Cost-effectiveness analysis of apixaban versus dabigatran in preventing venous thromboembolism (VTE) in total knee (TKR) or hip (THR) replacement.
MethodsModel with two periods: post-prophylaxis period of 90 days (short-term) and 5 years (Markov). VTE complications (distal and proximal deep vein thrombosis, pulmonary embolism, bleeding and post-thrombotic syndrome) were included. The comparative efficacy was obtained from a meta-analysis, and the costs from Spanish sources. An annual discount rate of 3.5% for costs and benefits was applied.
ResultsAccording to the meta-analysis, the relative risk (RR) of VTE or death, compared with enoxaparin, was lower with apixaban than with dabigatran in TKR (RR 0.89, 95% CI 0.32–1.65 and RR 1.35, 95% CI, 0.19–3.39) and THR (RR 0.35, 95% CI, 0.05–2.51 and RR 0.89, 95% CI 0.22–3.21, respectively). In the short-term, there were more life years (LYG) and more quality-adjusted life years (QALY) per patient in TKR (0.2037; 0.1908) and THR (0.2417; 0.1921) with apixaban than with dabigatran (0.1818; 0.1901 and 0.2345; 0.1918, respectively) were obtained. With apixaban lower costs per patient in TKR (€−14) were generated, so it was the dominant treatment. Additional costs (€15) could be incurred in THR, with a cost per LYG of €2083 and €50,000 per QALY gained. In 5 years, apixaban was cheaper and more effective in both TKR and THR.
ConclusionsAccording to this study, apixaban was shown to be a cost-effective treatment compared with dabigatran for VTE prevention.
Análisis de coste-efectividad de apixaban frente a dabigatrán en la prevención de la tromboembolia venosa (TEV) en la artroplastia total de rodilla (ATR) o cadera (ATC).
MétodosModelo con 2 periodos: posprevención de 90 días (corto plazo) y a 5 años (Markov). Se incluyeron las complicaciones de la TEV (trombosis venosa profunda distal y proximal, embolia pulmonar, sangrados y síndrome postrombótico). La eficacia comparada se obtuvo de un metaanálisis y los costes de fuentes españolas. Se aplicó una tasa de descuento del 3,5% anual para costes y beneficios.
ResultadosSegún el metaanálisis, el riesgo relativo (RR) de TEV o muerte, frente a enoxaparina, fue menor con apixaban que con dabigatrán en ATR (RR: 0,89; IC 95% 0,32-1,65 y RR: 1,35, IC 95% 0,19-3,39) y en ATC (RR: 0,35, IC 95% 0,05-2,51 y RR: 0,89, IC 95% 0,22-3,21, respectivamente). A corto plazo, con apixaban se obtendrían más años de vida (AVG) y más años de vida ajustados por calidad (AVAC) por paciente, tanto en ATR (0,2037; 0,1908) como en ATC (0,2417; 0,1921) que con dabigatrán (0,1818; 0,1901, y 0,2345; 0,1918, respectivamente). Habría menos costes por paciente con apixaban en ATR (-14 €) por lo que este sería el tratamiento dominante. En ATC se producirían costes adicionales (15 €) con un coste por AVG de 2.083 y de 50.000 € por AVAC ganado. A 5 años, apixaban fue más barato y más efectivo en ATR y en ATC.
ConclusionesSegún el presente estudio, apixaban es un tratamiento coste-efectivo en comparación con dabigatrán en la prevención de la TEV.
Deep vein thrombosis (DVT) and pulmonary embolism (PE) are 2 manifestations of venous thromboembolism (VTE).1 Although most of these thromboses are asymptomatic, they are a serious threat for the life of patients and represent the primary cause of preventable hospital mortality.2 One of the risk factors most commonly associated with VTE is major orthopaedic surgery, such as total knee replacement/arthroplasty (TKR/TKA) and total hip replacement/arthroplasty (THR/THA).3
There are a number of objective reasons that explain the need for prevention of thromboembolism in major orthopaedic surgery: the high prevalence of VTE without prevention (40–80% of distal DVT, 10–20% of proximal DVT, 4–10% of PE and 1–5% of fatal PE).4,5 In addition, the symptoms of VTE may be silent or asymptomatic, so the first manifestation could be a fatal PE, non-invasive diagnostic techniques (echo-Doppler) have little sensitivity for asymptomatic DVT, and lastly, untreated DVT is associated with high long-term morbidity due to post-thrombotic syndrome (PTS) and recurrent DVT. Anticoagulant prevention achieves a reduction of 50–75% in the risk of thromboembolism.4
In Spain, 96% of VTE prevention is conducted with low molecular weight heparins (LMWH), mainly with enoxaparin,6 which has proved very effective for this indication.7 However, LMWHs have 3 main problems, including a cumulative effect on kidney failure, non-availability of a fully effective antidote and, although less frequently than with unfractionated heparins, their involvement in heparin-induced thrombocytopenia (HIT).8,9 Moreover, enoxaparin is administered by subcutaneous injection. This represents a considerable inconvenience for patients during outpatient treatment, as it requires them to be trained in self-administration or else to receive home visits or go to a healthcare centre to receive the dose from a nurse.9 Finally, another potential drawback of enoxaparin is the recommendation of administering the first injection 12h before surgical intervention,10 thus requiring hospitalisation of patients with considerable advance in some cases.
The new oral anticoagulants have certain practical advantages over LMWHs regarding prevention of VTE: they are administered orally, should not be used before surgery, do not require routine monitoring (coagulation or platelet count) and do not need dose adjustements.7,8
Apixaban and dabigatran are 2 new oral anticoagulants. Apixaban is an inhibitor of the spread of the coagulation cascade which exerts a direct inhibition on Xa factor8 and is indicated for the primary prevention of VTE in adult patients undergoing TKA or THA. Dabigatran is an oral, direct inhibitor of thrombin,8 with the same indications as apixaban.
