Critical COVID-19 survivors are at risk of developing Post-intensive Care Syndrome (PICS) and Chronic ICU-Related Pain (CIRP). We determined whether a specific care program improves the quality of life (QoL) of patients at risk of developing PICS and CIRP after COVID-19.
MethodsThe PAIN-COVID trial was a parallel-group, single-centre, single-blinded, randomized controlled trial. The intervention consisted of a follow up program, patient education on PICS and pain, and a psychological intervention based on Rehm’s self-control model in patients with abnormal depression scores (≥8) in the Hospital Anxiety and Depression Scale (HADS) at the baseline visit. QoL was evaluated with the 5-level EQ 5D (EQ 5D 5 L), mood disorders with the HADS, post-traumatic stress disorder (PTSD) with the PCL-5 checklist, and pain with the Brief Pain Inventory short form, the Douleur Neuropathique 4 questionnaire, and the Pain Catastrophizing Scale. The primary outcome was to determine if the program was superior to standard-of-care on the EQ visual analogue scale (VAS) at 6 months after the baseline visit. The secondary outcomes were EQ VAS at 3 months, and EQ index, CIRP incidence and characteristics, and anxiety, depression, and PTSD at 3 and 6 months after baseline visits.
ConclusionsThis program was not superior to standard care in improving QoL in critical COVID-19 survivors as measured by the EQ VAS. However, our data can help establish better strategies for the study and management of PICS and CIRP in this population.
Trial registration# NCT04394169, registered on 5/19/2020.
Los supervivientes de COVID-19 crítica tienen riesgo de desarrollar Síndrome post-cuidados intensivos (SPCI) y dolor crónico relacionado con la UCI (CIRP). Nuestro objetivo fue comparar si los cuidados específicos mejoran la calidad de vida (CdV) de los pacientes con riesgo de desarrollar SPCI y CIRP tras la COVID-19.
MétodosEl ensayo PAIN-COVID se trató de un ensayo controlado aleatorizado de grupo paralelo, unicéntrico y uniciego. La intervención consistió en un programa de seguimiento, educación al paciente sobre SPCI y dolor, y una intervención psicológica basada en el modelo de autocontrol de Rehm en pacientes con puntuación de depresión anormales (≥8 de la escala HADS (Hospital Anxiety and Depression Scale) en la visita basal. La CdV fue evaluada mediante EQ 5D de 5 niveles (EQ 5D 5 L), los trastornos del ánimo con HADS, el trastorno de estrés post-traumático (TEPT) con la lista de comprobación PCL-5, y el dolor con el formulario abreviado BPI (Brief Pain Inventory), el cuestionario DN4 (Douleur Neuropathique 4), y PCS (Pain Catastrophizing Scale). El resultado primario fue determinar si el programa era superior al estándar de cuidados en la escala analógica visual (EVA) de EQ transcurridos 6 meses de la visita basal. Los resultados secundarios fueron EVA de EQ transcurridos 3 meses y el índice EQ, la incidencia y las características de CIRP, así como ansiedad, depresión y TEPT transcurridos 6 meses de las visitas basales.
ConclusionesEste programa no fue superior a los cuidados estándar para mejorar la CdV en los supervivientes de COVID-19 crítica, según la medición EVA de EQ. Sin embargo, nuestros datos pueden ayudar a establecer mejores estrategias para el estudio y manejo de SPCI y CIRP en esta población.
Registro del ensayo# NCT04394169, registrado el 5/19/2020.
The SARS-CoV-2 pandemic has significantly increased the number of patients hospitalized and, particularly, those admitted to the ICU. It is well known that ICU survivors can experience a significant deterioration of their mental and physical health, as well as their quality of life (QoL).1 In 2012, the Society of Critical Care defined post-intensive care syndrome (PICS), as a term referring to new-onset long-term physical or mental health status problems in critically ill patients after discharge from ICU.2 Recently, it was reported that critically ill COVID-19 survivors frequently reported physical, mental, and cognitive symptoms within a year of ICU admission.3 A high prevalence of moderate to extreme chronic pain has also been observed in survivors of critical illness,4 and the term chronic ICU-related pain (CIRP) has been suggested to describe pain that persists for at least 3 months after discharge from the ICU.4 These problems have a reciprocal relationship and have been associated with a worse prognosis.5
Despite the large population susceptible to develop PICS and CIRP in COVID-19 patients, with an associated high impact in terms of both QoL and economic costs, to our knowledge, only few studies6,7 have assessed the efficacy of a preventive intervention to improve the post-discharge wellbeing in COVID-19 patients.
Therefore, we hypothesize that a specific follow-up program that includes therapeutic education and psychological intervention can improve the quality of life of COVID-19 patients at risk of developing PICS and CIRP. The primary outcome of the study is to determine whether the specific program is superior to the standard of care in improving the QoL, as measured by the EQ VAS, at six months after the initial visit. Secondary outcomes include assessing the influence of the intervention program on EQ VAS at three months, EQ index, CIRP incidence and characteristics, as well as incidences of anxiety, depression, and PTSD at 3 and 6 months after baseline visits.
Material and methodsStudy designThe PAIN-COVID trial is a simple blinded, randomized, controlled, superiority trial, two parallel groups conducted in the Hospital Clinic of Barcelona, Spain. It was approval by the Comité Ético de Investigación Clínica del Hospital Clinic de Barcelona (HCB/2020/0549), and registered on May 9, 2020 at http://www.clinicaltrials.gov. (NCT04394169). The study protocol has been previously described.8
Eligible patientsAdult survivors from critically severe COVID-19 infection confirmed by polymerase chain reaction-based tests with at least one of the following inclusion criteria were eligible for participation: 1) Acute Physiology and Chronic Health Evaluation (APACHE) II score over 14, 2) ICU stay over 10 days, 3) Acquired weakness in ICU (Supplement, Definition D1), 4) Delirium during ICU admission (Supplement Definition, D2). These criteria were in accordance with those previously recommended for critical illness monitoring and rehabilitation program9; delirium was added as it has been associated with worst outcomes in multiple studies.10
The exclusion criteria were: (1) Central Nervous System degenerative diseases Terminal illness (Supplement, Definition D3), (2) Terminal illness (Supplement, Definition D4), (3) Insufficient understanding of the Spanish language, (4) Difficulty in completing follow-up (home distance >50 km from the Hospital), (5) Not providing informed consent.
