The conventional devices available for the percutaneous occlusion of a patent ductus arteriosus (PDA) may have limitations, especially in small children and adults with a large ductus arteriosus. The Amplatzer® Vascular Plug II (AVP II) device has been used in these scenarios with promising results. This study aims to report an early experience with the AVP II device at three reference centres.
MethodsThis was a prospective study with retrospectively collected data from patients who had undergone PDA occlusion with the AVP II since 2011. The devices were implanted using the anterograde approach under general anaesthesia, except for in one patient. Technical aspects, immediate occlusion, and complication rates were assessed.
ResultsForty patients (67.5% females) with a median age of 56.7 months (6 months to 654.7 months) and a median weight of 17.3kg (5kg to 93kg) were included. Of these, 36 had a type A PDA, three had a type E PDA, and one had a type C PDA. The mean diameters of the ductus and of the device were 3.7±1.5mm and 9.4±3.6mm, respectively. In 3 patients, the initial device had to be replaced by another device of a different size. In one patient, an unsuccessful attempt led to the use of two implant devices. This patient was later referred for surgical repair. In five patients, a mild protrusion of the disc towards the aorta was observed, but the protrusion did not cause a significant pressure gradient. Residual flow was observed in two patients. There were no significant complications.
ConclusionsThe AVP II device is a safe and effective alternative for PDA treatment, especially in patients with limitations to the conventional occlusion procedure.
Oclusão Percutânea do Canal Arterial com a Prótese Amplatzer® Vascular Plug II:Experiência Inicial em Três Centros de Referência
IntroduçãoAs próteses convencionais disponíveis para o fechamento percutâneo da persistência do canal arterial (PCA) podem apresentar algumas limitações a seu uso, principalmente em crianças pequenas e em adultos com canais de maior diâmetro. A prótese Amplatzer® Vascular Plug II (AVP II) tem sido utilizada nesses casos com resultados animadores. Objetivamos apresentar a experiência inicial com AVP II em três centros de referência.
MétodosEstudo prospectivo, com coleta de dados retrospectiva, dos pacientes submetidos a fechamento de PCA com AVP II desde 2011. Os dispositivos foram implantados sob anestesia geral por via anterógrada, exceto em um paciente. Foram avaliados aspectos técnicos, taxa de oclusão imediata e complicações.
ResultadosForam selecionados 40 pacientes (67,5% do sexo feminino) com mediana de idade de 56,7 meses (6 meses a 654,7 meses) e mediana de peso de 17,3kg (5kg a 93kg). Desses pacientes, 36 tinham PCA do tipo A, 3 do tipo E e 1 do tipo C. Os diâmetros médios do canal e da prótese foram de 3,7±1,5mm e de 9,4±3,6mm, respectivamente. Em 3 pacientes foi necessária a troca do dispositivo inicial por outro de diferente tamanho. Em uma paciente foi realizada tentativa de implante com 2 dispositivos, sem sucesso, a qual foi posteriormente encaminhada para correção cirúrgica. Em 5 pacientes foi observada discreta protrusão do disco para a aorta sem ocasionar gradiente pressórico significativo. Houve fluxo residual em 2 pacientes. Não ocorreram complicações significativas.
ConclusõesO dispositivo AVP II é uma alternativa segura e eficaz para o tratamento de PCA, principalmente naqueles que apresentam limitações ao fechamento convencional.
