Psoriasis (PsO) and psoriatic arthritis (PsA) are chronic rheumatic diseases with a significant impact on quality of life. Consensus has been described in the literature to determine the preliminary points for implementing a centralized patient care programme. However, there is no clarity regarding its effectiveness in real-life conditions.
ObjectiveTo collect the evidence systematically and exhaustively that meets the search and eligibility criteria for effectiveness of centralized care programmes in the population diagnosed with PsO and PsA.
Materials and methodsA systematic review of the literature was carried out over 5 years using the electronic bases Medline, PubMed, Cochrane, Virtual Health Library, and Embase of models focused on disease care or clinics of excellence versus programmes of conventional care.
ResultsA total of 8902 articles were identified, of which 16 studies were selected and grouped into 3 domains: multidisciplinary care, telemedicine, and training by health professional, and patient self-management, determining the programmes’ effectiveness through scales such as PASI, DAPSA, DAS28, EQ-5D-5L, DLQI, SF36, and MMAS-8, as well as adherence to medication, satisfaction levels, improvement perception, comorbidities assessment, and early diagnosis of joint involvement.
DiscussionThe clinical evidence that supports the effectiveness of centralized patient care programme strategies is scarce. However, the information collected demonstrates the efficacy of these interventions using activity and quality of life outcomes, demonstrating the importance of their use and implementation in PsO and PsA care.
La psoriasis (PsO) y la artritis psoriásica (APs) son condiciones reumáticas incurables, crónicas, con un impacto importante en la calidad de vida de las personas. En la literatura se encuentran consensos que determinan los puntos preliminares de la implementación de programas centralizados de atención de los pacientes, sin embargo, no existe claridad con respecto a su efectividad en condiciones de vida real.
ObjetivoReunir de forma sistemática y exhaustiva evidencia publicada que cumpla los criterios de búsqueda y elegibilidad de la efectividad de programas de atención centralizados en la población con diagnóstico de PsO y APs.
Materiales y métodosSe realizó una revisión sistemática de la literatura en un periodo de 5 años, utilizando las bases electrónicas Medline, PubMed, Cochrane, Biblioteca Virtual de la Salud y EMBASE, con el propósito de encontrar modelos centrados en la atención de enfermedades o clínicas de excelencia comparados con programas de atención convencional.
ResultadosSe identificó un total de 8.902 artículos, de los cuales se seleccionaron 16. Estos últimos se agruparon en tres dominios: atención multidisciplinaria, telemedicina y educación al personal de salud y autogestión del paciente, identificando la eficacia de estos programas por medio de escalas como PASI, DAPSA, DAS28, EQ-5D-5L, DLQI, SF36 y MMAS-8, además de adherencia a medicación, niveles de satisfacción, percepción de mejoría, valoración de comorbilidades y diagnóstico temprano de compromiso articular.
DiscusiónLa evidencia clínica que avala la efectividad del uso de programas centralizados de atención de pacientes es escasa, no obstante, la información recopilada demuestra la efectividad de estas intervenciones usando desenlaces de actividad y calidad de vida, y ello señala la importancia de su implementación en la atención de los pacientes con PsO y APs.
Psoriasis (PsO) is an inflammatory skin condition mainly affecting people between 20 and 30 years, and 50-6 years old, with similar frequency between men and women. PsO affects 1–2% of the population. PsO patients may have joint involvement in 40% of cases. Skin manifestations may precede the onset of arthritis by 10 years.1–3
PsO and psoriatic arthritis (PsA) are chronic, incurable rheumatic conditions, but respond favourably to different available therapies. According to the recent guidelines for the treatment of PsA, in case of poor response in the control of axial compromise, enthesitis, and dactylitis, with the use of nonsteroidal anti-inflammatory drugs (NSAID), biological therapy can be used, including anti-tumour necrosis factor (TNF), anti-interleukin 17 (IL17), or anti-IL12/23 therapies or alternatives such as phosphodiesterase 4 inhibitors or Janus kinase inhibitors.4 When there is a poor response to topical measures and disease-modifying anti-rheumatic drugs (DMARD) for skin involvement, DMARDs, biological therapy can be also used.4
PsO and PsA are part of the spectrum of a chronic disease, with various clinical manifestations that require a periodic evaluation and sequential use of medications. The preceding imposes many challenges in patient care, especially adherence in a comorbid population with high cardiovascular risk.2,4
One option to improve adherence and increase the probability of good results with therapies is to manage these patients with care models based on multidisciplinary groups, with transdisciplinary interaction.5
Understanding the complexity of skin manifestations together with a wide variety of joints involvement, it is logical to develop care programmes focused on improving the quality of life of these patients through disease control.5 The concept of centralized care programmes for pathologies has been structured in several types of chronic diseases, such as heart failure, haemophilia, chronic renal failure, chronic obstructive pulmonary disease, among others. These programmes are multidisciplinary and transdisciplinary, in which the participating professionals are distributed according to the conditions of each disease. Adherence to treatment schemes is reinforced through educational programmes, periodic follow-up of clinical outcomes, evaluation of health systems barriers, and maintaining treatment continuity, among other interventions. Within rheumatic diseases, this concept has been developed in rheumatoid arthritis (RA).6 Centres with comprehensive centralized patient care schemes, called centres of excellence, had been created to improve health outcomes and reduce healthcare costs. In PsO and PsA, it is evident that the development of outpatient care clinics with dermatologists and rheumatologists improves diagnostic certainty and treatment access for multiple skin conditions related to rheumatic diseases.5 There are multiple reports in the literature ranging from narrative reviews to expert consensus, to establish models of care, but there is heterogeneous information among groups. One example, Gratacos et al. performed a systematic review to develop a consensus document to determine the preliminary points for implementing a centralized care programme for patients with PsO and PsA.7 Still, there is no information summarizing the implementation of these programmes.
