The purpose of this study is to compare the biocompatibility and the effect in osteoblasts of polymethyl methacrylate alone, and mixed with hydroxyapatite in different concentrations of 5, 10, 15 and 20%, without exceeding 20%, as it can alter mechanical properties of the composite.
Materials and methodsExperimental study comparing osteoblast response to polymethyl methacrylate alone and with hydroxyapatite in different concentrations.
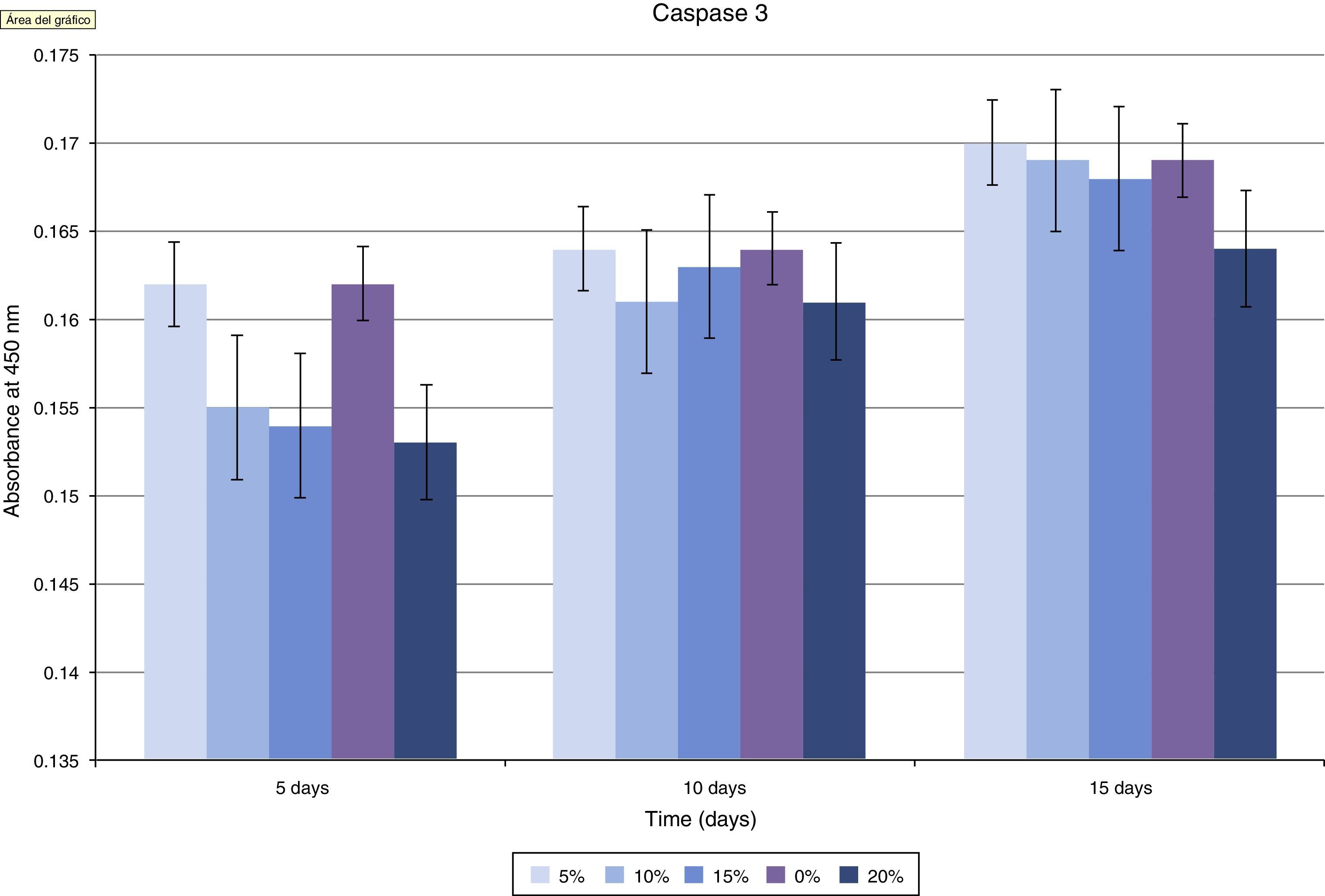
ResultsComposites at 15 and 20% obtained better osteoblast response, with higher osteoblastic activity markers, and lower apoptosis markers. Electron microscopy images show improved adhesion of osteoblasts.