Patent ductus arteriosus (PDA) is one of the most common congenital cardiac lesions and accounts for up to 7% of all congenital heart diseases.1,2 Treatment is indicated in the first year of life when there is heart failure or later, when there are negative hemodynamic consequences, including increased left ventricular dimensions on echocardiography.2 The timely closure of a PDA prevents the onset of complications such as heart failure, pulmonary arterial hypertension, arrhythmias, and possibly infectious endarteritis.1,2
The percutaneous treatment of PDAs with lastgeneration intra-cardiac prostheses has been safely and effectively performed in several age groups, with the exception of neonates and young infants weighing < 4 to 5kg.3
Several devices are used for closing a PDA; coils and plugs are the most common devices. The latter are prostheses designed exclusively for closing a PDA and consist of a body and a retention disc, which is positioned in the aortic side of the defect.3,4 However, in some anatomic variations5 (large-calibre tubular ductus, type A ductus with shallow aortic ampulla and small diameter, and angulated ductus), especially in low-weight patients, the distal disc can protrude to the aortic side and generate a pressure gradient in the aorta. The protrusion into the left pulmonary artery can also be occasionally observed in patients weighing < 8 to 10kg. Moreover, the plug-type prosthesis, usually indicated for a ductus with a minimum diameter > 2.5 to 3mm, is not provided by the Brazilian Unified Health System (Sistema Único de Saúde – SUS) in Brazil, which limits the percutaneous treatment of PDAs in significant parts of the population of patients with this condition, including adults who typically have a larger diameter ductus and a wide and deep aortic ampulla. To overcome some of these limitations, the Amplatzer® Vascular Plug II device (AVP II, St. Jude Medical – St Paul, USA), originally designed for vessel occlusion, has been used for the percutaneous treatment of PDA with promising initial results.6–8 However, there have been few reports in the literature on studies of patients in whom this type of prosthesis was used for PDA closure.
The aim of this study was to present the experience of three hemodynamic services specialising in the treatment of congenital heart disease with the use of the AVP II prosthesis for PDA closure. The technical aspects, immediate occlusion, and complication rates were evaluated.
METHODSStudy planningThis was an observational, prospective study with retrospective data collected from patients who underwent PDA closure with the AVP II prosthesis at three reference centres for the percutaneous treatment of congenital heart disease.
Selection criteriaPatients with a patent ductus arteriosus with hemodynamic effects, determined by left ventricular enlargement on echocardiogram, were included. Patients weighing < 5kg, patients with fixed pulmonary artery hypertension and patients with active infection at the time of the procedure were excluded.
ProcedureAll patients and/or guardians signed an informed consent, and the patients were taken to the catheterisation laboratory.
All patients underwent general anaesthesia and received prophylaxis for infectious endocarditis during the procedure; the antibiotic of choice was cefuroxime at a dose of 30 mg/kg to 50 mg/kg, followed by two additional doses. A femoral puncture was performed with the femoral sheaths positioned in the arterial and venous pathways. After the arterial puncture, heparin was administered at a dose of 50 U/kg to 100 U/kg, according to the institution’s routine protocol.
With a pigtail catheter positioned at the end of the aortic arch, angiographies were performed using the left profile view to determine the anatomy of the ductus arteriosus and to acquire measurements of the pulmonary extremity diameters, as well as the aortic ampulla and its length. In some cases, it was necessary to use other views (left oblique 100 degrees, with 10 degrees of cranial angulation, or right oblique, 10 to 30 degrees) for better visualisation of the entire trajectory of the channel. Aortic and pulmonary trunk pressures were measured in all patients.
The implantation route was defined after choosing the prosthesis. In general, the route of choice was the antegrade venous route. The retrograde route was occasionally used for a ductus < 3mm. A therapeutic multipurpose or Judkins right coronary catheter with an internal diameter compatible with the passage of the device (defined by the table provided by the manufacturer) was used to deliver the prosthesis. New angiographies were performed before and after the prosthesis was released to evaluate the final position.