RandomizationThe patient was assigned to either the control or intervention group in a 1:1 proportion according to a list of computer-generated random numbers. The data for the screening were obtained from the clinical records.
Study protocolThe intervention program was compared to the standard-of-care clinical practice. The intervention included a follow up program, therapeutic education about the PICS and pain, and a psychological intervention in patients with abnormal depression ´s values (≥8) in the hospital anxiety and depression scale (HADS) at the baseline visit (Supplement, questionnaire Q1).
BlindingVisits were carried out by an investigator with adequate training in questionnaire administration who did not participate in the intervention or the evaluation of the results. The follow up program and the therapeutic education were performed by a pain physician with knowledge about PICS and CIRP (AO) and the psychological intervention was performed by two psychologists (MMSR, ACC). These researchers did not participate in the questionnaire and baseline data collection. Researchers who analyze the results were blinded to the randomizations arm.
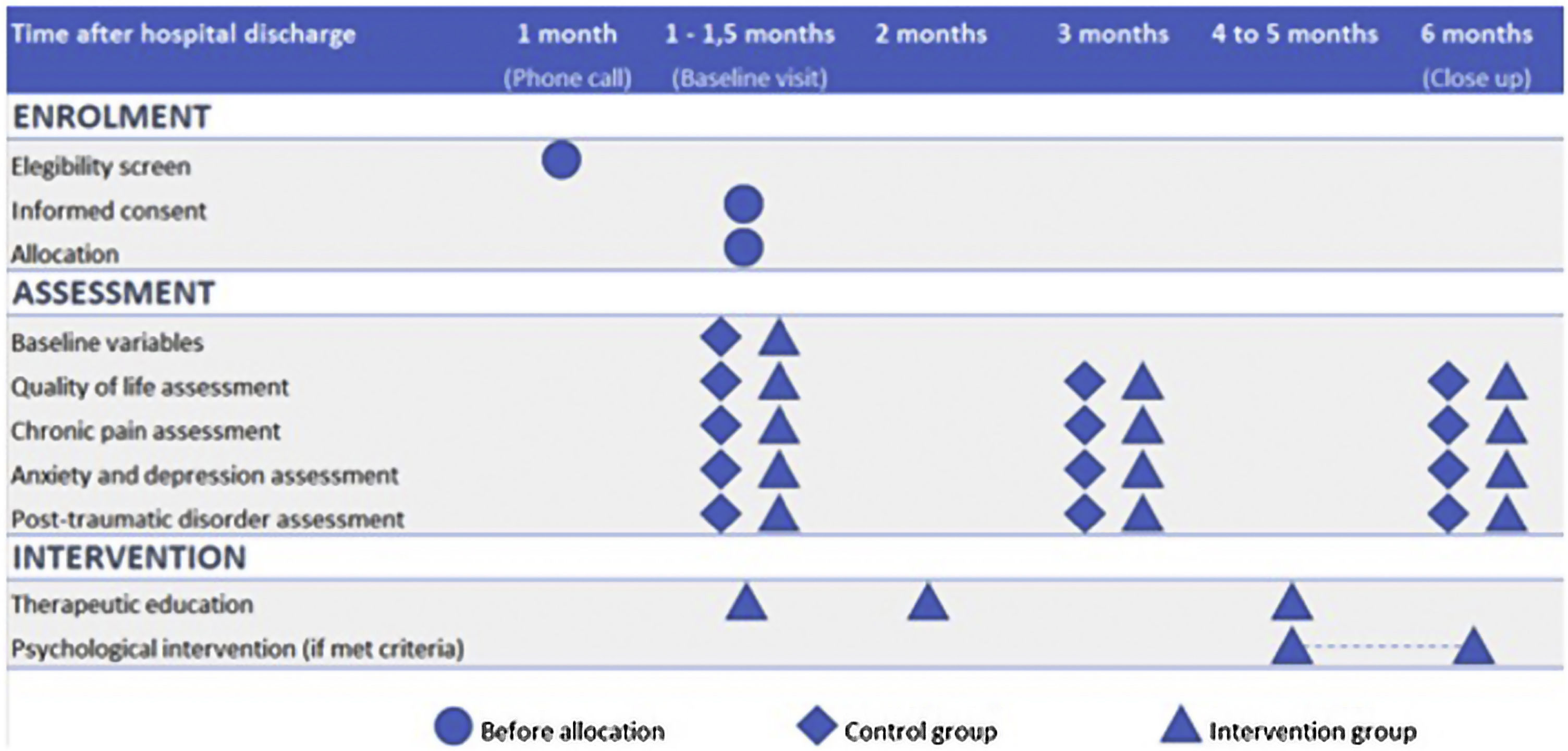
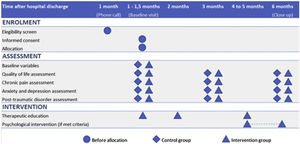
Recruitment and participant timelineAll patients who were discharged from the ICU were identified using the site's clinical database. Those who were eligible were contacted one month after hospital discharge to determine their interest in participating in the study, and informed consent was obtained. After obtaining consent, the patients were randomized. Fig. 1 shows the timeline of participants in the study.
Baseline Visit (V1): All patients were scheduled to a face-to-face baseline visit during which demographic data, medical history, and ICU and hospitalization variables were collected. The definitions of these variables can be found in the Supplement. To assess participants' QoL, anxiety, depression, PTSD, and pain characteristics, several scales were administered, including EQ 5D/5 L, HADS, PCL-5 questionnaire, brief pain questionnarie (BPI), Dolour Neuropathique 4 (DN4) interview questionnaire, and pain catastrophizing scale (PCS). Detailed explanations of each test can be found in the outcome measurements section and in the Supplement. Mini-mental state exam (MMSE) test, which is a widely used test of cognitive function among the elderly that analyses orientation, attention, memory, language, and visual-spatial skills, was done before answering the questionnaires.
Follow up Visits (V2, V3): These visits were conducted by phone at 3 and 6 months (close out visit) after the baseline visit. During these visits, the participants completed the outcomes measurements test: EQ 5D/5 L, HADS, PCL-5, BPI-SF, DN4 interview questionnaire, and PCS.
Intervention groupSpecific follow up program and therapeutic educationConsisted of three face-to-face sessions (Supplement, Table S1).