Meanwhile, the benefits of care programmes focused on specific pathologies seem straightforward, but their effectiveness presents several methodological challenges for their implementation. This systematic review aims to systematically and exhaustively gather the published evidence that meets the search and eligibility criteria regarding the effectiveness of care programmes focused on PsO and PsA.
Materials and methodsProtocol and registryA systematic literature review was conducted according to the PRISMA (Preferred Reporting Items for Systematic Review and Meta-analysis) guidelines.8 The protocol was pre-registered in PROSPERO in August 2022 (CRD42022360788). The main objective was written in PICO format (population-intervention-comparator-outcome). A modification in the indices for both diseases such as PASI, DAPSA, NAPSI, DLQI, PsAQoL, and SF36 was defined as the primary outcome and patient satisfaction as the secondary outcome.
Eligibility criteriaStudies in humans older than 18 years, diagnosed with PsO and/or PsA were included. The search was carried out with a time limit of 5 years, original studies (clinical study, clinical trial protocol, comparative study, controlled clinical trial, observational study, practice guideline, pragmatic clinical trial, systematic review, and meta-analysis), in Spanish and English until May 15th, 2022. Letters to the editor, case reports, book chapters, preliminary publications in congresses, studies in animals/cells or duplicates, as well as studies with poor methodological quality were excluded.8
Information, resources, and searchA systematic electronic search of Medline, PubMed, Cochrane, Virtual Health Library, and Embase was performed. Data from clinical trials (clinicalTrials.gov) and additional sources were reviewed through references in reviews and systematic reviews. MeSh, DeCs terms, and keywords (Appendix 1) were used. In case of missing data or articles not found, the study authors were contacted directly to access publications.
Data collection and methodological evaluationThe methodological evaluation process of the articles was carried out by three independent authors using the Cochrane evaluation strategy (https://handbook.cochrane.org)9; in case of disputes, a fourth author resolved these. Data were extracted from: reference, country of study, study population, type of study, sample size (n), age, disease duration, type of health provider, and intervention performed by health providers. Outcomes considered were: PASI, DAPSA, NAPSI, DLQI, PsAQoL, and SF36. Patient satisfaction was a secondary outcome. The methodological evaluation of the included studies was performed according to the National Institutes of Health (NIH) quality assessment tool for cross-sectional and observational cohort studies.10 For clinical trials, the revised Cochrane risk of bias assessment tool for randomized clinical trials was used (RoB 2).11 References that appeared to be relevant to this review were hand searched. Finally, a descriptive analysis was carried out, grouping according to the type of intervention performed, reporting the results of each study. Considering the findings of the articles included, which reported multiple measures of efficacy, and since not all of them were used in each study, no heterogeneity tests were performed, so the results were not susceptible to meta-analysis.