El objetivo de este estudio es comparar la biocompatibilidad y efecto sobre osteoblastos de polimetilmetacrilato solo y PMMA al que se ha añadido, hidroxiapatita en concentraciones del 5, 10, 15 y 20%, no superando nunca esta cifra del 20%, dado que si se supera esta cifra pueden verse alteradas las propiedades biomecánicas del PMMA.
Material y métodosEstudio experimental que consiste en el estudio de la adhesividad, diferenciación y muerte celular sobre discos de PMMA y composite PMMA/HA a diferentes concentraciones.
ResultadosLos composites al 15 y especialmente al 20% presentaron mejor respuesta osteoblástica, mayores marcadores de actividad y menores marcadores de apoptosis. En las imágenes de microcopía electrónica se aprecia una mayor adhesión celular.
For over 50 years, polymethylmethacrylate (PMMA) has been used in Traumatology and Orthopedic Surgery to graft implants on the organism,1 particularly since the 1960s by Charnley and Smoth. A resistant and durable union is achieved between PMMA and the correct implant, but the same is not true between the cement and the bone, as PMMA limits the activity of osteoblasts which are in contact with it. It is common to observe fibroblastic cells in the interface between cement and bone.2
In recent years, the use of PMMA has extended to spinal surgery, particularly in reinforcement techniques for the treatment of vertebral fractures. Treatment with these techniques started in 1987, for both traumatic and pathological fractures, and cement also began to be used as a supplement for fixation of pedicular screws in specific cases.
PMMA used in vertebral reinforcement techniques has specific characteristics which differ from those of conventional PMMA, mainly increased viscosity and lower exothermic capacity. Its osseointegration characteristics and the biological response to it are not different from conventional PMMA, but small areas of bone necrosis have been detected in the vertebrae following vertebral reinforcement techniques.3
There have been studies of vertebral reinforcement with biocompatible materials, such as hydroxyapatite (HA) and tricalcium phosphate (TCP), but their biomechanical properties in terms of rigidity, strength, resistance and Young modulus are notably inferior to those of PMMA,4,5 so they are not reliable for the support of loads in the damaged vertebra.
PMMA and HA cements are being investigated using different HA concentrations in order to obtain a composite with similar biomechanical properties to PMMA and biocompatibility with similar osteoconductive properties to HA.6–13
The objective of this study is to compare the biocompatibility and effect on osteoblasts of PMMA alone and PMMA with added HA in concentrations of 5%, 10%, and 20%, never exceeding the level of 20% as this could alter the biomechanical properties of PMMA.14
Materials and methodsPreparation of materialsHydroxyapatite was obtained from Keramat (Ames, A Coruña, Spain). The size of HA powder particles (Ca10 [PO4]6 [OH]2, Ca/P=1.67) was examined with a particle size analyzer (Beckman Coulter, Fullerton, CA, USA), and the microstructure of each powder was examined by scanning electron microscopy (SEM), (SEM, Hitachi S-2400, Japan). Detection mapping and X-ray of the chemical composition of the powders was carried out using an energy dispersive X-ray spectrometer (EDX spectrometer, Kevex MS3 Sigma, USA).
As polymethylmethacrylate cement we used PMMA HV-R Kyphon (Medtronics, Spain), which is commonly used in our Unit for percutaneous vertebral reinforcement techniques.
Powder HA was mixed with the PMMA monomer at different concentrations by weight (5%, 10%, 15% and 20%) in our laboratory under sterile conditions in a vertical laminar flow cabinet. The mix was prepared in a mixing mill (Retsich MM400, Germany) for 10min with a vibration frequency of 20s.