Session 1: scheduled four weeks after discharge in accordance with published recommendations.9 The session lasted 60 min and involved a standardized interview and physical examination to evaluate the patient's condition. Based on the findings, patients were advised to seek specialized follow-up. Additionally, this session marked the beginning of therapeutic education on PICS and pain, which will be further explained in later sections.
Session 2 and Session 3: Sessions were scheduled 8 and 18 weeks after discharge, respectively. These sessions lasted 30 min and involved a standardized interview to assess any new symptoms related to PICS and chronic pain. Patients were advised to seek help from a specialist if new findings were present or if they had not yet been evaluated by the appropriate specialist. In this session, it was also evaluated whether the patient had read and completed the delivered rehabilitation manual.11 If the patient reported pain, specific therapeutic education on pain management was provided.
The Therapeutic education about PICS was delivered orally and through specific documents. The documents included a descriptive sheet developed by the researchers (Supplement, Other documents 1) and a rehabilitation manual developed by Jones group at St Helens and Knowsley Teaching Hospital in the UK, which was recommended by the Follow-up and Rehabilitation Committee of the Argentine Society of Intensive Care, this rehabilitation manual consists of three sections: the first section contains a six-week rehabilitation program, the second section provides recommendations and information about common problems that may arise after intensive care, and the third section outlines the exercise program that should be followed during the rehabilitation process.11 Subjects were asked to carefully read the documents and completed the rehabilitation manual at home.
The Therapeutic education program on pain was delivered orally to all subjects (Supplement, Table S2). They were provided with information about the purpose of acute pain and the differences between acute and chronic pain. Additionally, they were explained that pain serves as a protective mechanism rather than just being a symptom of damage. Subjects who reported new onset pain were provided with additional information, including how pain can become chronic, the importance of proper diagnosis and treatment of neuropathic pain, the rational use of prescribed analgesics, strategies for managing daily activities while experiencing pain, the importance of preventive pain management for proper rehabilitation, central sensitization, and its causes, and the relationship between stress, emotions, and chronic pain. The sessions were designed to encourage patient questions and feedback, with an emphasis on individualized information. The topics covered in this program were based on pain neuroscience education, which have robust scientific evidence for improving outcomes in various chronic pain syndromes.12
Psychological interventionThe intervention protocol consisted of 7 weekly sessions lasting one and a half hours each (Supplement, Table S3). The intervention in depression is based on Rehm’s model of self-control. Psychological interventions may cause adverse events resulting in worsening of patients’ clinical course (overdose, self-harm, and self-harm attempts). Therefore, the investigators monitored any related symptom, report it as an adverse event, and refer the patient for treatment by a specialist unit.
A patient was considered to have completed intervention if they attended at least 66% of the scheduled face-to-face visits (2 of 3 for the follow up program and therapeutic education visits and 5 of 7 for the psychological intervention visits).
Control groupThe patients in the study were provided with the standard of local care, which encompassed routine follow-up appointments with their referring physicians (primary care physicians or specialists), were not directly involved in the study. The study did not interfere with the patients' usual medical care or treatment plans. No preventive psychological intervention was administered to the patients as part of the study. Therefore, any psychological counseling or therapy that the patients received was a part of their regular care and not a component of the study.
Outcome measurementsThe impact of the intervention program on QoL was assessed through the EQ 5D/5L (Supplement, questionnaire Q2). This questionnaire evaluates five domains of QoL: mobility, self-care, usual activities, pain/discomfort, anxiety/depression, each scored on a scale of 1 (no problems) to 5 (indicating extreme problems), generating a 5-digit code corresponding to QoL. The visual analogue scale of the same test was also assessed (from 0 -the worst imaginable health- to 100 -the best imaginable health).
To asses pain, (presence and intensity) the BPI questionnaire was used (Supplement, questionnaire Q3). This is a multidimensional questionnaire that evaluates pain intensity in the last 24 h (worst, lowest, average) and current (right now). According to IMMPACT recommendations, a clinically significant pain were defined if the BPI average pain item mean is greater than or equal to 3.13 The definition of new onset-pain was an affirmative answer to the first question of the BPI-SF questionnaire. If the answer was positive, the investigator confirmed that the pain was not present before admission to ICU or prior to COVID-19 infection. Patients were instructed not to consider acute covid symptoms when asked whether they had pain before ICU admission. If BPI was positive for pain, pain catastrophizing and neurophatic pain (NP) was assessed by the PCS (Supplement, questionnaire Q4) and by the DN4 test interview (DN4-interview) (Supplement, questionnaire Q5) respectively. A PCS score greater than or equal to 30 was considered a clinically relevant level of catastrophizing. The presence of NP was considered if the DN4-interview score was greater than or equal to 3.
The impact of intervention program on anxiety or depression incidence was assessed by the HADS test (Supplement, questionnaire Q1), a cut-off point equal or greater that 8 was used as abnormal anxiety or depression values.14 Finally, the incidence of PTSD was evaluated with the PTSD checklist questionnaire (Supplement, questionnaire Q6) (PCL-5).
Statistical methodsSample sizeThe study protocol and the statistical analysis plan were presented before the enrollment.8 The sample size was calculated assuming an average of 50 points on the EQ VAS in the control group and a clinically relevant difference between the groups of 20%. For distribution of a tail with a type I error of 0.05 and a power of 80%, we have calculated a sample size of 84 patients, 42 for each arm. Estimating a loss of follow up of 20%, we would need a sample size of 102 patients (51 each group).
Data analysisQualitative variables are presented as proportions while for quantitative variables, mean (standard deviation) or median (interquartile range), after checking for normality using the Shapiro-Wilk test. To compare variables across groups, Student t-tests or Mann–Whitney U test for continuous data and Chi-square tests or exact tests for categorical variables were carried out. Before parametric hypothesis testing, equality of variances was studied with the use of Levene’s test and if assumptions are not met, contrasts were performed with Welch’s test. An intention-to-treat approach will be followed. Two-tailed P-values were presented and a significance level of 0.05 will be used. For secondary outcomes, adjustment with the Benjamini–Hochberg procedure was carried out. A sub-analysis of the effect of treatment on compliers was performed for the main outcome. Compliers were defined as those subjects that, being randomized to the intervention, complete at least two out of three medical visits and at least five out of seven psychological interventions. For statistical analysis, instrumental variable analysis was carried out. Statistical analysis was performed using R statistical software.