A recent meta-analysis of direct and indirect comparisons analysed the effects of different drugs indicated for the prevention of VTE in patients undergoing TKA and THA.11,12 This study found that the risk (odds ratio, OR) of total VTE and deaths from all causes was higher with dabigatran than with apixaban, both in TKA (OR: 1.72; 95% CI 1.22–2.42) and in THA (OR: 2.51; 95% CI 1.50–4.21). Regarding the risk of haemorrhage, although this was higher with dabigatran, there were no statistically significant differences between both treatments (OR: 1.16; 95% CI 0.86–1.56).11,12
The annual healthcare cost of VTE in Spain is estimated at €66.5 million. PE represents 67.7% of the cost (€45 million) and DVT the remaining 33.3% (€21.4 million). Up to 90% of the cost is derived from hospital care.3
The differences observed in efficacy between apixaban and dabigatran may have an impact on the results, as well as on healthcare expenditure due to prevention of VTE. Accordingly, the present study aims to conduct a cost-effectiveness analysis of VTE prevention with apixaban and dabigatran in patients undergoing TKA or THA.
MethodsCost-effectiveness analysis is a particularly relevant tool for decision making by the National Healthcare System. We performed this type of analysis in order to assess the efficiency of introducing apixaban for the prevention of VTE in patients undergoing TKA or THA.
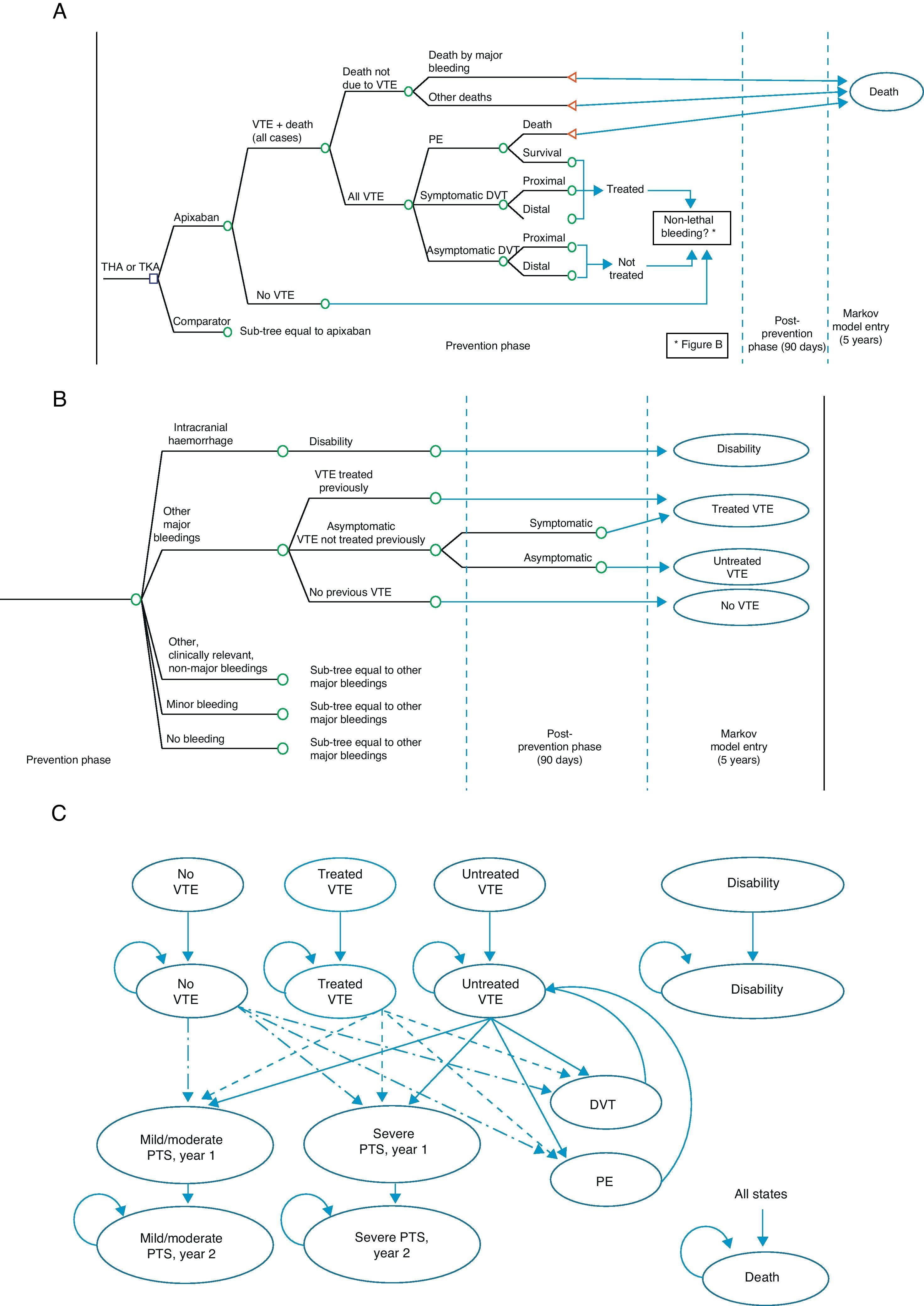
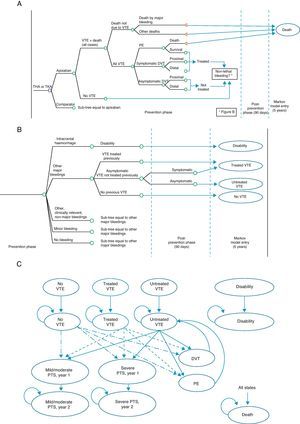
The economic model shows the evolution of a patient undergoing TKA or THA.12 It consists of a decision tree which simulates the short-term evolution (prevention phase and up to 90 days thereafter), and a Markov model13 simulating the evolution at 5 years (Fig. 1). The possibilities in the short-term included VTE occurring or not (symptomatic or asymptomatic DVT, PE), mortality associated or due to other causes (Fig. 1A), as well as potential adverse events including bleeding, fatal or not, classified as intracranial haemorrhage, other major bleeding events, clinically relevant minor bleeding and minor bleeding (Fig. 1B). At 5 years, 9 Markov states were considered (Fig. 1C): (1) without VTE, (2) untreated VTE (with asymptomatic DVT which does not become symptomatic in the short-term), (3) treated VTE (with symptomatic DVT or non-fatal PE in the short-term), (4) disability (caused by intracranial haemorrhage in the short-term), (5) mild to moderate PTS (patients entering the 5-year phase with treated or untreated VTE who suffer a mild to moderate episode of PTS; this state is divided into a first year, due to its higher cost, and after the second year), (6) severe PTS, (7) DVT occurring in the 5-year period, (8) PE occurring in the 5-year period, and finally (9) death due to all causes.12
Economic model for the prevention of venous thromboembolism. (A) Short-term: VTE and death. (B) Short-term: bleeding. (C) 5-year period: Markov process. DVT: deep vein thrombosis; PE: pulmonary embolism; PTS: post-thrombotic syndrome; THA: total hip arthroplasty; TKA: total knee arthroplasty; VTE: venous thromboembolism.