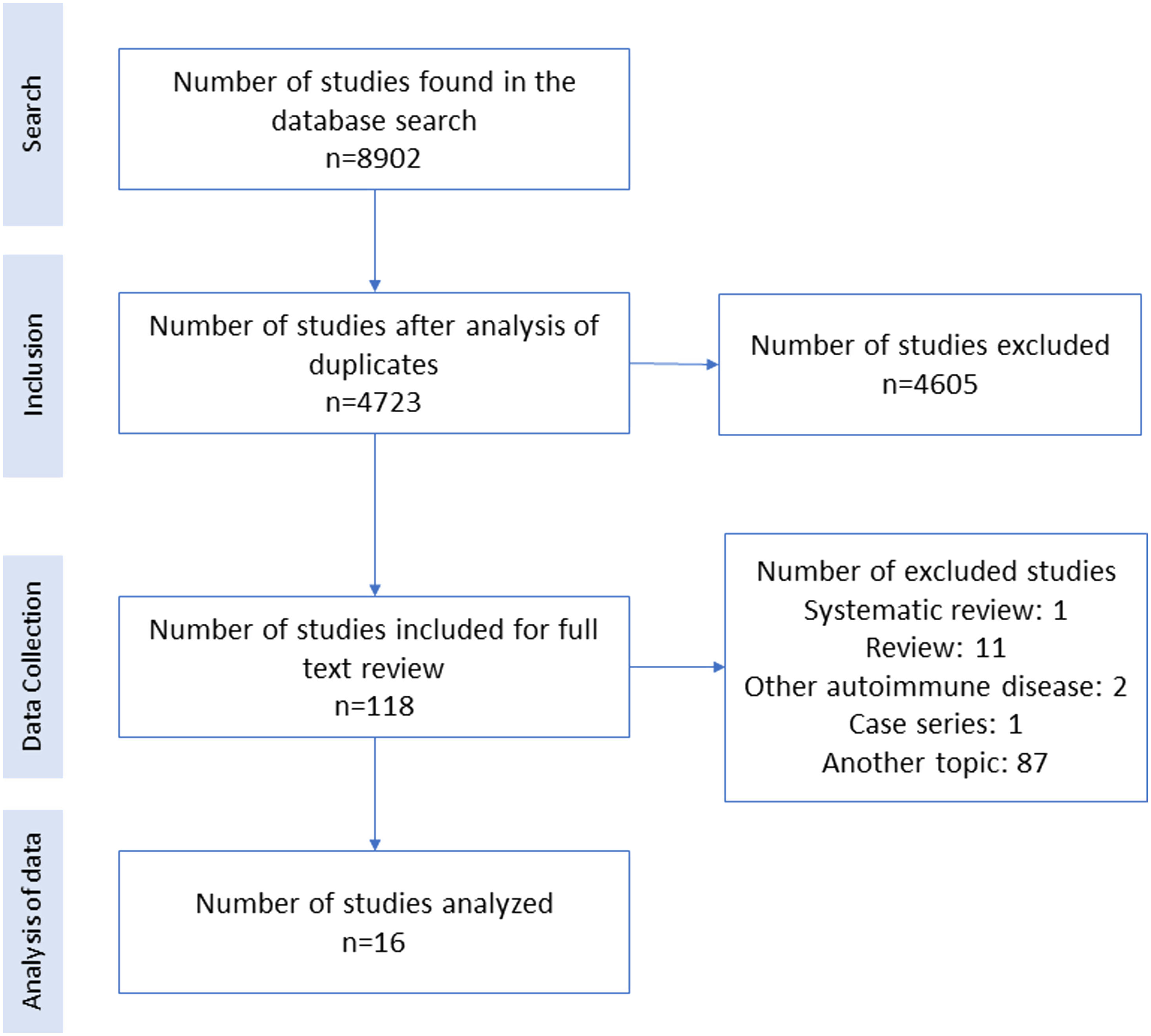
ResultsA total of 8902 articles were identified. After removing duplicates and searching by title and abstract, a total of 4723 articles remained, of which the full text of 118 articles were evaluated, and finally 16 were included in the analysis (Fig. 1).