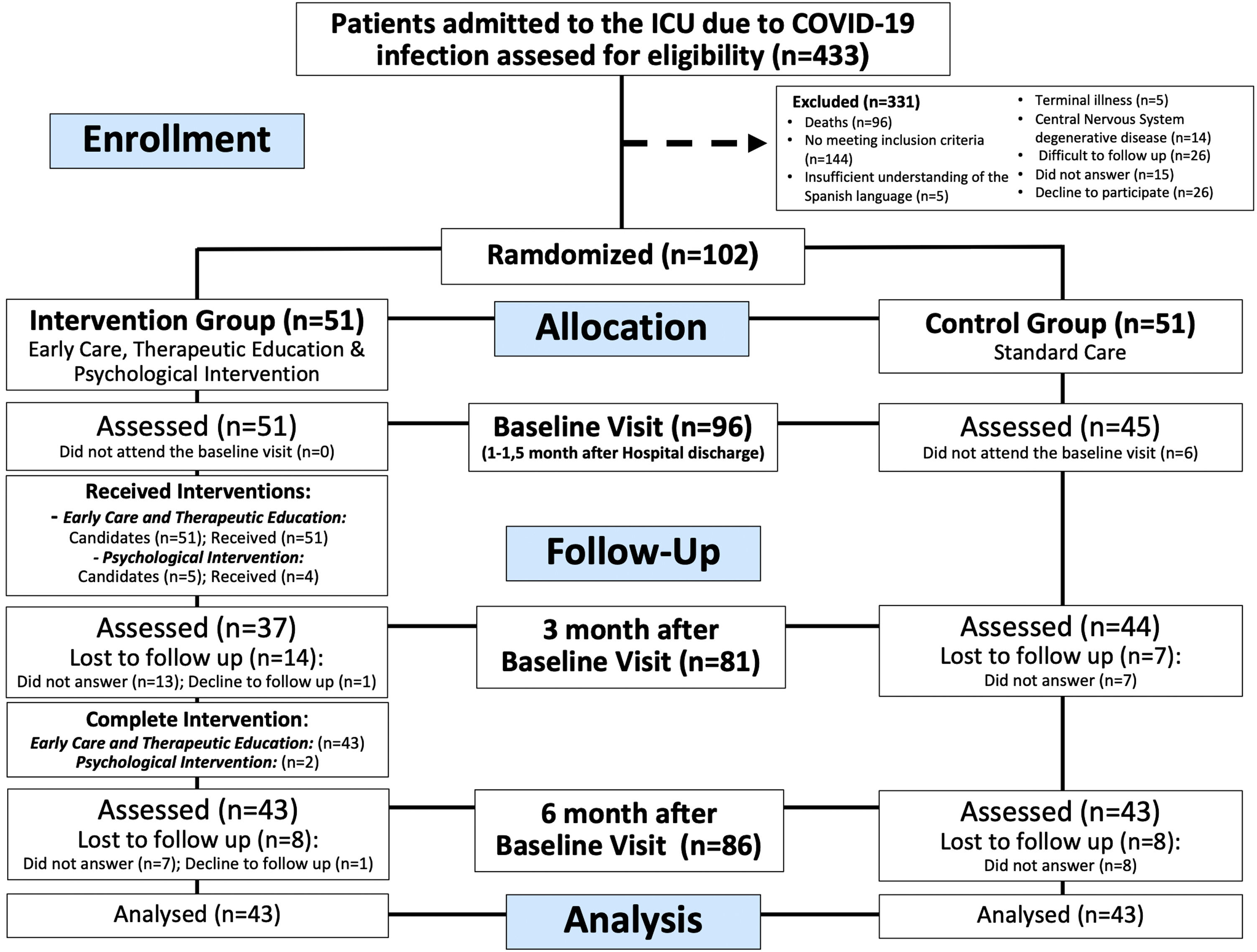
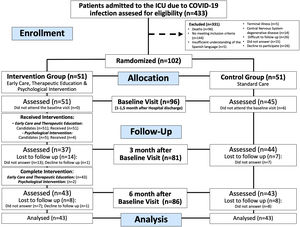
ResultsParticipantsEnrolment began on May 27, 2020, and ended on February 26, 2021, when the sample size was reached. Consecutively 433 patients were assessed for eligibility, 144 did not meet the inclusion criteria, and 187 were excluded based on the exclusion criteria. Finally, 102 patients agreed to participate in the study and were randomized, of which, 96 were evaluated at first visit (intervention group n = 51), 81 (intervention group n = 37) at 3 months, and 86 (intervention group n = 43) at 6 months (Fig. 2). The loss to follow-up rate at 3 months was 13.7% in the control group and 27.5% (p = 0.14) in the intervention group, at 6 months, both groups have the same loss to follow up rate (15.7%, p > 0.99).
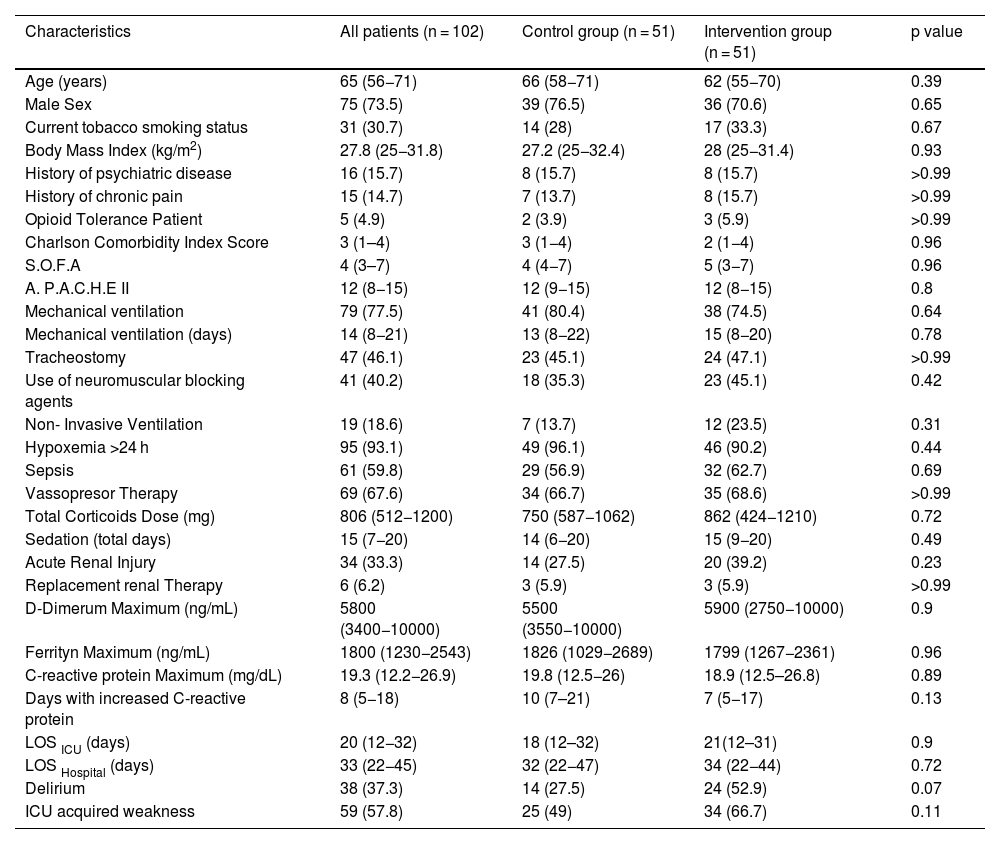
A summary of the baseline characteristics of all enrolled patients are showed in Table 1, and a summary of the baseline characteristics of all analyzed patients are showed in Supplement Table S4. The baseline characteristics of all enrolled and analyzed patients were similar between the groups.