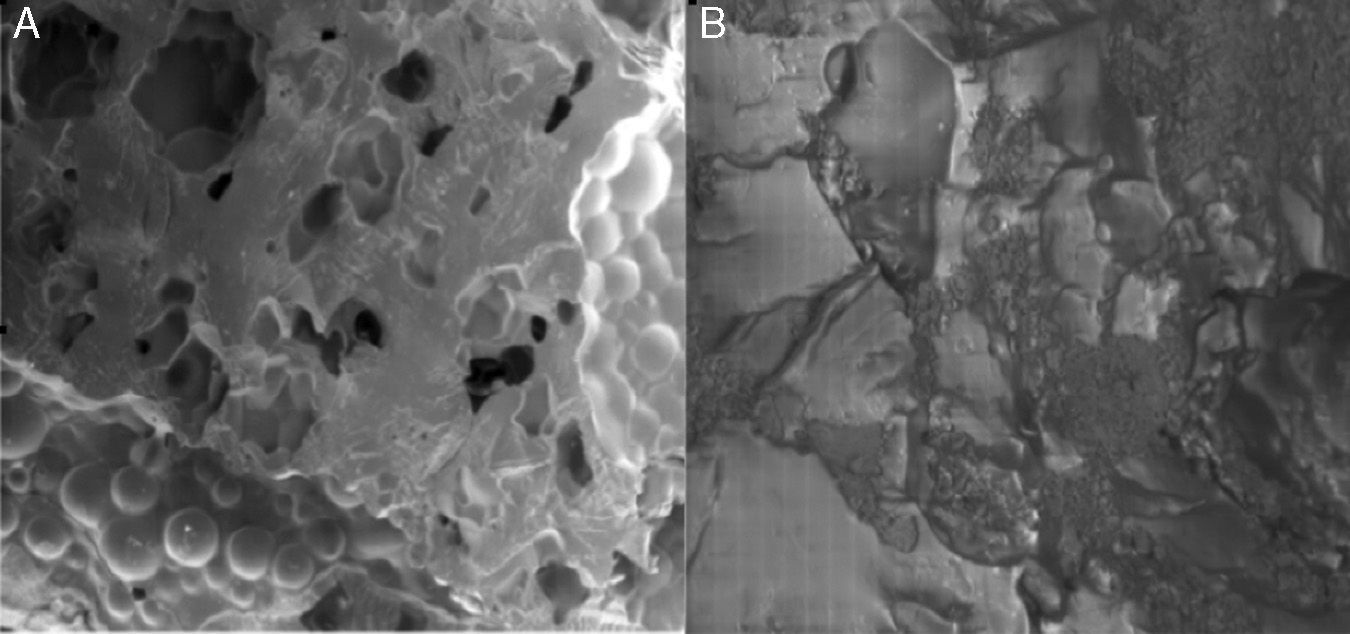
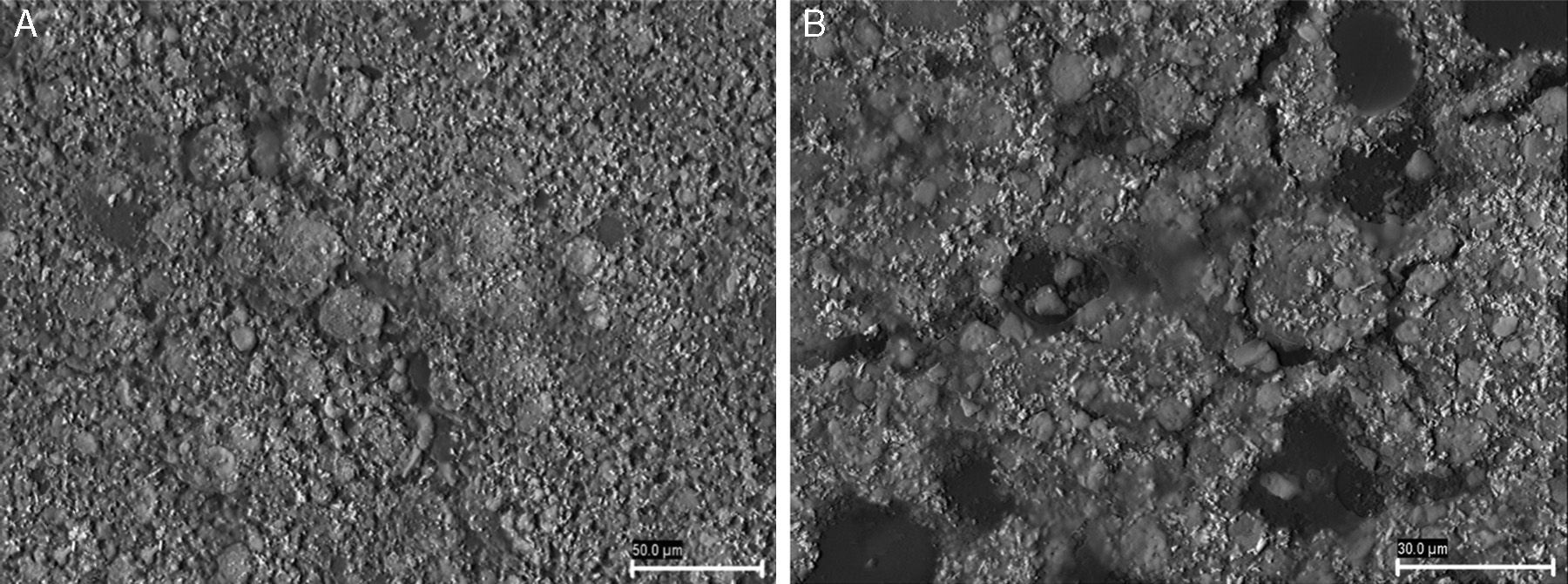
The microstructure of the surfaces of the composites created by different HA concentrations was studied using SEM to assess their rugosity (Fig. 1).
