To date, there are no studies evaluating the use of the titanium-nitride-oxide coated stent in patients with multivessel coronary artery disease. We have compared the performance of the Titan-2® stent to that of the second generation drug-eluting stents in this scenario.
MethodsFrom 2011 to 2012, 284 patients were treated with the Titan-2® stent, of which 100 (35.2%) had multivessel coronary artery disease. This group was compared to 100 patients, of a group of 304 (38.9%) patients with multivessel coronary artery disease treated with second generation drug-eluting stents with durable or biodegradable polymers. The primary endpoint was the occurrence of major adverse cardiovascular events at 1 year.
ResultsClinical, angiographic and procedure-related characteristics of the patients did not show differences between groups. Most patients in the Titan-2® group were male (70%), mean age was 68.4 ± 12.9 years and 25% were diabetic. Stable symptomatic patients were prevalent (68%), 51% had three-vessel disease and ventricular function was preserved (55.6 ± 12.7%). The incidence of major adverse cardiovascular events at 1 year in the Titan-2® group was 21% (vs. 17%; p = 0.59), death was observed in 3% (vs. 2%; p > 0.99) of the patients, acute myocardial infarction in 5% (vs. 4%; p > 0.99) and a new revascularization procedure in 13% (vs. 11%; p = 0.83). Definitive stent thrombosis was not observed in either group.
ConclusionsThe Titan-2® stent showed similar results to those of the second-generation drug-eluting stents, which makes it attractive for use in the complex scenario of patients with multivessel coronary artery disease.
RESUMODesempenho do Stent Recoberto por Titânio-Óxido Nítrico em Pacientes com Doença Coronária Multiarterial
IntrodçãoAté o momento, nenhum estudo avaliou o stent recoberto por titânio-óxido nítrico em pacientes com doença arterial coronariana multiarterial. Comparamos o desempenho do stent Titan-2® ao stents farmacológicos de segunda geragao nesse cenário.
MétodosNo período de 2011 a 2012, 284 pacientes foram tratados com o stent Titan-2®, dos quais 100 (35,2%) eram portadores de doença arterial coronariana multiarterial. Esse grupo foi comparado a 100 pacientes, de um grupo de 304 (38,9%), com doença arterial coronariana multiarterial, tratados com o stent farmacológico de segunda geração com polímeros duráveis ou biodegradáveis. O desfecho primário foi a ocorrência de eventos cardíacos adversos maiores em 1 ano.
ResultadosCaracterísticas clínicas, angiográficas e do procedimento não apresentaram diferenças entre os grupos. A maioria dos pacientes do grupo Titan-2® era do sexo masculino (70%), com idade de 68,4 ± 12,9 anos e 25% eram diabéticos. Predominaram os quadros clínicos estáveis (68%), 51% tinham acometimento triarterial e a função ventricular estava preservada. A incidência de eventos cardiovasculares adversos maiores em 1 ano no grupo Titan-2® foi de 21% (vs. 17%; p = 0,59), óbito ocorreu em 3% (vs. 2%; p > 0,99) dos pacientes, infarto do miocárdio em 5% (vs. 4%; p > 0,99) e nova revascularização miocárdica em 13% (vs. 11%; p = 0,83). Não foram constatadas tromboses de stent definitivas em nenhum grupo.
ConclusõesO uso do Titan-2® apresentou resultados similares aos do stent farmacológico de segunda geração, o que o torna atrativo para ser utilizado no complexo cenário de pacientes portadores de doença arterial coronariana multiarterial.
Coronary stent implantation has become the standard percutaneous coronary intervention, with a safer approach and better results than those obtained with balloon angioplasty.1,2 However, coronary restenosis, although reduced, still remains as a limitation of the procedure, resulting in the need for new procedures and increased costs.3 Drug-eluting stents (DES) have significantly reduced late luminal loss and angiographic restenosis, as well as the need for repeat revascularization, when compared to bare metal stents (BMS).4 This reduction has been significant in several clinical and anatomical scenarios,5–9 especially with second-generation stents.8
The Titan-2® bioactive stent (Hexacath – Paris, France) is approved for clinical use in Europe, Asia, and North, Central, and South America, including Brazil, with more than 5,000 units already used worldwide.10,11 This stent consists of stainless steel coated with titanium-nitride-oxide, which has shown in vitro to reduce platelet aggregation and fibrinogen binding when compared to conventional bare metal stents. Preliminary data have shown similar safety and efficacy profiles of first- and second-generation DES in several clinical scenarios.11–18 However, few studies have addressed the performance of the Titan-2® in the treatment of patients with multivessel coronary artery disease (CAD).