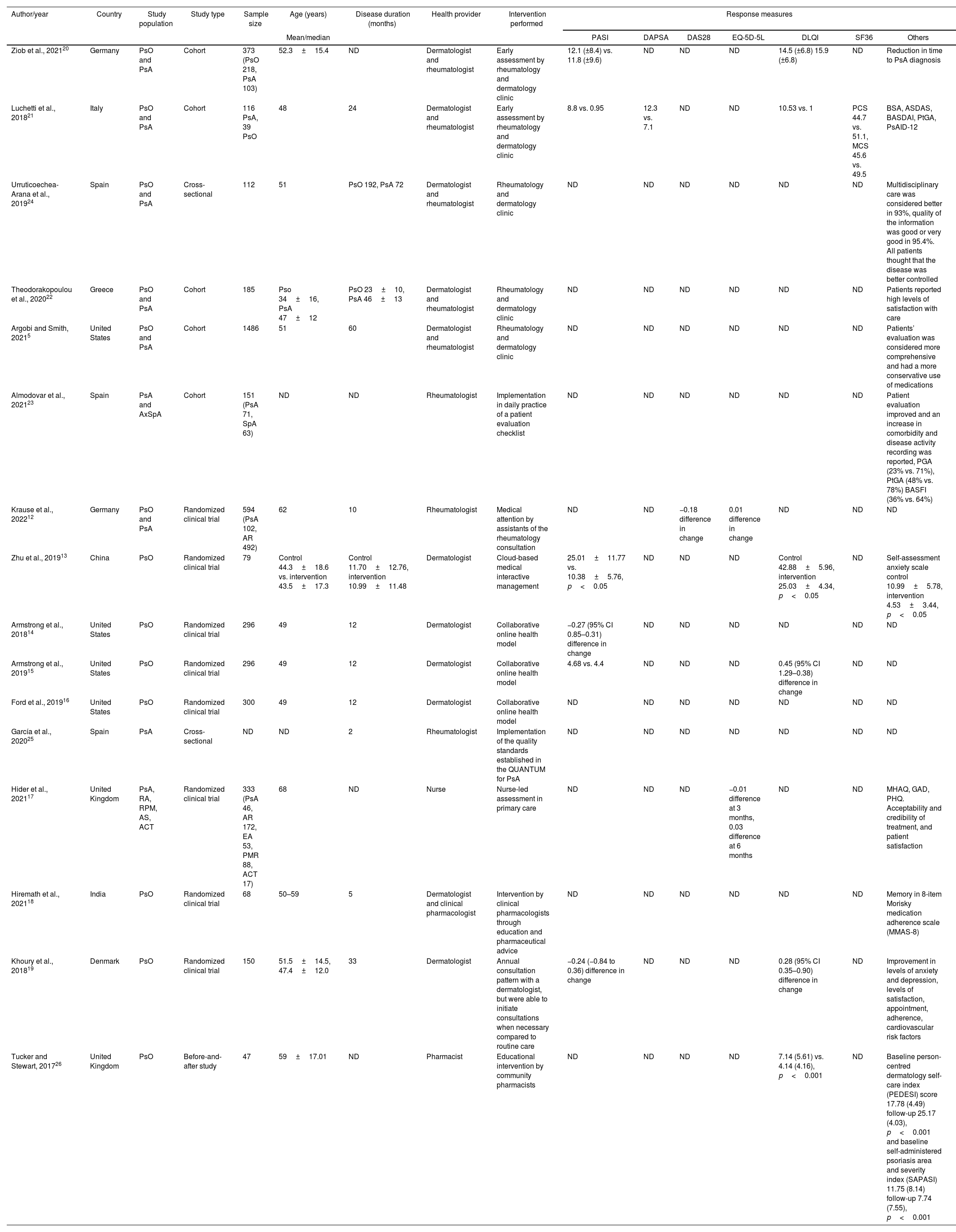
Characteristics of the studiesOf the total of 16 articles, 8 studies were randomized clinical trials,12–19 5 cohorts,5,20–23 2 cross-sectional studies and one was a before and after study.24–26 These studies could be classified into three main categories, the first is an evaluation of patient care systems in a multidisciplinary manner,5,12,20–24 the second category studies used online systems or telemedicine,13–16 and a category of patient self-management studies and education by health professionals to modify clinical care.17–19,25,26 Measures of effectiveness differed given the heterogeneity of the studies, which assessed, among other domains efficacy was assessed with PASI, DAPSA, DAS28, EQ-5D-5L, DLQI, SF36, and MMAS-8, and quality of life, disease control, or adherence to management were also informed. However, other effectiveness measures are reported for which no validated scales were used, including adherence to medication, evaluation of the patient's perception regarding levels of satisfaction and improvement in health care perception, assessment of comorbidities or diagnosis of early joint involvement (Table 1). Most studies were conducted in European countries (11/16),12,17,19–26 four from the United States,5,14–16 a study from China,13 and another from India.18 No studies were identified in Latin American or Colombian populations.
Overview of studies with models focused on disease care or clinics of excellence versus programmes of conventional in PsO and PsA.