The mixture was left to settle for 24h following the setting times indicated by Medtronics. We then sterilized the discs created using gamma radiation in a dry environment.
A total of 100 discs were used in the project, divided into 5 groups according to the concentration of HA. In addition, a small series of 10 discs was reserved for electron microscopy analysis. The analysis by SEM was conducted at the Microscopy Service of Santiago de Compostela University.
Cellular responseHuman osteoblasts of the cellular lineage ATTC Saos-2 were seeded in the specimens in a disc shape (height=3mm, diameter=6mm) at a density of 6×105cells/ml with Dulbecco's modified Eagle's medium (DMEM-HG, Sigma, St. Louis, MO, USA) with 10% fetal bovine serum (FBS, Gibco BRL, MO, USA) for 72h (37°C, 5% CO2). We then proceeded to study the differences in cellular proliferation on the different samples by means of an MTT assay (Sigma).
The total volume of osteoblasts cultured per sample was 60×103 on the discs with different concentrations of PMMA-HA. Prior to the cell culture, the discs were kept in contact with a volume of DMEM for 20min so as to functionalize the surface of the cements, thus avoiding any possible hydrophobic interactions which could ultimately interfere with cellular adhesion. The discs were cultured in plates with 24 wells (Corning, USA) for 5, 10 and 15 days (Fig. 2). The culture supernatant was methodically kept at −20°C after the ending times of the culture period. All the supernatants were tested at the same time for apoptosis, proliferation and osteoblastic markers.
The possible toxicity of the composite created was studied through apoptosis (cellular death), measured by immunoassay for the detection of caspase 3 levels and through a study with the TRAIL system (Sigma).
The TRAIL system consists in an enzyme immunoabsorption assay used to detect the TRAIL enzyme (TNF-related-apoptosis-inducing-ligand), a cytokine from the TNF family which promotes apoptosis through a mitochondrial pathway.15 For the detection we used the supernatants collected at 5, 10 and 15 days, following the protocol provided by the manufacturer, and the absorption readings were carried out with a spectrophotometer at 450nm.
Caspase 3 is a key mediator of apoptosis and is commonly used as a marker for it that can be measured using the ELISA technique.16 Like for the TRAIL enzyme, we used the supernatants collected at 5, 10 and 15 days. The plates were also read at a wavelength of 450nm.
The specific markers of osteoblast activity were tested through the production of alkaline phosphatase (ALP) in supernatant osteoblasts collected at 5, 10 and 15 days, following the protocol provided by the manufacturer, Sigma Technical Bulletin Procedure. The osteoblasts were isolated using 0.1% Triton-X 10mM Tris–HCI (pH 7.4) for (2 H, 4°C) and incubated with p-nitrophenyl phosphate (pNPP) (Sigma Kit No. 104) for 15min at 37°C. Absorption readings were carried out with a spectrophotometer at 405nm (MedSystems Bender).
Quantification of hydroxyapatite was carried out with BioVision kit. This alkaline phosphatase assay kit is very accurate, simple and direct. It is a colorimeter assay designed to measure ALP activity in serum and biological samples. It is comprised by 10 tablets of substrate for multiple uses. The kit uses p-nitrophenyl phosphate as phosphatase substrate, which turns yellow (λmax=405nm) when it is dephosphorylated by alkaline phosphatase. The kit can detect 10–250mU of alkaline phosphatase in samples.