Summary of demographics and characteristics of all enrolled patients.
| Characteristics | All patients (n = 102) | Control group (n = 51) | Intervention group (n = 51) | p value |
|---|---|---|---|---|
| Age (years) | 65 (56−71) | 66 (58−71) | 62 (55−70) | 0.39 |
| Male Sex | 75 (73.5) | 39 (76.5) | 36 (70.6) | 0.65 |
| Current tobacco smoking status | 31 (30.7) | 14 (28) | 17 (33.3) | 0.67 |
| Body Mass Index (kg/m2) | 27.8 (25−31.8) | 27.2 (25−32.4) | 28 (25−31.4) | 0.93 |
| History of psychiatric disease | 16 (15.7) | 8 (15.7) | 8 (15.7) | >0.99 |
| History of chronic pain | 15 (14.7) | 7 (13.7) | 8 (15.7) | >0.99 |
| Opioid Tolerance Patient | 5 (4.9) | 2 (3.9) | 3 (5.9) | >0.99 |
| Charlson Comorbidity Index Score | 3 (1–4) | 3 (1−4) | 2 (1−4) | 0.96 |
| S.O.F.A | 4 (3–7) | 4 (4−7) | 5 (3−7) | 0.96 |
| A. P.A.C.H.E II | 12 (8−15) | 12 (9−15) | 12 (8−15) | 0.8 |
| Mechanical ventilation | 79 (77.5) | 41 (80.4) | 38 (74.5) | 0.64 |
| Mechanical ventilation (days) | 14 (8−21) | 13 (8−22) | 15 (8−20) | 0.78 |
| Tracheostomy | 47 (46.1) | 23 (45.1) | 24 (47.1) | >0.99 |
| Use of neuromuscular blocking agents | 41 (40.2) | 18 (35.3) | 23 (45.1) | 0.42 |
| Non- Invasive Ventilation | 19 (18.6) | 7 (13.7) | 12 (23.5) | 0.31 |
| Hypoxemia >24 h | 95 (93.1) | 49 (96.1) | 46 (90.2) | 0.44 |
| Sepsis | 61 (59.8) | 29 (56.9) | 32 (62.7) | 0.69 |
| Vassopresor Therapy | 69 (67.6) | 34 (66.7) | 35 (68.6) | >0.99 |
| Total Corticoids Dose (mg) | 806 (512−1200) | 750 (587−1062) | 862 (424−1210) | 0.72 |
| Sedation (total days) | 15 (7−20) | 14 (6−20) | 15 (9−20) | 0.49 |
| Acute Renal Injury | 34 (33.3) | 14 (27.5) | 20 (39.2) | 0.23 |
| Replacement renal Therapy | 6 (6.2) | 3 (5.9) | 3 (5.9) | >0.99 |
| D-Dimerum Maximum (ng/mL) | 5800 (3400−10000) | 5500 (3550−10000) | 5900 (2750−10000) | 0.9 |
| Ferrityn Maximum (ng/mL) | 1800 (1230−2543) | 1826 (1029−2689) | 1799 (1267−2361) | 0.96 |
| C-reactive protein Maximum (mg/dL) | 19.3 (12.2−26.9) | 19.8 (12.5−26) | 18.9 (12.5–26.8) | 0.89 |
| Days with increased C-reactive protein | 8 (5−18) | 10 (7–21) | 7 (5−17) | 0.13 |
| LOS ICU (days) | 20 (12−32) | 18 (12–32) | 21(12–31) | 0.9 |
| LOS Hospital (days) | 33 (22−45) | 32 (22−47) | 34 (22−44) | 0.72 |
| Delirium | 38 (37.3) | 14 (27.5) | 24 (52.9) | 0.07 |
| ICU acquired weakness | 59 (57.8) | 25 (49) | 34 (66.7) | 0.11 |
Data are in numbers and proportions (%) for categorical variables and in median and interquartile range (IQR) for continuous variables. Abbreviations: APACHE II: Acute Physiology and Chronic Health Evaluation II; S.O.F.A: Sequential Organ Failure Assessment Score; LOS: Length of Stay. Non-Invasive Ventilation: Patients without Intubation all along the process. p value refers to the statistical comparison between “Intervention Group” and “Control Group” patients.
All patients assigned to the intervention group received the follow up program and the therapeutic education. Eight patients were considered non-compliers (only attended 1 medical visit), of those patients, six patients reported not having time to attend to visits, one patient reported not wanting to attend to visits, and one patient was hospitalized at the time of the medical visits.
Psychological interventionOnly five patients were candidates to receive specific psychological intervention since HADS Depression scale was equal to or greater than 8 in the baseline assessment. Of these subjects, one was already undergoing psychological treatment for substance use disorder. Of the four remaining patients, only two attended more than 5 visits.