The characteristics of the cohort simulated in the model were adjusted to those of the Spanish population, whenever data were available. We considered that 26.2% of patients undergoing TKA and 36.1% of those undergoing THA would be males14,15 and the age of the cohort at the beginning of the simulation would be 71.9 and 73.3 years, respectively.14,15
The effectiveness of prevention with apixaban and dabigatran was estimated by a meta-analysis of direct comparisons, or else indirect comparisons when not available (mixed treatment comparison, MTC),11 in order to provide solid evidence in the absence of clinical trials with direct comparisons of the treatments.16–18 The meta-analysis included only double blind, randomised clinical trials in patients undergoing TKA or THA who received pharmacological VTE prevention with either drug.11 In order to obtain all available studies with efficacy or bleeding results, we conducted a systematic literature search of electronic databases (The Cochrane Library, Medline, Embase, CINAHL) and reviewed the communications of conferences from medical societies related to the disease until July 8th, 2010.11 After eliminating duplicated publications, we obtained 1809 potentially relevant works includible in the analysis. Of these, 1610 were excluded after reviewing their abstracts. We reviewed the original articles of the 199 remaining publications and of another 4 identified by a manual search, excluding a further 164. Eventually, we included 39 publications with data from 42 trials which met the inclusion criteria established previously.11
All variables of interest (mortality, incidence of VTE, PE, DVT and bleeding) were measured dichotomously, performing an intention to treat (ITT) analysis (including all randomised patients).11 We conducted separate analyses for THA and TKA.
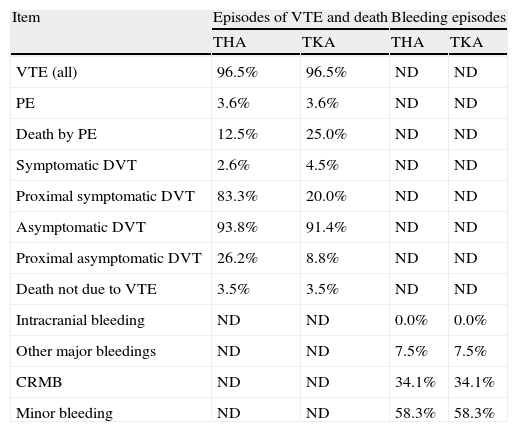
The meta-analysis was conducted with a random effects model using the DerSimonian and Laird method19 and estimating heterogeneity through the Mantel–Haenszel model.20 Indirect comparisons between apixaban and another treatment through a common comparator (enoxaparin) were performed by the Bucher method.21 The MTC was performed using Monte Carlo simulations in Bayesian Markov chains,11 using version 1.4.1 of the WinBUGS program.22–24 The model calculated the relative risks of VTE, PE, DVT and bleeding with different drugs, compared with enoxaparin (Table 1).11
Probabilities of subsequent events to venous thromboembolism or bleeding adopted in the economic model.
| Item | Episodes of VTE and death | Bleeding episodes | ||
| THA | TKA | THA | TKA | |
| VTE (all) | 96.5% | 96.5% | ND | ND |
| PE | 3.6% | 3.6% | ND | ND |
| Death by PE | 12.5% | 25.0% | ND | ND |
| Symptomatic DVT | 2.6% | 4.5% | ND | ND |
| Proximal symptomatic DVT | 83.3% | 20.0% | ND | ND |
| Asymptomatic DVT | 93.8% | 91.4% | ND | ND |
| Proximal asymptomatic DVT | 26.2% | 8.8% | ND | ND |
| Death not due to VTE | 3.5% | 3.5% | ND | ND |
| Intracranial bleeding | ND | ND | 0.0% | 0.0% |
| Other major bleedings | ND | ND | 7.5% | 7.5% |
| CRMB | ND | ND | 34.1% | 34.1% |
| Minor bleeding | ND | ND | 58.3% | 58.3% |
CRMB: clinically relevant minor bleedings; DVT: deep vein thrombosis; ND: these events were not described in the clinical trials included in the meta-analysis; PE: pulmonary embolism; THA: total hip arthroplasty; TKA: total knee arthroplasty; VTE: venous thromboembolism.
The efficacy variable considered in the economic analysis was the composite of symptomatic and asymptomatic DVT, non-fatal PE and death from any cause during treatment.11,12,25 The economic model used the results of the meta-analysis, expressed as relative risk (RR).11 The RR of the combination of VTE and death for apixaban and dabigatran versus a common comparator (enoxaparin, administered at the European dosage of 40mg/day) used in the model was the following11: (a) in patients undergoing TKA: 0.895 with apixaban and 1.354 with dabigatran and (b) in patients undergoing THA: 0.359 with apixaban and 0.883 with dabigatran.
The effectiveness of treatments was measured as life years gained (LYG) and as quality-adjusted life years (QALYs) gained with the most effective treatment.12 QALYs were estimated for both the short-term and at 5 years, starting from the baseline value of the general population in men and women (0.78 on a scale from 0 to 1, where 0 represented death or the worst imaginable health state and 1 represented perfect health),26 and applying to this the losses associated with each episode of VTE or bleeding27–32: −0.08 for PE and distal or proximal symptomatic DVT, −0.49 for intracranial haemorrhage, −0.03 for other major haemorrhages, −0.00029 for the annual impact of aging, −0.01 for treated VTE, −0.02 for mild to moderate PTS in the first year or in subsequent years, and finally −0.07 for severe PTS in the first year or in subsequent years. The age-related mortality was obtained from the National Statistics Institute.33 The probabilities of events subsequent to VTE or bleeding were obtained from the clinical trials ADVANCE-234 and ADVANCE-335 (Table 1).