A group of discs was reserved for fixation and subsequent analysis by SEM. This process used a paraformaldehyde (25%) and glutaraldehyde (5%) buffered solution. After 12h of incubation, the samples were washed twice with phosphate buffer saline (PBS) and then dehydrated through successive substitutions of ethanol as a culture medium until an absolute alcohol concentration was reached which eliminated all presence of water in the samples (30–100%).
Statistical analysisThe statistical analysis of all the experimental data was carried out with the software package SPSS (v 11.0, SPSS Inc., Chicago, IL, USA). We proceeded to confirm that the data obtained in the assays was adjusted to a normal distribution (0.1) using the Kolmogorov–Smirnov test. We also confirmed homogeneity of variances, which indicated homoscedasticity of samples, enabling us to study differences between normal distributions. After this confirmation we applied the Student t test. The data were presented as mean±standard deviation at a significance level of P=.05.
ResultsThe physical characterization of HA resulted in the following data:
- •
Specific surface: 8.82m2/g.
- •
Chemical composition: calcium orthophosphate.
- •
Mineral composition: hydroxyapatite.
- •
Mean size: 300nm.
- •
Quantitative analysis by DRX: hydroxyapatite [Ca10(PO4)6(OH)2] >99%.
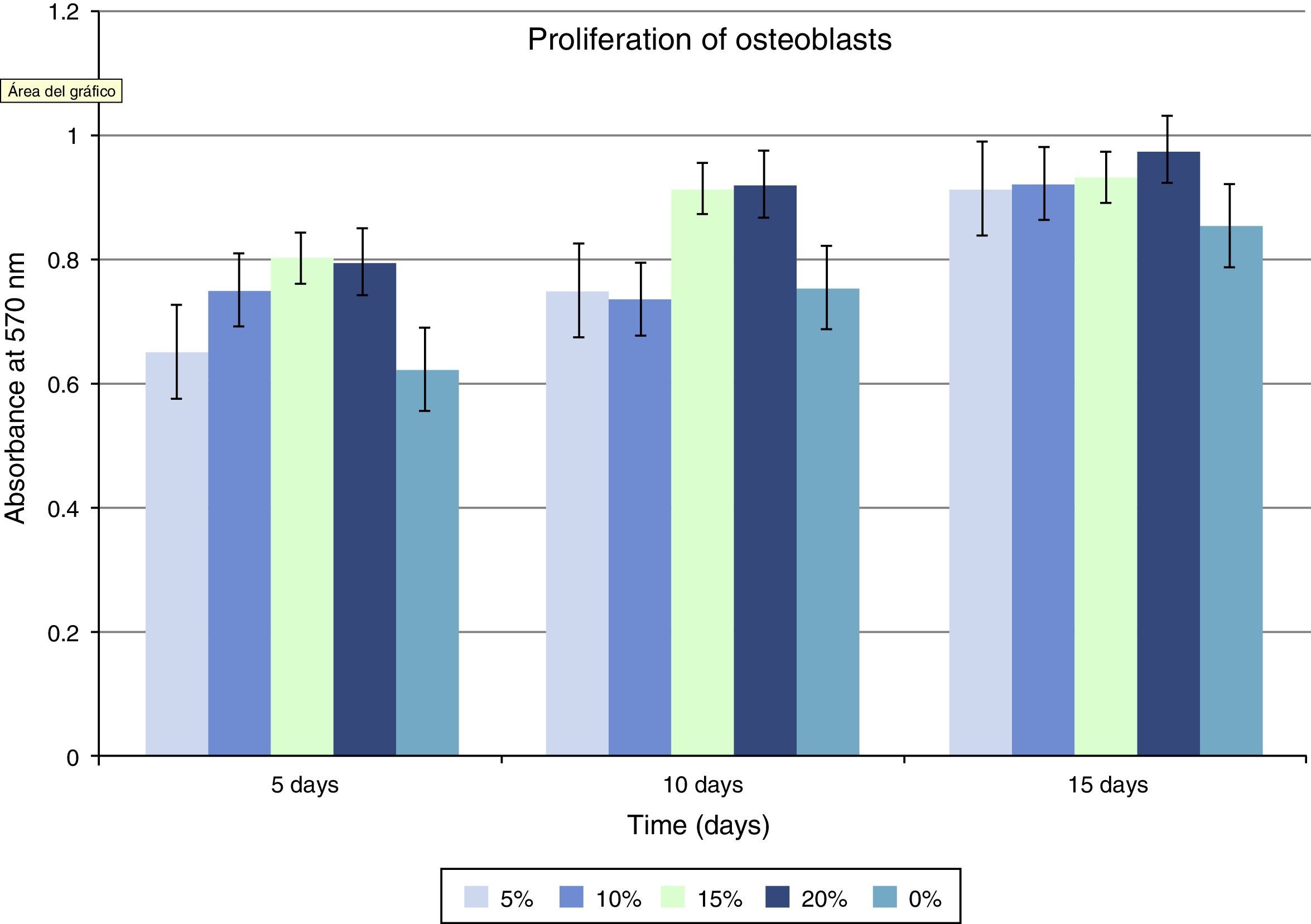
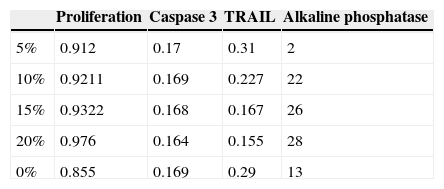
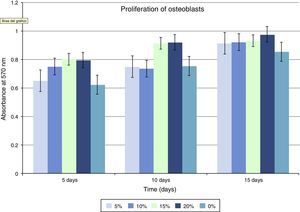