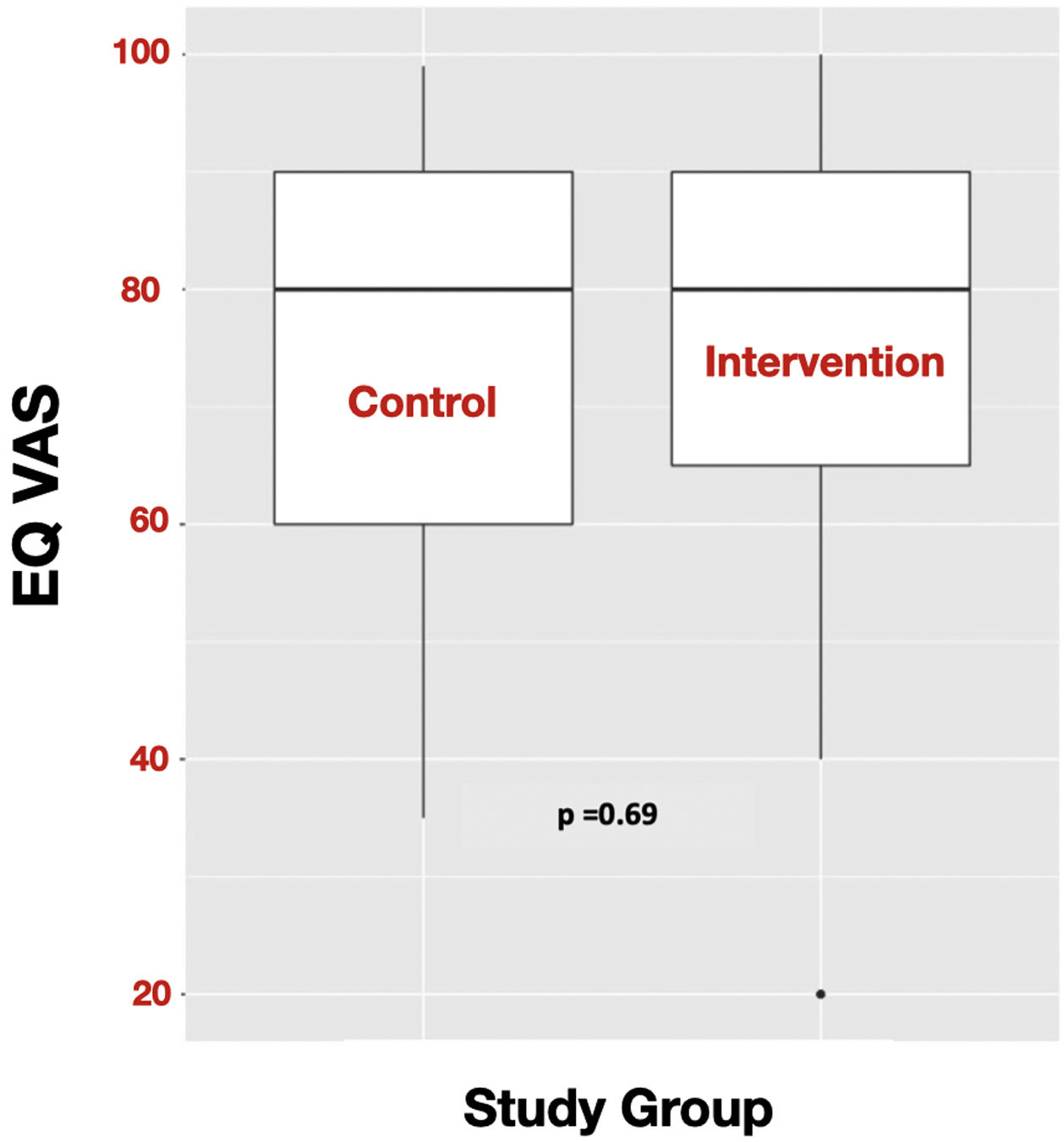
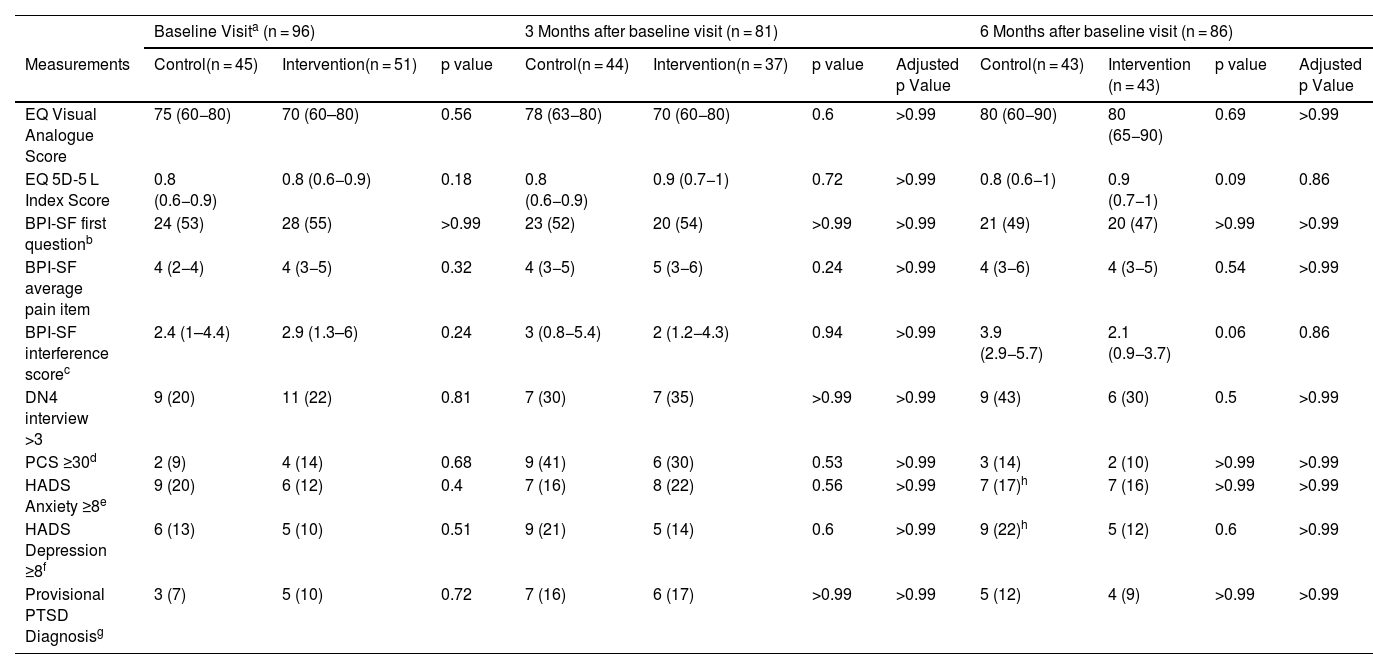
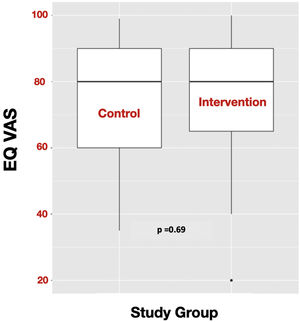
OutcomesIn these critically ill COVID-19 survivors’ population, there were no statistically significant differences in the QoL measured by the EQ VAS at six months after the baseline visit between the subjects of the intervention group and the control group (EQ VAS: 80 (65−90) vs. 80 (60−90); p = 0.69) (Fig. 3). There were also no statistically significant differences in the secondary outcomes (Table 2). In addition, being compliant with the follow up program and the therapeutic education program were not related to response to the intervention for the primary outcome.