We considered both the costs of acquiring the drugs being compared (apixaban and dabigatran) and of the possible complications of VTE in the short-term and at 5 years (distal and proximal DVT, PE, intracranial bleeding, other major bleeding, minor bleeding with and without clinical relevance and PTS).
We included the following treatment guidelines, approved in Spain for the prevention of thrombosis26: 2.5mg twice daily of oral apixaban (12 days for TKA and 35 days for THA) and 220mg daily of oral dabigatran (10 days for TKA and 31.5 days for THA).12,36 We considered the cost of drugs to be included in the cost of hospitalisation, so we only included it as a separate cost after discharge.
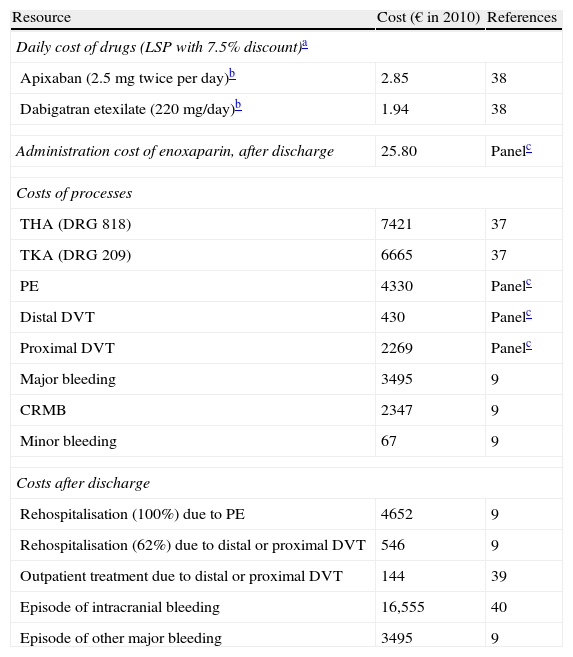
The mean length of hospital stay (7.8 days for TKA and 11.5 days for THA) was obtained from the mean stays of diagnosis related groups (DRGs) 219 and 818, reflected in the statistics of the Health and Consumption Ministry.37 The healthcare cost of PE, distal DVT and proximal DVT was estimated through a Delphi panel composed of 3 Spanish clinical experts (2 specialists in internal medicine and 1 specialist in traumatology). This panel was chosen based on the following criteria: (a) proven experience in the management of VTE and (b) covering the clinical areas involved in the management of the disease (traumatology and/or internal medicine).Unit costs (drugs, administration thereof, TKA, THA, PE, DVT, bleeding and rehospitalisation) considered in the model were obtained from Spanish sources and are summarised in Table 2. We considered the laboratory sale price (LSP) of drugs with a 7.5% discount.28 The costs of DRGs for TKA, THA, VTE and bleeding were obtained from other Spanish sources.37–40
Unit costs considered in the economic model.
| Resource | Cost (€ in 2010) | References |
| Daily cost of drugs (LSP with 7.5% discount)a | ||
| Apixaban (2.5mg twice per day)b | 2.85 | 38 |
| Dabigatran etexilate (220mg/day)b | 1.94 | 38 |
| Administration cost of enoxaparin, after discharge | 25.80 | Panelc |
| Costs of processes | ||
| THA (DRG 818) | 7421 | 37 |
| TKA (DRG 209) | 6665 | 37 |
| PE | 4330 | Panelc |
| Distal DVT | 430 | Panelc |
| Proximal DVT | 2269 | Panelc |
| Major bleeding | 3495 | 9 |
| CRMB | 2347 | 9 |
| Minor bleeding | 67 | 9 |
| Costs after discharge | ||
| Rehospitalisation (100%) due to PE | 4652 | 9 |
| Rehospitalisation (62%) due to distal or proximal DVT | 546 | 9 |
| Outpatient treatment due to distal or proximal DVT | 144 | 39 |
| Episode of intracranial bleeding | 16,555 | 40 |
| Episode of other major bleeding | 3495 | 9 |
CRMB: clinically relevant minor bleedings; DRG: diagnosis-related groups; DVT: deep vein thrombosis; LSP: laboratory sale price; PE: pulmonary embolism; THA: total hip arthroplasty; TKA: total knee arthroplasty.
We analysed the incremental cost-effectiveness of apixaban compared to dabigatran regarding 2 time horizons (short-term, up to 90 days after arthroplasty, and up to 5 years). All costs (€2010) were discounted by 3.5% annually.
We performed separate analyses for patients undergoing TKA and THA. We analysed a base case with the mean values of all parameters in the short-term and at 5 years. We also conducted several simple univariate sensitivity analyses for the following variables: (a) costs of VTE and bleeding episodes (±20%), (b) losses associated with episodes of VTE or bleeding (±10%), and finally (c) annual discount rate for costs, life years and losses (0% and 6%). The last 2 studies were only conducted for short-term analysis, since the effects of losses and discounts can only be appreciated or have meaning, respectively, over prolonged periods.
Finally, we performed a probabilistic analysis (Monte Carlo simulation, 2000 simulations) of the Markov model, which included the following variables: (a) effectiveness of treatment, (b) risk of VTE in a 5-year period, (c) costs, and (d) usefulness. The statistical distributions employed for the different variables were: (a) β for effectiveness of treatments, probabilities of transition and short-term usefulness and (b) γ for costs and losses in the 5-year period.12
The results of the comparison of apixaban and dabigatran are presented as follows: (a) number of episodes obtained with either drug, (b) cost difference per patient, (c) difference in usefulness per patient, and (d) incremental cost-effectiveness ratio (ICER), cost of a LYG and cost of a QALY gained with the most effective treatment.