Regarding cellular proliferation, we observed that Saos-2 cells cultured on the different surfaces proliferated incrementally according to the concentration of HA on PMMA. The maximum growth spikes were registered with concentrations around 15% and 20%, with values of 0.86±0.026 and 0.90±0.008 (mean proliferation at 5, 10 and 15 days). The group with PMMA alone showed almost no proliferation, but this gradually increased along with the concentration of HA. There seems to exist a clear point of inflexion for proliferation between the concentrations of 10% and 15%, with the increase in cellular proliferation showing statistical significance after 15% HA (Fig 3 and Table 1).
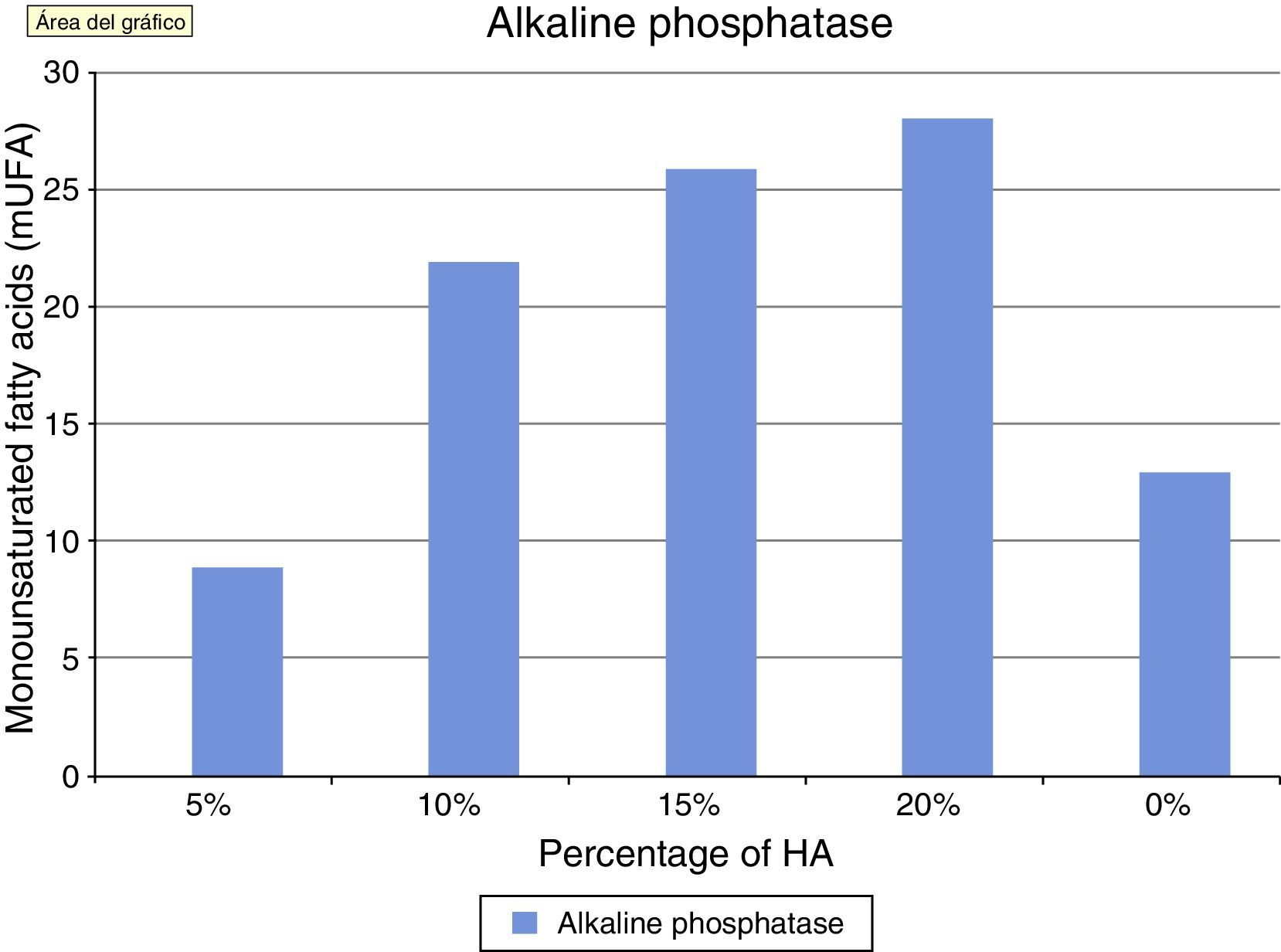
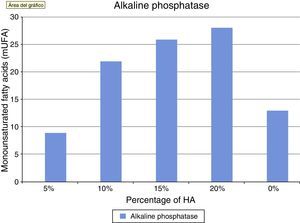
Alkaline phosphatase is an in vitro marker of osteoblastic activity in systems and materials. Fig. 4 shows how the values of alkaline phosphatase increased in parallel with the concentration of HA. The values in the control group were superior to cement at 5%. This concentration did not seem to positively modulate the phenotype of osteoblasts, with wide variation in the groups being observed after concentrations of 10% HA. These results at the end of the test can also be seen in Table 1.
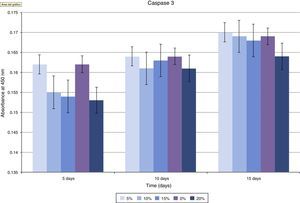
The assessment of the toxicity on osteoblasts of the composite created included a study of caspase 3 expression (Fig. 5 and Table 1). As shown in the graph, there was a progressive decrease of this cell death marker according to the increase of the HA/PMMA ratio. The concentration of 20% HA showed the best results, with the lowest measurement of caspase 3. The statistical analysis showed significant differences.
The values obtained using the TRAIL method showed how an increasing concentration of HA imbibed in PMMA improved inflammation values when compared to control groups. In this case, the statistical analysis of the TRAIL values for the 15% and 20% specimens showed the existence of a significant difference in the values measured by spectrophotometry in TRAIL, as shown in Table 1.