This study aimed to evaluate the performance of the Titan-2® stent in patients with multivessel CAD and compare it to that of second-generation DES.
METHODSPatientsFrom January 2011 to December 2012, 284 patients were treated at Hospital Beneficência Portuguesa de São Paulo with the Titan-2® stent, of whom 100 (35.2%) had multivessel CAD and were selected for this analysis. This study excluded cases whose initial clinical presentation was myocardial infarction with ST-segment elevation, and those who had lesions > 50% in the left main coronary artery or when the percutaneous coronary intervention was performed with saphenous vein grafts. This group was compared to 100 patients from a group of 304 (38.9%) patients with multivessel CAD treated with second-generation DES with durable polymers – Endeavor® (Medtronic, Minneapolis, United States) or Xience V® (Abbott Vascular, Santa Clara, United States), and biodegradable polymers – BioMatrix® (Biosensors International, Singapore), in the same period, at the present institution.
The study was conducted in agreement with the Declaration of Helsinki, and all patients signed an informed consent.
The StentsThe Titan-2® balloon-expandable stent combines a stainless steel platform (316 L) of thin struts (0.0040 inch) with open cells and helical connections. It is not coated with polymers or antiproliferative drugs, but rather with a titanium matrix system bound to nitride oxide, applied by vapor deposition on the stent surface.
Stents that were used as controls included the Endeavor®, which releases zotarolimus from a biocompatible phosphorylcholine polymer applied to a cobalt-chromium platform of thin struts; the XienceV®, which releases everolimus from a biocompatible fluorinated acrylic polymer applied to a platform of cobalt-chromium with thin struts; and the BioMatrix®, which releases biolimus A9 from a biodegradable polylactic acid polymer applied to a stainless steel platform with thin struts.
ProcedurePercutaneous coronary interventions were performed according to current guidelines,19,20 and the final strategy for the procedure was left to the discretion of the surgeon.
During the procedure, unfractionated heparin was administered at a dose of 70-100 IU/kg, and the use of glycoprotein IIb/IIIa inhibitors was at the discretion of the surgeon. Pre-dilation was not compulsory, and post-dilation of stents was recommended in case of residual stenosis > 20% by visual estimation. The administration of dual antiplatelet therapy (acetylsalicylic acid [loading dose 200 mg/100 mg maintenance] and clopidogrel [loading dose 300-600mg/75mg maintenance]) should be started at least 24 hours before the procedure. In patients with acute coronary syndromes, a loading dose of 600mg of clopidogrel, 60mg of prasugrel, or 180mg of ticagrelor was recommended. After PCI, therapy with aspirin was maintained indefinitely; clopidogrel, prasugrel (10 mg/day), or ticagrelor (90 mg/day) were maintained for a period of 1 to 3 months for the Titan-2® group, and for at least one year for the DES group.
Outcomes and clinical follow-upThe primary study endpoint was the occurrence of major adverse cardiac events (MACE) during a 12 month follow-up. MACE was defined as death, non-fatal myocardial infarction, and need for repeat revascularization. All deaths were considered cardiac unless a non-cardiac cause could be clearly established by clinical and/or pathological study. The diagnosis of myocardial infarction was based on the development of new pathological Q waves in more than two contiguous ECG leads and/or elevation of creatine kinase MB isoenzyme (CK-MB) greater than three times the upper limit of the normal level after the procedure (during the index hospitalization), or twice the upper limit of the normal level after discharge. All new revascularizations, whether percutaneous or surgical, were considered. Definite stent thrombosis was diagnosed according to the criteria of the Academic Research Consortium.21
Clinical follow-up was performed at 12 months after the procedure, consisting of telephone contact performed according to pre-established institutional protocol.