ResultsEpisodes of VTEThe meta-analysis showed a greater efficacy of apixaban in preventing VTE. The relative risk (RR) of developing a VTE or suffering death by any cause, compared with enoxaparin, was lower with apixaban than with dabigatran, both in TKA (RR: 0.89; 95% CI 0.32–1.65, and RR: 1.35; 95% CI 0.19–3.39) and in THA (RR: 0.35; 95% CI 0.05–2.51, and RR: 0.89; 95% CI 0.22–3.21, respectively).11,12
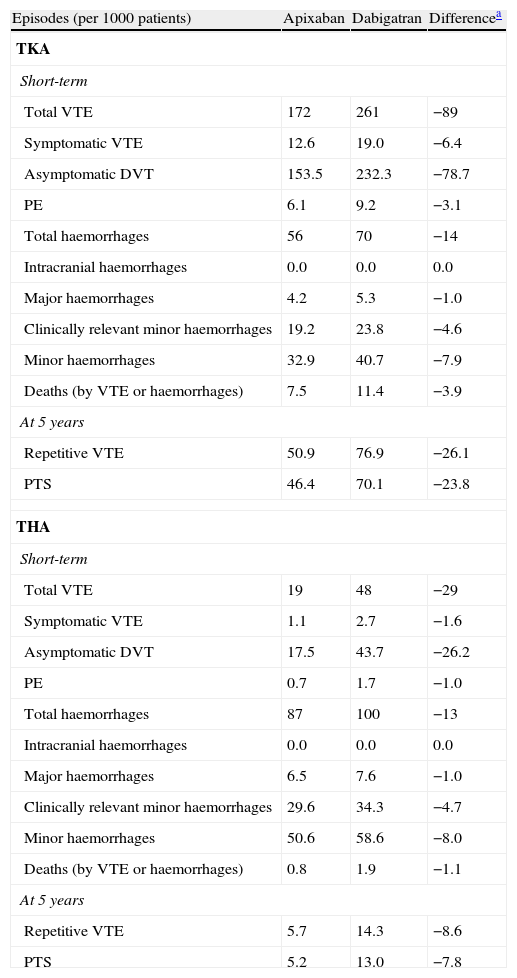
In the short-term, in a cohort of 1000 patients undergoing TKA, apixaban would prevent 89 episodes of VTE compared with dabigatran. Cases prevented in patients undergoing THA would be 29. After 5 years, prevented cases would be 26 repetitive VTE and 24 PTS in TKA, and 9 and 8 cases, respectively, in THA (Table 3).
Episodes expected in a cohort of 1000 patients intervened for total hip arthroplasty and total knee arthroplasty, treated preventively with apixaban or dabigatran in the short-term and at 5 years.
| Episodes (per 1000 patients) | Apixaban | Dabigatran | Differencea |
| TKA | |||
| Short-term | |||
| Total VTE | 172 | 261 | −89 |
| Symptomatic VTE | 12.6 | 19.0 | −6.4 |
| Asymptomatic DVT | 153.5 | 232.3 | −78.7 |
| PE | 6.1 | 9.2 | −3.1 |
| Total haemorrhages | 56 | 70 | −14 |
| Intracranial haemorrhages | 0.0 | 0.0 | 0.0 |
| Major haemorrhages | 4.2 | 5.3 | −1.0 |
| Clinically relevant minor haemorrhages | 19.2 | 23.8 | −4.6 |
| Minor haemorrhages | 32.9 | 40.7 | −7.9 |
| Deaths (by VTE or haemorrhages) | 7.5 | 11.4 | −3.9 |
| At 5 years | |||
| Repetitive VTE | 50.9 | 76.9 | −26.1 |
| PTS | 46.4 | 70.1 | −23.8 |
| THA | |||
| Short-term | |||
| Total VTE | 19 | 48 | −29 |
| Symptomatic VTE | 1.1 | 2.7 | −1.6 |
| Asymptomatic DVT | 17.5 | 43.7 | −26.2 |
| PE | 0.7 | 1.7 | −1.0 |
| Total haemorrhages | 87 | 100 | −13 |
| Intracranial haemorrhages | 0.0 | 0.0 | 0.0 |
| Major haemorrhages | 6.5 | 7.6 | −1.0 |
| Clinically relevant minor haemorrhages | 29.6 | 34.3 | −4.7 |
| Minor haemorrhages | 50.6 | 58.6 | −8.0 |
| Deaths (by VTE or haemorrhages) | 0.8 | 1.9 | −1.1 |
| At 5 years | |||
| Repetitive VTE | 5.7 | 14.3 | −8.6 |
| PTS | 5.2 | 13.0 | −7.8 |
DVT: deep vein thrombosis; PE: pulmonary embolism; PTS: post-thrombotic syndrome; THA: total hip arthroplasty; TKA: total knee arthroplasty; VTE: venous thromboembolism.
According to the meta-analysis, compared with enoxaparin, the RR of bleeding was lower with apixaban (RR: 0.81; 95% CI 0.48–1.31) than with dabigatran (RR: 1.00; 95% CI 0.44–2.49).11,12
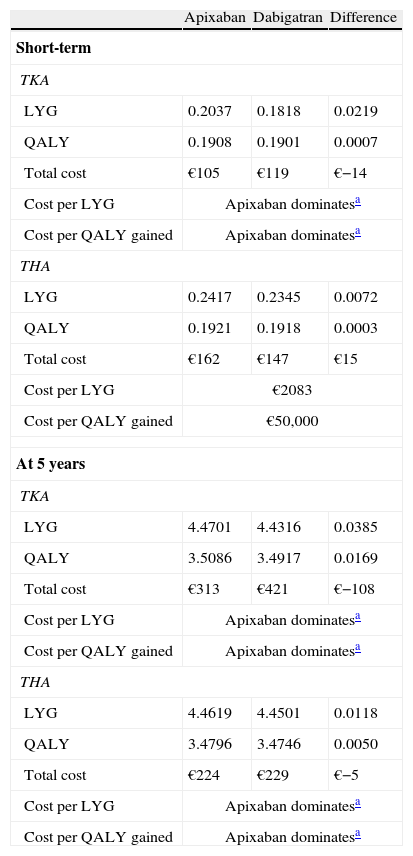
Cost-effectivenessConsequently, in the short-term analysis, apixaban would yield more years of life (LYG) and quality-adjusted life years (QALYs) per patient, both for TKA (0.2037; 0.1908) and THA (0.2417; 0.1921) than dabigatran (0.1818; 0.1901 and 0.2345; 0.1918, respectively). Apixaban would generate lower costs per patient than dabigatran for THA (€−14); therefore, apixaban would be the dominant treatment in TKA. In THA there would be additional costs (€15) with a cost of €2083 per LYG and €50,000 per QALY gained (Table 4). In the analysis at 5 years, apixaban was dominant in both TKA and THA (Table 4).