| Author/year | Country | Study population | Study type | Sample size | Age (years) | Disease duration (months) | Health provider | Intervention performed | Response measures | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean/median | PASI | DAPSA | DAS28 | EQ-5D-5L | DLQI | SF36 | Others | ||||||||
| Ziob et al., 202120 | Germany | PsO and PsA | Cohort | 373 (PsO 218, PsA 103) | 52.3±15.4 | ND | Dermatologist and rheumatologist | Early assessment by rheumatology and dermatology clinic | 12.1 (±8.4) vs. 11.8 (±9.6) | ND | ND | ND | 14.5 (±6.8) 15.9 (±6.8) | ND | Reduction in time to PsA diagnosis |
| Luchetti et al., 201821 | Italy | PsO and PsA | Cohort | 116 PsA, 39 PsO | 48 | 24 | Dermatologist and rheumatologist | Early assessment by rheumatology and dermatology clinic | 8.8 vs. 0.95 | 12.3 vs. 7.1 | ND | ND | 10.53 vs. 1 | PCS 44.7 vs. 51.1, MCS 45.6 vs. 49.5 | BSA, ASDAS, BASDAI, PtGA, PsAID-12 |
| Urruticoechea-Arana et al., 201924 | Spain | PsO and PsA | Cross-sectional | 112 | 51 | PsO 192, PsA 72 | Dermatologist and rheumatologist | Rheumatology and dermatology clinic | ND | ND | ND | ND | ND | ND | Multidisciplinary care was considered better in 93%, quality of the information was good or very good in 95.4%. All patients thought that the disease was better controlled |
| Theodorakopoulou et al., 202022 | Greece | PsO and PsA | Cohort | 185 | Pso 34±16, PsA 47±12 | PsO 23±10, PsA 46±13 | Dermatologist and rheumatologist | Rheumatology and dermatology clinic | ND | ND | ND | ND | ND | ND | Patients reported high levels of satisfaction with care |
| Argobi and Smith, 20215 | United States | PsO and PsA | Cohort | 1486 | 51 | 60 | Dermatologist and rheumatologist | Rheumatology and dermatology clinic | ND | ND | ND | ND | ND | ND | Patients’ evaluation was considered more comprehensive and had a more conservative use of medications |
| Almodovar et al., 202123 | Spain | PsA and AxSpA | Cohort | 151 (PsA 71, SpA 63) | ND | ND | Rheumatologist | Implementation in daily practice of a patient evaluation checklist | ND | ND | ND | ND | ND | ND | Patient evaluation improved and an increase in comorbidity and disease activity recording was reported, PGA (23% vs. 71%), PtGA (48% vs. 78%) BASFI (36% vs. 64%) |
| Krause et al., 202212 | Germany | PsO and PsA | Randomized clinical trial | 594 (PsA 102, AR 492) | 62 | 10 | Rheumatologist | Medical attention by assistants of the rheumatology consultation | ND | ND | −0.18 difference in change | 0.01 difference in change | ND | ND | ND |
| Zhu et al., 201913 | China | PsO | Randomized clinical trial | 79 | Control 44.3±18.6 vs. intervention 43.5±17.3 | Control 11.70±12.76, intervention 10.99±11.48 | Dermatologist | Cloud-based medical interactive management | 25.01±11.77 vs. 10.38±5.76, p<0.05 | ND | ND | ND | Control 42.88±5.96, intervention 25.03±4.34, p<0.05 | ND | Self-assessment anxiety scale control 10.99±5.78, intervention 4.53±3.44, p<0.05 |
| Armstrong et al., 201814 | United States | PsO | Randomized clinical trial | 296 | 49 | 12 | Dermatologist | Collaborative online health model | −0.27 (95% CI 0.85–0.31) difference in change | ND | ND | ND | ND | ND | ND |
| Armstrong et al., 201915 | United States | PsO | Randomized clinical trial | 296 | 49 | 12 | Dermatologist | Collaborative online health model | 4.68 vs. 4.4 | ND | ND | ND | 0.45 (95% CI 1.29–0.38) difference in change | ND | ND |
| Ford et al., 201916 | United States | PsO | Randomized clinical trial | 300 | 49 | 12 | Dermatologist | Collaborative online health model | ND | ND | ND | ND | ND | ND | ND |
| García et al., 202025 | Spain | PsA | Cross-sectional | ND | ND | 2 | Rheumatologist | Implementation of the quality standards established in the QUANTUM for PsA | ND | ND | ND | ND | ND | ND | ND |
| Hider et al., 202117 | United Kingdom | PsA, RA, RPM, AS, ACT | Randomized clinical trial | 333 (PsA 46, AR 172, EA 53, PMR 88, ACT 17) | 68 | ND | Nurse | Nurse-led assessment in primary care | ND | ND | ND | −0.01 difference at 3 months, 0.03 difference at 6 months | ND | ND | MHAQ, GAD, PHQ. Acceptability and credibility of treatment, and patient satisfaction |
| Hiremath et al., 202118 | India | PsO | Randomized clinical trial | 68 | 50–59 | 5 | Dermatologist and clinical pharmacologist | Intervention by clinical pharmacologists through education and pharmaceutical advice | ND | ND | ND | ND | ND | ND | Memory in 8-item Morisky medication adherence scale (MMAS-8) |
| Khoury et al., 201819 | Denmark | PsO | Randomized clinical trial | 150 | 51.5±14.5, 47.4±12.0 | 33 | Dermatologist | Annual consultation pattern with a dermatologist, but were able to initiate consultations when necessary compared to routine care | −0.24 (−0.84 to 0.36) difference in change | ND | ND | ND | 0.28 (95% CI 0.35–0.90) difference in change | ND | Improvement in levels of anxiety and depression, levels of satisfaction, appointment, adherence, cardiovascular risk factors |
| Tucker and Stewart, 201726 | United Kingdom | PsO | Before-and-after study | 47 | 59±17.01 | ND | Pharmacist | Educational intervention by community pharmacists | ND | ND | ND | ND | 7.14 (5.61) vs. 4.14 (4.16), p<0.001 | ND | Baseline person-centred dermatology self-care index (PEDESI) score 17.78 (4.49) follow-up 25.17 (4.03), p<0.001 and baseline self-administered psoriasis area and severity index (SAPASI) 11.75 (8.14) follow-up 7.74 (7.55), p<0.001 |
The evaluation of the risk of bias according to the RoB2 for clinical trials was low in 62.5% of the studies (5/8). In comparison, the observational studies with their evaluation by the NIH tool was good in 12.5% of the studies (1/8). Detailed information is in supplementary material.
