p<.05). The median value for the surface category of condyles in both group 3 and group 4 was the same as that from group 2 (0–0–0) (p>.05). In the category of cellular distribution and matrix, groups 3 and 4 included more samples with hyaline characteristics than group 2, but these differences were not statistically significant.
DiscussionIn the present study, we could not show that, in rabbits, the treatment of full-thickness chondral lesions with Hylan G-F 20® significantly improved the regeneration of joint cartilage at 3 months. Moreover, we found no differences in the macroscopic and histological characteristics of newly formed tissue when comparing the protocols with a single dose and with 3 weekly doses. In these groups, the defects were filled by mixed tissue, with fibrocartilage and hyaline characteristics and variable presentation between samples. According to these results, the use of Hylan G-F 20® in acute chondral lesions has some effect, but this does not seem to be significant.
There are multiple clinical options for the treatment of chondral lesions. However, none of these strategies have been able to regenerate the joint cartilage.3,4 Currently, intraarticular viscosupplementation with HA is used as an alternative in patients with osteoarthritis and several multicenter, double-blind, randomized and placebo studies comprising a large number of patients have been published, which have demonstrated that HA infiltration is a safe, well-tolerated intervention that provides continued pain relief and improves patient function with few adverse reactions.10,18,19 However, these studies were mostly conducted on osteoarthritic patients whose pathologies had little in common with isolated chondral lesions.
Furthermore, some experimental studies have reported good results with HA in the cellular viability and regeneration of chondrocytes, but these models are not comparable with our work, since they assess partial chondral lesions,20,21 rather than full thickness ones, as in our work.
Viscosupplementation with HA has been studied in isolated joint cartilage lesions associated with microfractures.21,22 Working with rabbits, Strauss et al.22 demonstrated that microfractures associated with 3 weekly doses of HA filled the chondral defect and improved the macroscopic appearance of the tissue. Additionally, degenerative joint changes decreased significantly through the use of HA. This could be explained by the synergistic effect of both procedures, rather than each of them separately.
The precise mode of action of HA in cartilage repair is not yet fully understood. However, some studies have shown that the effect does not only take place through its mechanical properties, but it also has other functions, such as increasing the endogenous production of HA, inhibition of PG degradation and anti-inflammatory effects in vitro,23–25 which would generate a more favorable environment in the joint, delaying the development of degenerative changes and promoting regeneration of hyaline cartilage. Nevertheless, according to our results, these benefits would only be theoretical.
We did not find experimental or clinical studies comparing the use of a single dose of HA vs 3 doses. Strauss et al.22 associated microfractures with a protocol of 3 or 5 weekly doses of HA, indicating a statistically significant difference at 3 months in favor of the use of 3 doses, although at 6 months the macroscopic and histological appearance of the tissue formed in the lesion was similar to the control group.
A limitation of our study is that we only assessed morphological parameters, rather than functional. However, our objective was to describe the effect of 2 different treatment protocols using HA, analyzing their regenerative capacity for the treatment of chondral injuries, rather than their impact on joint function. Probably the greatest strength of this work is the use of a simple and reproducible method for the use of a single anesthetic procedure, surgical technique and perioperative management. The fact that the macroscopic and histological results were similar to each other also suggests that our analysis was accurate and represents a double assessment of the tissue generated.
In the present study, we demonstrated that, compared to a single dose, the use of a protocol including 3 doses of HA does not significantly improve the regeneration of hyaline cartilage in acute full thickness chondral lesions in rabbits.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Figueroa D, Espinosa M, Calvo R, Scheu M, Valderrama JJ, Gallegos M, et al. Tratamiento de lesiones condrales agudas de espesor completo con ácido hialurónico de alto peso molecular; un modelo experimental. Rev Esp Cir Ortop Traumatol. 2014;58:261–266.