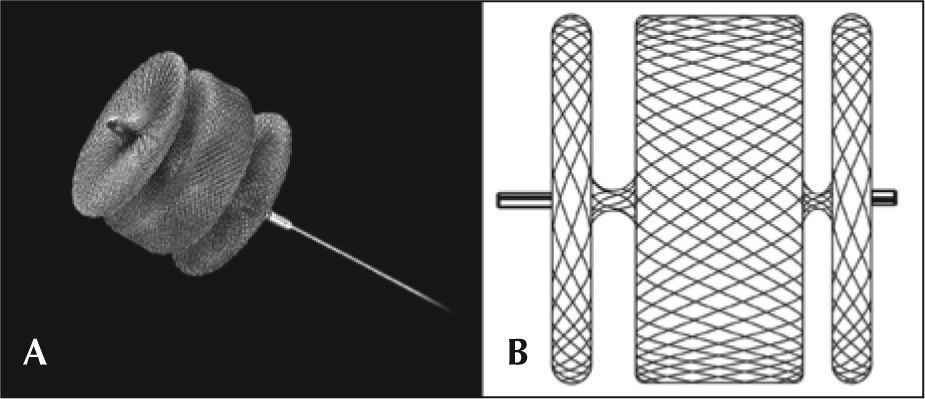
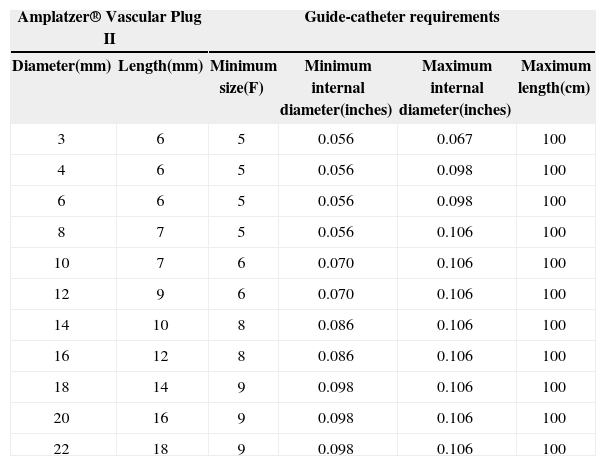
AVP IIThe AVP II prosthesis, initially conceived for the occlusion of vessels (fistulas, collaterals),9 consists of a nitinol mesh with varying numbers of wires (144 to 360) with different designs (one to three layers of wires) (Figure 1 A and B). It has three discs of equal diameter without the presence of tissue inside. A small flexible joint between the discs allows their articulation. The prosthetic diameter starts at 3mm and continues up to 22mm, in 2mm increments, beginning at 4mm. The AVP II can be used with a guide catheter or extended introducers of varying sizes according to the manufacturer’s recommendations. The specifications for diameters, lengths, and compatibilities are shown in Table 1.
Amplatzer® Vascular Plug II Models and Minimal Requirements
| Amplatzer® Vascular Plug II | Guide-catheter requirements | ||||
|---|---|---|---|---|---|
| Diameter(mm) | Length(mm) | Minimum size(F) | Minimum internal diameter(inches) | Maximum internal diameter(inches) | Maximum length(cm) |
| 3 | 6 | 5 | 0.056 | 0.067 | 100 |
| 4 | 6 | 5 | 0.056 | 0.098 | 100 |
| 6 | 6 | 5 | 0.056 | 0.098 | 100 |
| 8 | 7 | 5 | 0.056 | 0.106 | 100 |
| 10 | 7 | 6 | 0.070 | 0.106 | 100 |
| 12 | 9 | 6 | 0.070 | 0.106 | 100 |
| 14 | 10 | 8 | 0.086 | 0.106 | 100 |
| 16 | 12 | 8 | 0.086 | 0.106 | 100 |
| 18 | 14 | 9 | 0.098 | 0.106 | 100 |
| 20 | 16 | 9 | 0.098 | 0.106 | 100 |
| 22 | 18 | 9 | 0.098 | 0.106 | 100 |
The prosthesis was selected after considering the minimum diameter of the channel, the diameter of the aortic ampulla, the total length of the channel, and the total length of the aortic ampulla. In general, the diameter of the discs was at least two times greater than the minimum diameter of the channel, and was at most 1mm larger than the largest diameter of the aortic ampulla. The length of the device (which increases according to the increase in disc diameter) did not exceed 1 to 2mm of the total length of the ductus arteriosus. Because this is a device with three retention discs, two discs were positioned on the aortic side, and the third disc was positioned on the pulmonary side of the ductus arteriosus (Figures 2 and 3).