Multidisciplinary managementRegarding the studies that evaluated multidisciplinary management, the most frequently reported was the use of outpatient clinics in which the intervention of rheumatology and dermatology is simultaneously involved in the approach of patients (five studies).5,12,20–24
Luchetti et al. evaluated the impact of a multidisciplinary outpatient clinic in 155 PsA patients with 48 weeks follow-up, identifying the presence of peripheral or axial joint involvement. This involvement led to adjustments in the treatment selection according to its efficacy and presence of uveitis or gastrointestinal symptoms, with evidence of reduction in disease activity indices both at skin and joint level, demonstrated by changes in PASI [8.8 (5.6–13) vs. 0.95 (0–2), p<0.001], DAPSA 12.3 [(5.9–17) vs. 7.1 (2.0–11.4), p<0.001], ASDAS [2.6 (1.4–3.8) vs. 1.7 (1.2–2.4), p<0.001], and BASDAI [4.2 (2.6–6.1) vs. 2.6 (1.4–4.1), p<0.001], as well as quality of life measures such as DLQI 10.53 [(5.5–15) vs. 1 (0–2), p<0.001] and SF-36 in the physical and emotional component ([44.7 (33.5–52.8) vs. 51.1 (42–57.3), p<0.001] and [45.6 (36.6–53.4) vs. 49.5 (40.8–55.7), p<0.001, respectively).21
The study by Ziob et al. evaluated in a retrospective cohort the impact of creating a specialized clinic in PsA care, where once a week a shared consultation between dermatology and rheumatology was carried out, comparing two periods of time in 404 consultants and after the implementation of the clinic, identifying similar values at the PASI (12.1±8.4 vs. 11.8±9.6) and DLQI (14.5±6.8 vs. 15.9±6.8) between the two evaluation times. Furthermore, an increase in the diagnosis of PsA (independent of the use of CASPAR criteria) and a decrease in symptoms duration and rheumatological complaints manifested by the patients were reported.20
The other studies that evaluated multidisciplinary model reported the improvement in patient's perception regarding the type of care received.22,24 It has been reported in a different group that this approach improved disease knowledge and increased the perception of higher disease control.24 And additionally, another group described a higher perception of integrative care and more conservative use of medications.5
A study by Almodovar et al. implemented a checklist in the care of patients with PsA and axial spondyloarthritis, where they found a trend towards an increase in the identification of comorbidities and registration of disease activity indices.23
In Germany, a clinical trial evaluated by non-inferiority analysis the care of patients with rheumatic diseases (RA and PsA) through delegation to physicians who are part of team-based care (including activities such as follow-up visits, optimization of management based on the strategy of treatment by objectives and improvement of drug safety). It was a population of 601 patients with 297 in the team-guided treatment group and 304 in the standard care group, finding non-inferiority regarding changes in disease activity measured by DAS28; standard care resulted in increase of 0.04 points compared to a decrease of 0.14 points in the team-based group (difference −0.18), p<0.001. In addition, they found no superiority in quality of life by EQ-5D-5L with a difference of 0.01 (p=0.285).12
Online systems or telemedicineWe found 4 studies whose care model focused on telemedicine.13–16 Zhu et al. described the results of a case-control study conducted in a Chinese population where 79 patients were randomly included. Patients in the control group had a regular follow-up, while those in the intervention group were followed up through a digital platform where patient education was provided and patients feedback was provided. Clinimetry was performed using PASI, DLQI, and SCL-90-R. There were no differences at baseline, but a statistically significant difference was observed at 12-month follow-up, with a PASI of 25.01 for the control group vs. 10.38 for the intervention group (p<0.05) and a DLQI of 42.88 vs. 25.03 (p<0.05), thus concluding that the follow-up and use of digital platforms focused on patient care models generate better outcomes in terms of disease activity and quality of life.13
Armstrong et al. conducted a randomized controlled clinical trial in the United States that included 296 participants assigned to receive online or personalized care with two publications.14,15 In the online care group, the mean change in PASI score was −1.37; in the face-to-face group the mean change in PASI score was −0.82. As a secondary outcome, body surface area (BSA) involvement was measured, finding a difference between the two groups of −0.05% (95% CI, −1.58% to 1.48%). The difference in DLQI between the two groups was evaluated, finding a difference of −0.45 (95% CI: −1.29 to 0.38), concluding that the online model was also effective.