Statistical AnalysisCategorical variables were described as percentages and compared by the chi-squared or Fisher’s exact test, when appropriate. Continuous variables were described as means and standard deviations and compared using Student’s t-test. p-values < 0.05 were considered statistically significant.
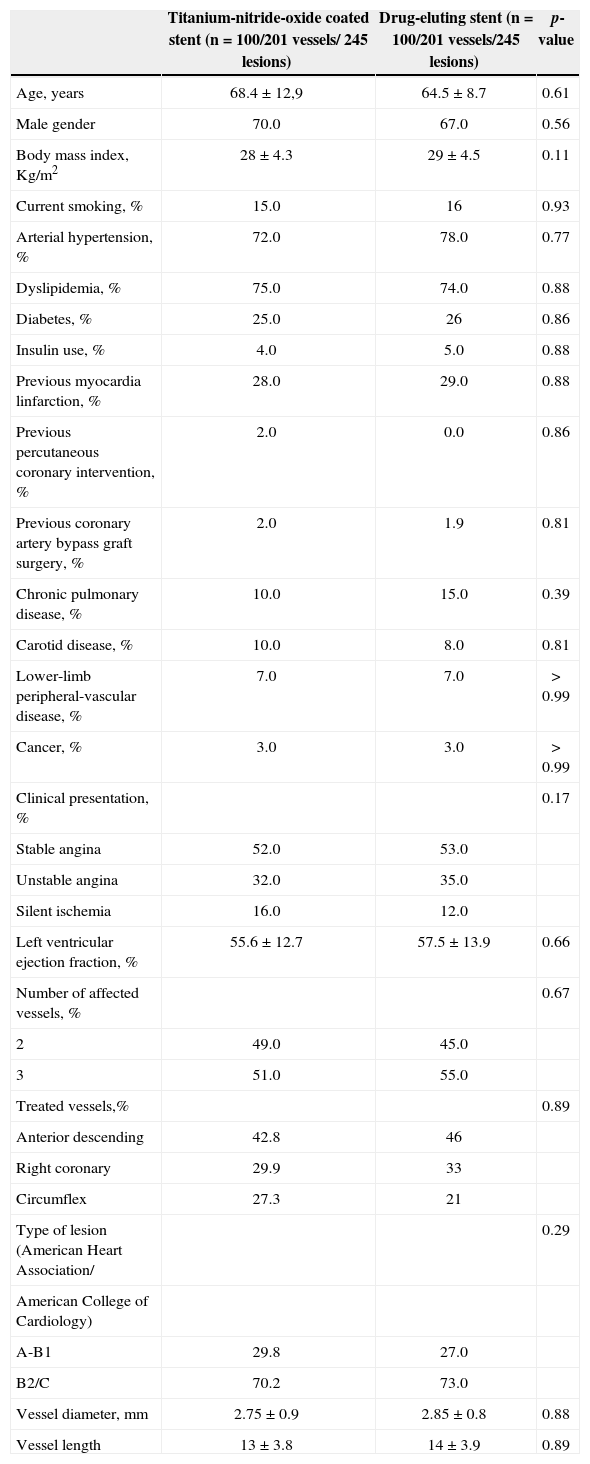
RESULTSThe clinical, anatomical, and procedural characteristics of the patients are summarized in Table 1, demonstrating no statistically significant differences between the groups. Most patients from the Titan-2® group were males (70%), with a mean age of 68.4 ± 12.9 years, and 25% were diabetics (4.0% used insulin). Stable clinical pictures predominated in the clinical presentation (68%), 51% had three-vessel CAD, and ventricular function was preserved (55.6 ± 12.7%). In patients with three-vessel CAD treated with the titanium-nitride-oxide stent, 135 lesions were treated. Of these, 78% were treated with three or more stents; the remaining cases (22%) were treated with two stents. In patients with two-vessel CAD, 110 lesions were treated; 80% of cases were treated with two or more stents and only one stent was used in the others. In patients with three-vessel CAD treated with drug-eluting stents; 141 lesions were treated, with three or more stents in 77% of cases, and with two stents used in the others. In patients with two-vessel CAD, 115 lesions were treated with two or more stents in 78% of cases, and only one stent was used in the others.