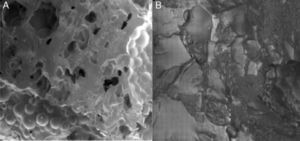
The images obtained through SEM objectively reflected greater cellular adhesion on the surface of implants. Fig. 6 shows the comparison of the surfaces of 2 discs as observed by SEM: the first with composite at 20% HA and the second with pure PMMA. A greater coverage of the surface by osteoblasts can be observed in the first image. This can be compared with the images of the non-cultured material in Fig. 1.
DiscussionOsseointegration of an implant that will remain within the bone tissue is essential for its long-term success.
The design of biomaterials must take into account any interactions with the tissues surrounding them in order to understand the existence of interface phenomena.17 Cellular adhesiveness and the toxicity of the material are crucial when designing biomaterials. Therefore, the initial proliferation and cellular recruitment at the surface of a biomaterial are vitally important when assessing its bioactivity.
PMMA has been proven to have no osteogenic capacity and several studies have shown that it has no capacity for adhesiveness of extracellular fluid proteins and, thus, for cells, maybe even resulting toxic for them.2 In addition, the exothermic reaction generated whilst setting18 is also damaging for surrounding cells, even causing an initial necrosis reaction.19 Recent studies have even found that PMMA produces higher rates of disc degeneration than calcium phosphate cements20 in experiments conducted on animals.