Ford et al. evaluated the impact of online care model on access to specialized care for patients with PsO through a randomized controlled trial that included 300 patients. They were followed up for 12 months, comparing the distance travelled and the time spent in receiving healthcare, concluding that online care models reduce considerably these measures.16
Training for health care professionals and patient self-managementIn Spain, García et al. published the results of an accreditation project (QUANTUM). It was a quality initiative to ensure an optimal level of quality and focused on the evaluation of care standards of primary health care units in the Spanish national system. The 59 criteria were grouped into 4: shorten time to diagnosis, optimize management, increase multidisciplinary collaboration, and improve patient follow-up. Of a total evaluation of 41 units, the analysis found compliance in 64.1% and 63.9% for the group focused on managing the disease through a multidisciplinary collaboration that involves rheumatology, dermatology, primary care staff and nursing. The units that had a specific care protocol corresponded to 61% (n=25/41), but only 41.5% carried out joint sessions between dermatology and rheumatology (17 units).25 To achieve the previously described accreditation, the units underwent self-assessment processes that allowed each centre to identify strengths and weaknesses, but also opportunities for improvement, with the possibility of accessing face-to-face audits and advice for improvement plans.25
We also found other studies based on these training processes for clinical care professionals. Hider et al, in 2021 conducted a controlled pilot trial in the United Kingdom, oriented towards an integrated and holistic care model led by nurses in primary care focused on identifying and managing comorbidities in patients with inflammatory diseases, including PsA. Nurses with experience in rheumatology and research received a training package for using the INCLUDE template and the software system used during patient assessment.17 It included 333 patients, 46 of them with PsA. The rate of acceptance by patients was greater than 70% for the nurse-led programme, making it feasible to offer this intervention in clinical practice.17
Two studies were found to determine the importance of the clinical pharmacist in the care of patients with chronic diseases based on education processes for this staff.18,26 The first one developed in India included 63 patients with PsO from a dermatological centre, which evaluated one group under standard care with periodic telephone follow-up and dermatology visits; the other received clinical pharmacist care focused on providing information of importance to adherence and how it is reflected in the control of the disease. Medication adherence was 59% in the intervention group versus 7% in the control group (p=0.0001) using the medication adherence scale Morisky medication 8 items (MMAS-8).18
The second of these two studies, Tucker and Stewart, evaluated the impact of a patient care model that involves the community pharmacist in education processes for patients with PsA in the United Kingdom. Furthermore, based on a pre-and post-intervention design, 7 community pharmacies were selected. The evaluation included 42 patients who underwent an initial measurement and another at 6 weeks, finding a significant increase in PeDeSI scores (person-centred dermatological self-care index) of 25.1 versus 17.7, SAPASI (self-assessed PsO area and severity index) 11.6 versus 7.7, and DLQI 7.2 versus 4.1 (p<0.001). In addition to enhancing patients’ knowledge, it reduced severity and impacted the quality of life.26
Finally, Khoury et al. conducted a randomized controlled trial of a decision-centred care model in 150 PsO patients from Denmark.19 In the intervention group, the patient had an annual dermatology appointment scheduled with the possibility of adding extra consultations when considered pertinent, versus the control group where assessments were assigned every 12–16 weeks. Outcomes were evaluated at week 52, and no statistically significant difference was found in DLQI (0.28, 95% CI −0.35 to 0.9) or PASI (−0.24, 95% CI −0.84 to 0.36). Adherence and safety were similar in both groups, but health interventions quality was perceived significantly better in the intervention group (p=0.003).