Outcome measurements.
| Baseline Visita (n = 96) | 3 Months after baseline visit (n = 81) | 6 Months after baseline visit (n = 86) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Measurements | Control(n = 45) | Intervention(n = 51) | p value | Control(n = 44) | Intervention(n = 37) | p value | Adjusted p Value | Control(n = 43) | Intervention (n = 43) | p value | Adjusted p Value |
| EQ Visual Analogue Score | 75 (60−80) | 70 (60–80) | 0.56 | 78 (63−80) | 70 (60−80) | 0.6 | >0.99 | 80 (60−90) | 80 (65−90) | 0.69 | >0.99 |
| EQ 5D-5 L Index Score | 0.8 (0.6−0.9) | 0.8 (0.6−0.9) | 0.18 | 0.8 (0.6−0.9) | 0.9 (0.7−1) | 0.72 | >0.99 | 0.8 (0.6−1) | 0.9 (0.7−1) | 0.09 | 0.86 |
| BPI-SF first questionb | 24 (53) | 28 (55) | >0.99 | 23 (52) | 20 (54) | >0.99 | >0.99 | 21 (49) | 20 (47) | >0.99 | >0.99 |
| BPI-SF average pain item | 4 (2−4) | 4 (3−5) | 0.32 | 4 (3−5) | 5 (3−6) | 0.24 | >0.99 | 4 (3−6) | 4 (3−5) | 0.54 | >0.99 |
| BPI-SF interference scorec | 2.4 (1–4.4) | 2.9 (1.3–6) | 0.24 | 3 (0.8−5.4) | 2 (1.2−4.3) | 0.94 | >0.99 | 3.9 (2.9−5.7) | 2.1 (0.9−3.7) | 0.06 | 0.86 |
| DN4 interview >3 | 9 (20) | 11 (22) | 0.81 | 7 (30) | 7 (35) | >0.99 | >0.99 | 9 (43) | 6 (30) | 0.5 | >0.99 |
| PCS ≥30d | 2 (9) | 4 (14) | 0.68 | 9 (41) | 6 (30) | 0.53 | >0.99 | 3 (14) | 2 (10) | >0.99 | >0.99 |
| HADS Anxiety ≥8e | 9 (20) | 6 (12) | 0.4 | 7 (16) | 8 (22) | 0.56 | >0.99 | 7 (17)h | 7 (16) | >0.99 | >0.99 |
| HADS Depression ≥8f | 6 (13) | 5 (10) | 0.51 | 9 (21) | 5 (14) | 0.6 | >0.99 | 9 (22)h | 5 (12) | 0.6 | >0.99 |
| Provisional PTSD Diagnosisg | 3 (7) | 5 (10) | 0.72 | 7 (16) | 6 (17) | >0.99 | >0.99 | 5 (12) | 4 (9) | >0.99 | >0.99 |
Data are in numbers and proportions (%) for categorical variables and in median and interquartile range (IQR) for continuous variables; p values were adjusted for false discovery rate by the Benjamini–Hochberg method.
Abbreviations: EQ: European Quality of Life Scale; 5D-5 L: 5 Dimensions- 5 Levels; BPI-SF: Brief Pain Inventory Short Form; DN4: Douleur Neuropathique 4 Questionnaire; PCS: Pain Catastrophizing Scale; HADS: Hospital Anxiety and Depression Scale; PTSD: Post Traumatic Stress Disorder.
BPI-SF questionnaire first question: “Throughout our lives, most of us have had pain from time to time (such as minor headaches, sprains, and toothaches). Have you had pain other than these everyday kinds of pain?”.
PCS ≥30: clinically relevant level of catastrophizing measured through the Pain Catastrophizing Scale.
HADS Anxiety ≥8: abnormal level of anxiety measured through the Hospital Anxiety and Depression Scale.
HADS Depression ≥8: abnormal level of depression measured through the Hospital Anxiety and Depression Scale.
To our knowledge, this is the first randomized clinical trial assessing the efficacy of a specific follow up program for the management of the mental components of PICS and chronic pain in COVID-19 critical illness survivors. Compared with standard care, the intervention program did not show any significant changes in the assessed outcomes. However, beyond these results, we have found a series of findings that provide valuable information for future research on this population. We have also proposed some hypotheses that could explain these findings.