Contrary to the case with PMMA, it has been proven that HA provides an adequate scaffold which favors the adhesion of extracellular matrix proteins, and, through it, enables the adhesion of cells, mainly mediated by integrins.21
It is known that addition of HA to the PMMA monomer does not affect its polymerization process, but it can affect its mechanical properties regarding hardness, compression, resistance to breakage, elasticity module, and resistance to traction, when the addition of HA is over 15% or 20%.14,22–24 In spite of this, recent studies, have reported that PMMA-HA composites in vivo improve the mechanical properties of isolated PMMA regarding long-term material fatigue.25In vitro studies have proven a greater osteoblastic adhesion to PMMA-HA composites.26
In terms of exothermic capacity, the addition of HA to PMMA reduces the peak temperature of the polymerization process, which decreases the damaging effect on cells.27
In our study, the maximum peaks of cellular growth of the osteoblastic lineage were obtained at concentrations between 15% and 20% with values of 0.86±0.026 and 0.90±0.008. There appears to be a clear inflexion point for proliferation at concentrations between 10% and 15%. Therefore, we consider concentrations over 15% to be optimal. It appears that HA concentrations of 15% mark the transition between encapsulation and bone growth processes.28 This increased proliferation and differentiation of osteoblasts in vitro is thought to be due to the presence of HA on the surface of the cement. It has been proven that rougher topographies are more favorable to cellular adhesion.29 It also seems that cells preferentially react with chemical stimuli on the surface of HA compared to the cement polymer, as HA is capable of absorbing elements from the extracellular matrix which would stimulate the adhesion of osteoblasts.30
We examined the osteoblasts adhered to the surface of PMMA-HA using SEM (Fig. 6) and observed a similar morphology and confluence to those obtained in other biomaterials with osteoinductive properties, such as HA alone and titanium.
The production of alkaline phosphatase is associated to the activity of osteoblasts in bone formation, therefore alkaline phosphatase is considered as a marker of this osteoblastic activity in most studies, both in vitro31 and in vivo. In our study, we observed how its activity increased in parallel to the increase of HA, with the highest levels being achieved at concentrations of 20%.
Assessment of the toxicity of the PMMA-HA mix created was carried out using caspase 3 as an indicator of apoptosis, by toxicity of the implanted material. It is well established that PMMA alone produces cellular death (apoptosis) of osteoblasts in vitro.32 We observed a progressive decrease in the concentration of caspase 3 as the concentration of HA in the composite increased. At concentrations of 20% HA, cellular death was similar to that observed in the control groups, so we can consider that its toxicity on osteoblasts is null at this concentration, with an optimal apoptotic index.
We also studied the toxicity of the cement on osteoblasts with the TRAIL system, and noted that at concentrations of 15% and 20% there was a significant difference with a decrease in apoptosis compared to composites with a lower content of HA and to PMMA alone.
In conclusion, we can establish that, in this study, PMMA-HA composites at concentrations between 15% and 20% showed conditions that favored osteoblastic proliferation without altering their osteogenic capacity, with lower toxicity than PMMA alone and composites with lower concentrations.
Using SEM, we subjectively observed better cellular adhesion on the composite at 15% and 20% than on the remaining samples in the study.
Further in vivo studies will be required to confirm our findings before they may be used in clinical practice.
Level of evidenceLevel of evidence I.
Ethical responsibilitiesProtection of people and animalsThe authors declare that this investigation did not require experiments on humans or animals.
Confidentiality of dataThe authors declare that this work does not reflect any patient data.
Right to privacy and informed consentThe authors declare that this work does not reflect any patient data.
Conflict of interestsThe authors have no conflict of interests to declare.
The authors wish to thank Medcomtech for providing the cement used to conduct the tests, as well as Keramat for the hydroxyapatite used to create the composites.
Please cite this article as: Pino-Mínguez J, Jorge-Mora A, Couceiro-Otero R, García-Santiago C. Estudio de viabilidad y adhesividad celular de osteoblastos a cementos óseos utilizados en técnicas de refuerzo vertebral, mezclados con hidroxiapatita a distintas concentraciones. Rev Esp Cir Ortop Traumatol. 2015;59:122–128.