DiscussionRheumatic diseases share as a characteristic their chronic nature with treatments that have shown improvement in activity indexes and quality of life.3 However, these results have decreased over time in real-life conditions due to multiple factors.27,28 Chronic disease care programmes have been described as a complementary strategy in managing these patients, which seek to improve treatment conditions that allow controlled situations that resemble clinical trials to achieve the best possible results.2,4
This systematic literature review focused on PsO and PsA, identifying patient programmes based on different domains, including the multidisciplinary approach, online systems or telemedicine, and training for health personnel or patient self-management.
Despite the low number of studies, the models focused on the training of health professionals, beyond rheumatologists/dermatologists, as well as specialized outpatient care units, had demonstrate positive impact on higher adherence, disease control, reduced severity, and improved quality of life. Nevertheless, there are limitations, such as the non-random choice of centers26 or the restricted statistical power to establish differences between the control and intervention groups.17
Patient-centred care models have been evaluated more extensively in other non-inflammatory chronic conditions such as cancer or inflammatory conditions such as RA. Only one study focused on PsO found to be non-inferior in terms of quality of care, but reported a possible impact in reducing time and costs compared to routine consultations.19
Recent developments highlight the novel role of telemedicine in expanding access to specialized and multidisciplinary consultation to patients who live in places distant from highly developed cities or who have little time to attend. Within our systematic review we found 4 randomized clinical trials whose care model focused on telemedicine; Armstrong et al.15 showed that equivalent results are obtained in a 12-month follow-up in PASI, BSA, and DLQI, with the advantage of being able to perform medical care asynchronous in places where there are no dermatologists and/or rheumatologists. Zhu et al.13 demonstrated the benefit of having platforms that provide education and monitorization of comorbidities, and Ford et al.16 showed that the distances travelled and the times for care are optimized without affecting the quality of care. Considering the sociodemographic conditions of the Latin American population where access to specialized medicine is low, telemedicine could become an efficient alternative to solve problems of opportunity in healthcare. Otherwise, it would have difficulties in its operation in places where access to networks and technology is limited. on the other hand, it would also have the limitation of not being able to perform a physician joint count in the consultation, which can considerably reduce the objective evaluation of disease activity.
The temporal restriction was another limitation in the present review. However, considering the novelty of the inclusion of multidisciplinary care models in chronic autoimmune/autoinflammatory diseases, 5 years was considered an adequate time interval to identify the most significant amount of information available in the literature. It is worth highlighting publications such as those by Vélez et al.29 and Luelmo et al.30 where multidisciplinary care models were evaluated with follow-up of patients for 4 years, concluding that there was an increase in the detection of comorbidities and early diagnosis of the disease. Although it is worth noting that they reported other effectiveness measures not validated efficacy measures as clinimetry was not used.
Regarding other limitation, the heterogeneity of the included studies vary from the type of patients and inclusion criteria among studies with diverse methodological designs (randomized clinical trials, cohorts and cross-sectional studies).
It should be noted that it is a developing area. As a result of the search, some studies proposed care models for patients with PsO and PsA31–35 based on experts consensus defining the conditions as reference pattern in healthcare and quality indicators. Likewise, although at the moment there are no published results, four studies are currently under recruitment process to evaluate different patient centred models such as attention routes for the diagnosis and treatment of patients with PsO,36 patient intervention in the support process of medical consultations,37 and two clinical trials,38,39 describing an implementation of the T2T (treat to target) strategy for primary health care patients. Therefore, more studies are required to validate the present results and establish an adequate implementation in patient healthcare.
ConclusionsThe care models suggest favouring the outcomes in different domains that have been evaluated from the complexity and comprehensiveness of these two chronic diseases, such as early diagnosis (including shorter periods from the onset of skin to joint manifestations), control of disease activity, but also early identification of comorbidities (with their prevention and treatment) and concomitant conditions linked to the primary diagnosis (such as uveitis and gastrointestinal involvement). However, in the scope of the bulk of these studies, no differences were reported with sufficient statistical power in the efficacy measures when using classical care models compared to those proposed in the studies mentioned above due to methodological limitations in the design.
From the preceding arises the interest to rely on unconventional measures graded on the spectrum of satisfaction, acceptability, and fidelity, among others, but also the need to expand the research models focused on this type of study.
Despite this subject's methodological challenges, attention guided by models of excellence is growing. We need to find specific information on the Latin American population, a region with a large field to explore concerning the development and implementation of centralized care programmes in autoimmune or autoinflammatory rheumatic diseases.
Ethical approvalThe project does not include experimentation with animals or humans; however, it is conceived in accordance with the considerations contained in the Declaration of Helsinki. It is based on the collection of previously published scientific information.
FundingNone declared.
Conflicts of interestThe authors declare that they have no conflict of interest, financial or otherwise.