Post-ICU care is currently under development and is a subject of debate when it comes to choosing the appropriate outcome to assess the impact of an intervention.1 Based on previous recommendations,9 we decided to use the EQ VAS as the primary outcome. Our study population's EQ VAS value at six months after the baseline visit was 80 (IQR 60−90), which is similar to that reported in the Spanish general population (77.53; SD 18.6).15 Our findings are similar to those of a previous longitudinal study,16 where EQ VAS values were similar to those of the general population, and those values hardly changed over time (Table 2). However, an EQ VAS value comparable to the general population that does not modify over time prevents evaluating the impact of an intervention on the QoL of the subjects studied. There could be several reasons to explain the mismatch between the symptoms and the deficiencies that a survivor of a critical illness has with respect to the way in which this person perceives their health person, some of these reasons are post-traumatic growth,17 resilience,18 other psychological phenomena such as blind optimism,19 and the social comparison,20 where people respond to health states compared to their reference groups. Recently, Turnbull AE et al. have described a term to define the difference between an individual's perceived health and the expected health perception among peers with similar self-reports. This term was called perspective deviation (PD) and was defined as the difference between a person's perception of health and the average or expected perception of health, given self-reported measures of physical, emotional, and social functioning after a period in a new state of health. In an analysis of the results of two longitudinal cohorts, they reported a moderate correlation between predicted and actual health perception, with approximately half of survivors reporting a perception that differed from predictions by more than the minimal clinically important difference for the EQ-5D VAS.21 It is important to note that the use of EQ VAS and EQ index as outcome measures for interventions targeting the mental components of PICS is limited by their ability to assess the physical aspect of a patient’s QoL. This is particularly relevant for post-ICU COVID-19 patients who often experience symptoms that mainly affect the physical domain of the EQ5D scale, such as dyspnea, muscle weakness.22,23 Therefore, to comprehensively evaluate the impact of a program on EQ5D, it is necessary to also assess muscle weakness and respiratory dysfunction. It is important to highlight that in our study we did not specifically assess these aspects, which could have affected the overall assessment of the patients' QoL.
Other limitation of our study is that the criteria for access to the psychological intervention program (HADS D ≥8 in the baseline evaluation) limited the number of candidates to receive this intervention, and only four patients were referred to this treatment. For this reason, comparative analysis to test the effectiveness of psychological interventions couldn't be performed. Furthermore, the ratio of depressive symptoms increased in the follow-up but was not included in the psychological treatment. The increased percentage of patients with depressive symptoms (from 5.2% at baseline to 16.3% at V3) suggests that psychological symptoms may increase some months after hospital discharge. This phenomenon has been observed in other adverse situations.24 We also found practical and organizational issues in achieving the complete implementation of the psychological intervention. As has been shown in other series, most of the patients had significant physical limitations.22,23 Therefore, some options to be considered include coordinating psychological interventions with physical rehabilitation programs in the hospital or implementing online treatment to improve access.
In contrast to other studies,25 our sample had low rates of depressive symptoms at baseline, potentially due to selection bias in our hospital district, where participants tended to have high socioeconomic and sociocultural status. Both are protective factors for psychopathology buffering the negative effect of stress factors on mental health.26
After treating post-ICU patients, we questioned whether self-control therapy was appropriate since it was designed for major depressive disorders, while our population faced mental, emotional, and physical issues related to PICS and COVID-19 crisis,22 that conditioned the implementation of behavioural interventions. Psychological interventions should be designed considering the specificity of this population, with a comprehensive intervention that include not only depressive symptoms but also other psychological symptoms, such as anxiety or post-traumatic symptoms, and the neuropsychological treatment of possible cognitive impairment.
It important to emphasize that almost half of the population in our study reported experiencing new-onset pain during the three evaluation periods (V1: 54%, V2: 53%, V3: 48%). Furthermore, the incidence of NP in these patients increased over time (V1: 21%, V2: 33%, V3: 37%). The high incidence of new-onset chronic pain, which is alarmingly higher than previously reported in other studies,27 could be a long-term post-COVID sequelae.28 Our intervention was not effective in reducing the incidence of this problem, which highlights the necessity to develop multidisciplinary and multimodal intervention programs.
Although not statistically significant, it's worth noting the clinically relevant higher incidence of delirium and ICU-acquired weakness in the intervention group, which may have contributed to the lack of significant differences in outcomes observed between the groups.
Our study has other limitations worth mentioning. Firstly, there is a potential selection bias since patients with severe mobility limitations, who met the inclusion criteria, may have been unable to attend trial visits. Secondly, our study was conducted at a single center, which may restrict the generalizability of our findings. Additionally, we acknowledge that telephone assessments conducted at 3 and 6 months follow-up might be less ideal than in-person assessments with a structured interview, which has been previously criticized. Future studies should address those limitations to guarantee an accurate and comprehensive evaluation of patients' outcomes.
Finally, due to the health emergency caused by the first wave of the COVID-19 pandemic, our ability to offer a more comprehensive program. It is crucial to emphasize the importance of preventing PICS and CIRP by targeting modifiable risk factors with physical pre-habilitation, cognitive stimulation, and psychological interventions. In addition, post-discharge care from the ICU during hospitalization is essential. A preliminary data analysis of an early intervention program in 47 non-COVID patients showed improvements in mental health components, patients' ability to perform basic activities of daily living, and reductions in perceived caregiver burden from ICU discharge to hospital discharge.29
These findings require confirmation through larger, randomized multicenter studies, specifically focusing on assessing the long-term impact of intervention programs on patients' quality of life, using appropriate outcome measures tailored and recommended to this population.30
ConclusionsThis specific program was not superior to the standard care in improving the QoL of critically ill COVID-19 survivors as measured by the EQ VAS, but we have shown findings that may help improve future studies in ICU survivors.
Authors' contributionsAll authors contributed to the study conception and design. The design was performed by A. Ojeda. Preparation, patient recruitment and data collection were performed by A. Calvo, T. Cuñat, O. Comino-Trinidad, J. Aliaga and M. Arias. The first draft of the manuscript was written by A. Ojeda with all the authors commented on previous versions of the manuscript. The intervention was performed by A. Ojeda, A. Costas-Carrera, and MM Sanchez. Data analysis was performed by R. Mellado-Artigas. C. Dürsteler, G. Martinez-Palli and C. Ferrando have reviewed the different contributions of all the authors and collaborated with the structure of this manuscript. All authors read and approved the final manuscript.
Ethics approvalThis study was performed in accordance with the tenets of the Helsinki Declaration and has been approved by the authors’ institutional review board. This study was approved by the Comité Ético de Investigación Clínica del Hospital Clinic de Barcelona, approval number: HCB/2020/0549, Chairperson: Prof. Joaquin Fores Viñeta, on May 14, 2020.
Availability of data and materialThe data that support the findings of this study will be available on www.clinicaltrials.gov or by request to the corresponding author (Dr. Ojeda), upon reasonable request.
FundingThe authors received no financial support for the research, the payment for copyright and use of all questionnaires was paid with own resources.
Authors state no conflict of interest.
The authors thank the staff of the Pain Unit and the Surgical Intensive Care Unit and to all the patients who have made this study possible.