Results of cost-effectiveness analysis of apixaban versus dabigatran in the short-term and at 5 years.
| Apixaban | Dabigatran | Difference | |
| Short-term | |||
| TKA | |||
| LYG | 0.2037 | 0.1818 | 0.0219 |
| QALY | 0.1908 | 0.1901 | 0.0007 |
| Total cost | €105 | €119 | €−14 |
| Cost per LYG | Apixaban dominatesa | ||
| Cost per QALY gained | Apixaban dominatesa | ||
| THA | |||
| LYG | 0.2417 | 0.2345 | 0.0072 |
| QALY | 0.1921 | 0.1918 | 0.0003 |
| Total cost | €162 | €147 | €15 |
| Cost per LYG | €2083 | ||
| Cost per QALY gained | €50,000 | ||
| At 5 years | |||
| TKA | |||
| LYG | 4.4701 | 4.4316 | 0.0385 |
| QALY | 3.5086 | 3.4917 | 0.0169 |
| Total cost | €313 | €421 | €−108 |
| Cost per LYG | Apixaban dominatesa | ||
| Cost per QALY gained | Apixaban dominatesa | ||
| THA | |||
| LYG | 4.4619 | 4.4501 | 0.0118 |
| QALY | 3.4796 | 3.4746 | 0.0050 |
| Total cost | €224 | €229 | €−5 |
| Cost per LYG | Apixaban dominatesa | ||
| Cost per QALY gained | Apixaban dominatesa | ||
LYG: life year gained; QALY: quality-adjusted life year; THA: total hip arthroplasty; TKA: total knee arthroplasty.
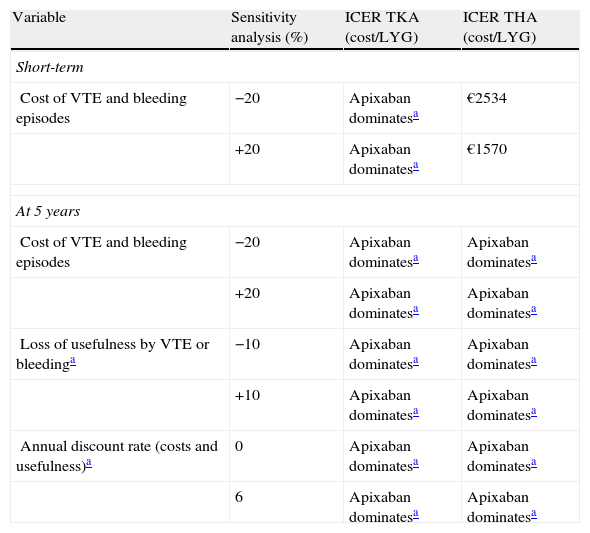
Sensitivity analyses confirmed the stability of the base case. As shown in Table 5, apixaban was the dominant treatment (more effective, with fewer costs) in all analyses conducted over a period of 5 years, but not in the short-term. When the cost of VTE and bleeding episodes was modified (±20%), the cost of LYG ranged between €1570 and €2534 (well below the willingness to pay €30,000 per QALY gained, considered acceptable in Spain).41
Results of cost-effectiveness analysis of apixaban versus dabigatran. Simple univariate sensitivity analysis in short-term and at 5 years.
| Variable | Sensitivity analysis (%) | ICER TKA (cost/LYG) | ICER THA (cost/LYG) |
| Short-term | |||
| Cost of VTE and bleeding episodes | −20 | Apixaban dominatesa | €2534 |
| +20 | Apixaban dominatesa | €1570 | |
| At 5 years | |||
| Cost of VTE and bleeding episodes | −20 | Apixaban dominatesa | Apixaban dominatesa |
| +20 | Apixaban dominatesa | Apixaban dominatesa | |
| Loss of usefulness by VTE or bleedinga | −10 | Apixaban dominatesa | Apixaban dominatesa |
| +10 | Apixaban dominatesa | Apixaban dominatesa | |
| Annual discount rate (costs and usefulness)a | 0 | Apixaban dominatesa | Apixaban dominatesa |
| 6 | Apixaban dominatesa | Apixaban dominatesa | |
ICER: incremental cost-effectiveness ratio; LYG: life year gained; THA: total hip arthroplasty; TKA: total knee arthroplasty; VTE: venous thromboembolism.
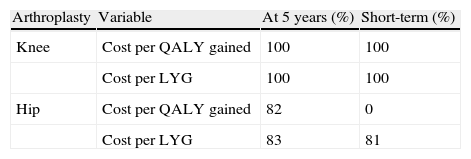
The probabilistic sensitivity analysis in TKA confirmed that apixaban was cost-effective in 100% of the analyses performed in both the short-term and at 5 years, for a willingness to pay €30,000 (Table 6).
Results of probabilistic sensitivity analysis: probability that apixaban is cost-effective versus dabigatran for a willingness to pay €30,000 per quality-adjusted life year gained or per life year gained.
| Arthroplasty | Variable | At 5 years (%) | Short-term (%) |
| Knee | Cost per QALY gained | 100 | 100 |
| Cost per LYG | 100 | 100 | |
| Hip | Cost per QALY gained | 82 | 0 |
| Cost per LYG | 83 | 81 |
LYG: life year gained; QALY: quality-adjusted life year.