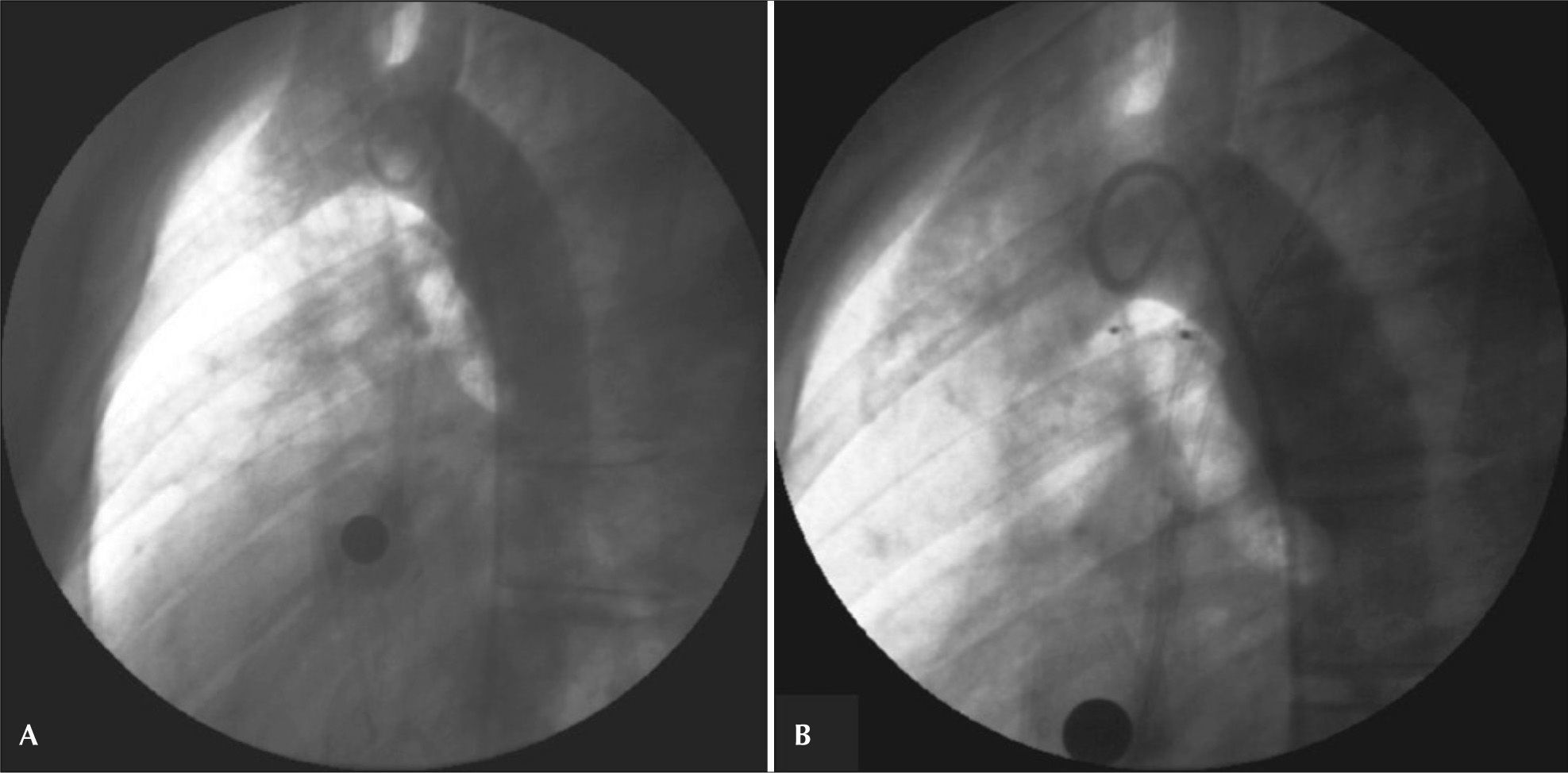
– Ductus arteriosus type A with a 2.8mm diameter at the pulmonary extremity. An 8mm Amplatzer® Vascular Plug II device was successfully employed. In A, initial aortography in left view. In B, control aortography showing the device well positioned within the ductus, with two discs inside the aortic ampulla and with no residual flow.
Echocardiographic assessments, chest radiographs, and electrocardiographic evaluations were performed in all patients before hospital discharge, which occurred the day after the procedure. A new echocardiogram was performed six months after implantation.
RESULTSFrom March of 2011 to March of 2012, 40 patent ductus arteriosus closure procedures were performed in 40 patients using the AVP II device.