Clinical, angiographic, and procedural characteristics
| Titanium-nitride-oxide coated stent (n = 100/201 vessels/ 245 lesions) | Drug-eluting stent (n = 100/201 vessels/245 lesions) | p-value | |
|---|---|---|---|
| Age, years | 68.4 ± 12,9 | 64.5 ± 8.7 | 0.61 |
| Male gender | 70.0 | 67.0 | 0.56 |
| Body mass index, Kg/m2 | 28 ± 4.3 | 29 ± 4.5 | 0.11 |
| Current smoking, % | 15.0 | 16 | 0.93 |
| Arterial hypertension, % | 72.0 | 78.0 | 0.77 |
| Dyslipidemia, % | 75.0 | 74.0 | 0.88 |
| Diabetes, % | 25.0 | 26 | 0.86 |
| Insulin use, % | 4.0 | 5.0 | 0.88 |
| Previous myocardia linfarction, % | 28.0 | 29.0 | 0.88 |
| Previous percutaneous coronary intervention, % | 2.0 | 0.0 | 0.86 |
| Previous coronary artery bypass graft surgery, % | 2.0 | 1.9 | 0.81 |
| Chronic pulmonary disease, % | 10.0 | 15.0 | 0.39 |
| Carotid disease, % | 10.0 | 8.0 | 0.81 |
| Lower-limb peripheral-vascular disease, % | 7.0 | 7.0 | >0.99 |
| Cancer, % | 3.0 | 3.0 | >0.99 |
| Clinical presentation, % | 0.17 | ||
| Stable angina | 52.0 | 53.0 | |
| Unstable angina | 32.0 | 35.0 | |
| Silent ischemia | 16.0 | 12.0 | |
| Left ventricular ejection fraction, % | 55.6 ± 12.7 | 57.5 ± 13.9 | 0.66 |
| Number of affected vessels, % | 0.67 | ||
| 2 | 49.0 | 45.0 | |
| 3 | 51.0 | 55.0 | |
| Treated vessels,% | 0.89 | ||
| Anterior descending | 42.8 | 46 | |
| Right coronary | 29.9 | 33 | |
| Circumflex | 27.3 | 21 | |
| Type of lesion (American Heart Association/ | 0.29 | ||
| American College of Cardiology) | |||
| A-B1 | 29.8 | 27.0 | |
| B2/C | 70.2 | 73.0 | |
| Vessel diameter, mm | 2.75 ± 0.9 | 2.85 ± 0.8 | 0.88 |
| Vessel length | 13 ± 3.8 | 14 ± 3.9 | 0.89 |
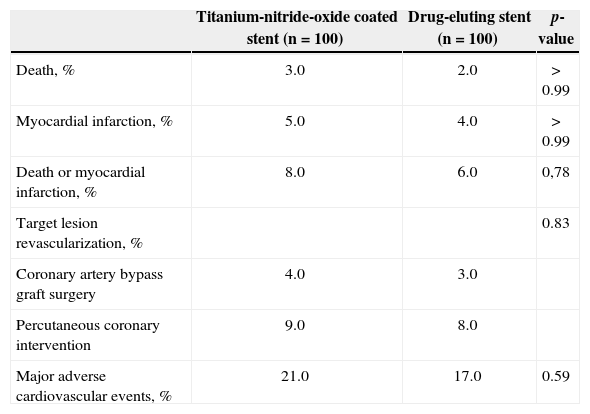
The incidence of MACE at 1 year in the Titan-2® group was 21% (vs. 17%; p = 0.59) and death occurred in 3% (vs. 2%; p > 0.99) of patients, myocardial infarction in 5% (vs. 4%; p > 0.99), and new revascularization procedure in 13% (vs. 11%, p = 0.83), predominantly at the expense of a new PCI in more than three-quarters of the cases (Table 2). No definite stent thrombosis was observed in either group.