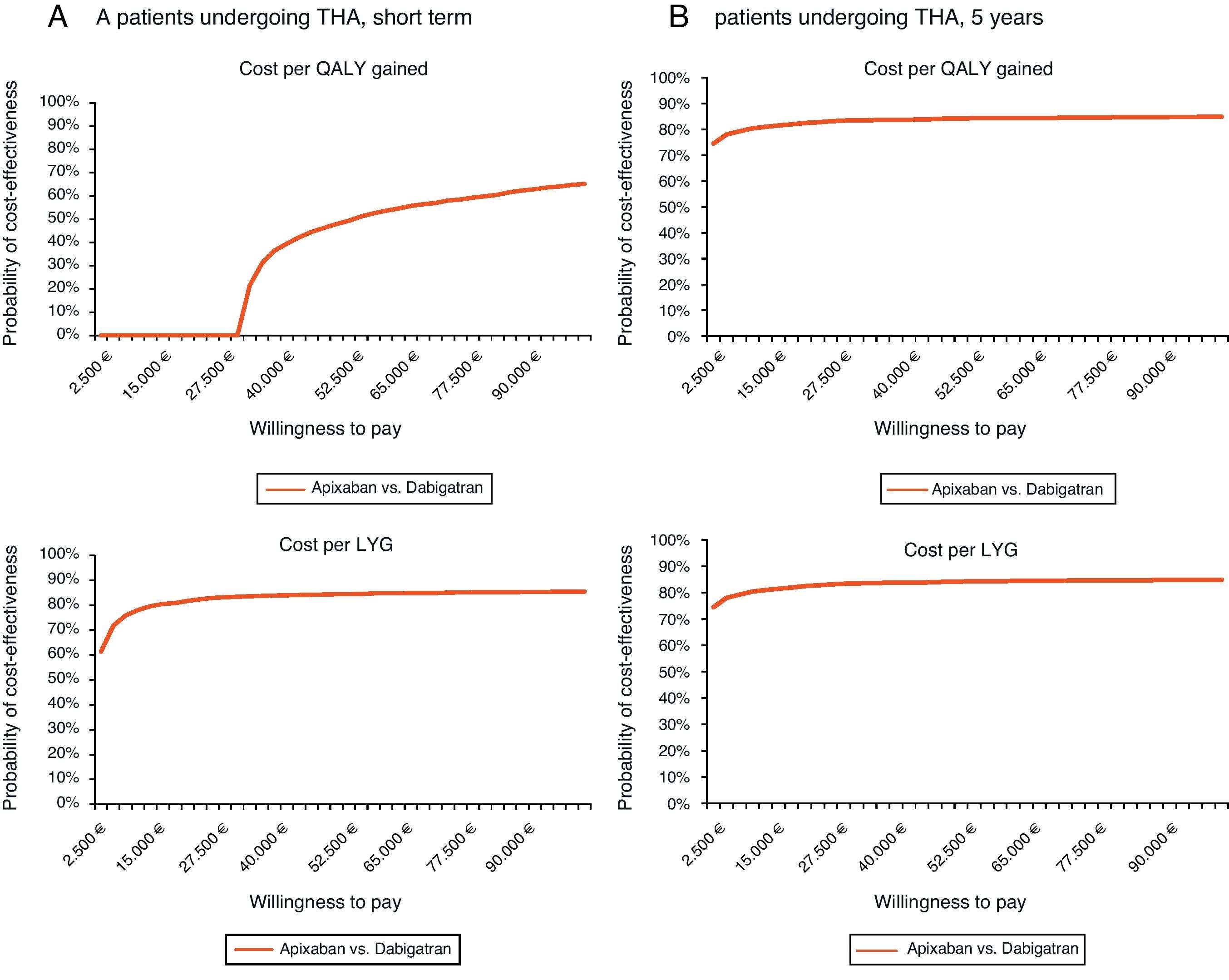
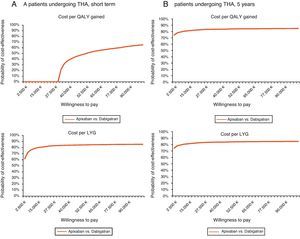
Regarding THA and for the same willingness to pay, apixaban would not be cost-effective in the short-term (cost per QALY gained), but it would be in both the short-term (81% probability for the cost per LYG) and at 5 years (probability of 82% and 83% for the cost per QALY gained and per LYG, respectively) (Table 6, Fig. 2).
Acceptability curve in total hip arthroplasty (THA): probability of apixaban being cost-effective versus dabigatran for different willingness to pay. (A) Short-term. In the short-term, for a willingness to pay €30,000 per QALY gained and per LYG, apixaban would not be cost-effective, whereas it would be with a probability of 81%, respectively. (B) 5-year period. In a 5-year period, for a willingness to pay €30,000 per QALY gained and LYG, apixaban would be cost-effective with a probability of 82 and 83%, respectively. LYG: life years gained; QALY: quality-adjusted life years; THA: total hip arthroplasty.
According to this model, apixaban is a cost-effective treatment compared with dabigatran in the prevention of VTE, in both TKA and THA.
When assessing the results of the study we must take into account both its potential limitations and consistencies. First, we must remember that this is a theoretical model which is, by definition, a simplified simulation of reality. Regarding the differences in effectiveness between apixaban and dabigatran, these were higher in the 5-year analysis than in the short-term (90 days). In the 5-year analysis, each patient would gain 0.0385 life years and 0.0169 QALYs in TKA, and 0.0118 life years and 0.0050 QALYs in THA. However, in the short-term, compared to dabigatran, apixaban would offer a gain of 0.0219 life years and 0.0007 QALYs in TKA, as well as 0.0072 life years and 0.0003 QALYs in THA. The clinically relevant minimum difference in the usefulness of 2 healthcare treatments or interventions observed with the EQ-5D, HUI2, HUI3 and SF-6D instruments was 0.074,42 0.030,43 0.030,43 and 0.033,44 respectively.45 In case the small differences in QALYs gained in the short-term, favourable to apixaban, were not considered clinically relevant, it would be concluded that treatment with apixaban generated fewer costs for patients with TKA in both the short-term and at 5 years, with savings per patient of €14 and €108, respectively. Regarding the indication in THA, in the 5-year period there would also be savings with apixaban (€5 per patient). However, in the short-term there would be an extra cost of €15 for each patient treated with apixaban.
Regarding the above, we must consider that the more realistic approach in this study is that including events at 5 years linked to TKA or THA, in the same way as most Spanish economic analyses published previously.9,46–48 In the present study, we modelled a chronic phase of 5 years for an age of 72 or 73 years at the time of the intervention, as in a Spanish study published in the year 2006.48 However, the work of Monreal et al.9 considered a much longer follow-up period, covering the 60 years following orthopaedic surgery, for patients of 40 years of age. In other Spanish studies, the mean age of patients who underwent surgery was 62–70 years and the simulated follow-up was 15–19 years according to the life expectancy.46,47 The duration of follow-up of the cohort in this study was considerably less and more restrictive than that applied in most previously published Spanish models. For monitoring the cohort between 15 and 20 years, QALYs gained for each patient treated with apixaban instead of dabigatran would be 0.037–0.041 QALYs in TKA and 0.010–0.011 QALYs in THA.
Nevertheless, these results for individual patients should be considered in the context of the population which would benefit from the difference in efficacy between apixaban and dabigatran. According to the meta-analysis conducted by Cohen et al.,11,12,25 the OR of total VTE and mortality due to all causes was higher with dabigatran than with apixaban, both in TKA (OR: 1.72; 95% CI 1.22–2.42) and in THA (OR: 2.51; 95% CI 1.50–4.21), with statistically significant differences. As shown in Table 3, this indicates that 89 episodes of VTE would be prevented in the short-term and 26 episodes of repetitive VTE at 5 years in a cohort of 1000 patients undergoing TKA and treated with apixaban. In a cohort of 1000 patients undergoing THA and treated with apixaban, 29 and 9 episodes would be prevented, respectively. Moreover, in a 5-year period, apixaban would prevent 24 episodes of PTS in patients undergoing TKA and 4 episodes in patients undergoing THA.
Although the published results of the meta-analysis by Cohen et al.11 were presented only as OR, they were also calculated as RR, and were used as such in the economic analysis. In this regard, we must indicate that the results obtained in OR were very similar to those in RR, since the frequency of thromboembolic events was much lower than 20%.49 For example, in the ADVANCE-3 study,35 the frequency of VTE was 0.8% with apixaban and 2.5% with enoxaparin.
There are other, recently published meta-analyses comparing the efficacy of apixaban, dabigatran and enoxaparin in preventing VTE. According to the meta-analysis by Huang et al.,50 apixaban reduced the risk of DVT compared with enoxaparin (OR: 0.47; 95% CI 0.27–0.82) in TKA. According to the meta-analysis of indirect comparisons by Gómez-Outes et al.,51 there would be no difference in the risk of VTE between apixaban and dabigatran (RR: 1.16; 95% CI 0.31–4.28) among patients undergoing both TKA and THA. This discrepancy with the meta-analysis by Cohen et al.11 could appear because, unlike in the latter, the TKA and THA results were analysed jointly rather than separately, and the clinical result analysed was not a compound of total VTE and deaths, but only symptomatic VTE.11,51 In the meta-analysis by Nieto et al.,52 apixaban was superior in reducing VTE (RR 0.63; 95% CI 0.36–1.01) compared with 40mg/day of enoxaparin, whilst dabigatran showed a similar efficacy to that of enoxaparin (RR 1.02; 95% CI 0.86–1.20).
Regarding the risk of bleeding, the new meta-analyses indicate that apixaban is associated with a lower rate of haemorrhages than enoxaparin (OR: 0.55; 95% CI 0.32–0.96),50 less clinically relevant haemorrhages that dabigatran (RR: 0.73; 95% CI 0.57–0.94)51 and a lower risk of bleeding than new oral anticoagulants (RR: 0.81; 95% CI 0.64–1.01).52
As for the strength of the model, the efficacy and adverse events data were obtained from the meta-analysis of randomised clinical trials mentioned previously, thus providing a high level of evidence in this regard. In addition, the costs of the various VTE and bleeding episodes related to thromboprophylaxis were obtained entirely from Spanish sources and were verified with a panel of clinical experts.
The present economic analysis, adapted to the Spanish healthcare system, is based on a model published previously for the national healthcare system of the United Kingdom.53 The results obtained in that country indicated that apixaban was a more efficient treatment that dabigatran in the prevention of VTE among patients undergoing major orthopaedic surgery. Apixaban prevented more VTE and more haemorrhages than dabigatran, and yielded more QALYs and more life years at 5 years follow-up.
There are no other cost-effectiveness analyses of apixaban conducted in Spain. However, the methodology employed in this study was similar to that in Spanish cost-effectiveness studies of VTE prevention published previously.9,46–48
According to the assumptions of the study, we may conclude that the introduction of apixaban could reduce the rate of VTE compared with dabigatran, whilst also reducing the expenditure for thromboprophylaxis among patients undergoing major orthopaedic surgery.
Ethical disclosuresProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of Data. The authors declare that no patient data appears in this article.
Right to privacy and informed consent. The authors declare that no patient data appears in this article.
Conflict of interestsThis study was jointly funded by Pfizer S.L.U. and Bristol-Myers Squibb S.A. Marina de Salas is employed by Pfizer S.L.U. and Lourdes Betegón is employed by Bristol-Myers Squibb S.A. The remaining authors have no conflict of interests to declare.
The authors wish to thank Dr. Emilio J. Alegre del Rey, from the Pharmacy Department of Hospital Universitario Puerto Real in Cadiz, Spain, for reviewing the manuscript and for his helpful comments.
Please cite this article as: Gómez-Cerezo JF, et al. Análisis coste-efectividad de apixaban frente a dabigatrán en la prevención de la tromboembolia venosa en pacientes intervenidos de artroplastia total de rodilla o de cadera. Rev Esp Cir Ortop Traumatol. 2012;56:459–70.