Frequency of major adverse cardiovascular events during a one-year period
| Titanium-nitride-oxide coated stent (n = 100) | Drug-eluting stent (n = 100) | p-value | |
|---|---|---|---|
| Death, % | 3.0 | 2.0 | >0.99 |
| Myocardial infarction, % | 5.0 | 4.0 | >0.99 |
| Death or myocardial infarction, % | 8.0 | 6.0 | 0,78 |
| Target lesion revascularization, % | 0.83 | ||
| Coronary artery bypass graft surgery | 4.0 | 3.0 | |
| Percutaneous coronary intervention | 9.0 | 8.0 | |
| Major adverse cardiovascular events, % | 21.0 | 17.0 | 0.59 |
In this study, it was demonstrated that clinical outcomes at one year of Titan-2® stent implantation in a clinical and anatomical scenario of greater complexity (patients with multivessel CAD, of whom approximately 25% were diabetics with predominantly type B2/C lesions according to the classification of the American Heart Association/American College of Cardiology) were similar to those of the group of patients who received second-generation DES implantation with durable or biodegradable polymers.
Modifications of the stent surface and material properties may have important implications for neointimal hyperplasia after stent implantation. Metallic implants can induce the exchange of electrons and protons, as well as the formation of ion-organic molecular bonds in living tissue. The resulting protein activation, cell toxicity, and stimulation of platelets, monocytes/macrophages, and fibroblasts may contribute to restenosis.22
Both stainless steel and cobalt-chromium have significant amounts of nickel, chromium, and molybdenum, which can be released from the stent and induce the aforementioned tissue reactions. The inflammatory and allergic reactions that induce the formation of new tissue around metal alloys containing nickel in patients with orthopedic and dental implants are well known.23 It is reasonable to assume that stents that contain nickel and other elements can increase neointimal hyperplasia.
Several types of stent coating have been used to create a biologically inert barrier between the metallic prosthesis surface and the circulating blood, but none has reduced rates of restenosis, and others, such as gold, increased such rates. An evaluation of five different biodegradable polymers and three no biodegradable polymers showed inflammatory reactions with subsequent coronary neointimal thickening, which was not expected based on in vitro tests.24 It is speculated that this inflammatory process might contribute to coronary restenosis and, eventually, to late thrombosis. Thus, it is necessary to improve the characteristics of the metallic stents, even as a platform for the release of antiproliferative drugs.
Titanium is the material of choice for biomedical implants, especially dental and orthopedic implants, because of its superior biocompatibility and resistance to corrosion. However, the processing of this metal is difficult, and its production costs are prohibitive. In contrast, titanium alloys, such as titanium-nitride oxide, can be more easily used and applied to stainless steel surfaces through physical vapor deposition at a reasonable cost. Titanium-nitride-oxide surfaces have been experimentally demonstrated to reduce the binding of platelets and fibrinogen in comparison to stainless steel.25 Moreover, neointimal hyperplasia was reduced by 50% in a porcine model, when compared to a conventional stainless steel stent.26
When compared to conventional stents, stents coated with titanium-nitride-oxide showed to be superior in reducing restenosis and major adverse cardiovascular all,27,28 demonstrated that the Titan-2® showed similar benefits to those of DES and did not require prolonged dual antiplatelet therapy (>1 month for stable patients and > 3 months for those with acute coronary syndromes), as late thrombosis was not observed.17
Study limitationsThe results of this study should be interpreted in the context of the following limitations: it was a nonrandomized study and, therefore, subject to selection biases; the patient population was too small to detect differences in clinical events of minor importance; clinical information was obtained predominantly by the present team or by telephone contact with the assistant physician, and there was no long-term follow-up (>1 year).
CONCLUSIONSThis study suggests that the Titan-2® stent has a similar safety and efficacy profile to that of second- generation drug-eluting stents, which makes it attractive for use in the treatment of the complex scenario of patients with multivessel coronary artery disease, particularly when long-term dual antiplatelet therapy is not intended.
CONFLICTS OF INTERESTThe authors declare no conflicts of interest.
FUNDING SOURCENone.