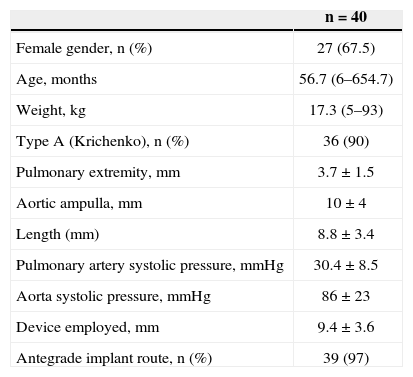
Of these patients, 27 were females (67.5%), and 13 were males (32.5%). The median age and weight were 56.7 months (6 months to 654.7 months) and 17.3kg (5kg to 93kg), respectively. Ten patients weighed < 10kg. The ductus arteriosus was classified, according to the Krichenko classification, as type A in 36 patients (90%), type E in three patients, and type C in one patient. Measurements of the ductus arteriosus at the time of catheterisation were 3.7±1.5mm at the pulmonary extremity, 10±4mm in the aortic ampulla, and 8.8±3.4mm in length. The mean diameter of the device used was 9.4±3.6mm. The systolic pulmonary artery pressure was 30.4±8.5mmHg, and the aortic systolic pressure was 86±23mmHg. The clinical, angiographic, and procedural data are shown in Table 2.
Clinical, Angiographic, and Procedural Data
| n=40 | |
|---|---|
| Female gender, n (%) | 27 (67.5) |
| Age, months | 56.7 (6–654.7) |
| Weight, kg | 17.3 (5–93) |
| Type A (Krichenko), n (%) | 36 (90) |
| Pulmonary extremity, mm | 3.7±1.5 |
| Aortic ampulla, mm | 10±4 |
| Length (mm) | 8.8±3.4 |
| Pulmonary artery systolic pressure, mmHg | 30.4±8.5 |
| Aorta systolic pressure, mmHg | 86±23 |
| Device employed, mm | 9.4±3.6 |
| Antegrade implant route, n (%) | 39 (97) |
n=number of patients.
The antegrade implantation route was the preferred approach, with the exception of one procedure where the prosthesis was implanted using a retrograde route, which is the choice for patients with a small ductus because this route provides procedural dexterity. In this patient, who weighed 59kg, the retrograde approach was used to treat a 1.5-mm ductus arteriosus with a 3-mm prosthesis and a 5F catheter (Figure 4).
– Small (1.5mm) ductus arteriosus in which a 3-mm Amplatzer® Vascular Plug II device was successfully implanted via a retrograde route. In A, initial aortography in left view. In B, control aortography showing the device well positioned within the ductus and with no residual flow.
The procedure was successfully completed in all but one of the patients. This patient weighed 7.4kg, and the ductus was type C, measuring 5.5mm at both ends (Figure 5). The pulmonary artery systolic pressure was 38mmHg, and attempts were made with 8-mm and 10-mm AVP II prostheses. The first prosthesis was not released and was replaced. The second prosthesis led to embolisation of the right pulmonary artery after its release and was successfully recovered with the aid of a bioptome. The patient was subsequently referred for surgery, and complications were not recorded.
– Tubular ductus arteriosus type C, of high flow, with 5.5mm diameter at both ends. Unsuccessful attempts were made to implant 8-mm and 10-mm Amplatzer® Vascular Plug II devices, with a resulting embolisation of the 10-mm prosthesis to the right pulmonary artery; the device was recovered with a bioptome. The control angiography after prosthesis recovery showed no signs of injury to the vascular wall. In A, initial aortography in left profile. In B, control aortography showing the 8-mm device poorly positioned within the ductus. In C, control aortography showing the 10-mm device well positioned within the ductus before its release. In D, the pulmonary trunk angiography showing the 10-mm device with protrusion toward the pulmonary artery after its release. In E, the embolised device in the pulmonary artery during recovery of the device, which was achieved using a bioptome.
The prosthesis showed a slight disk protrusion to the aortic side in five patients, but none of them showed a significant pressure gradient as measured both in the catheterisation lab and on echocardiography.
In addition to the patient in whom embolisation occurred, device substitutions were required in three other patients. The first patient, who had a 3-mm ductus arteriosus at the pulmonary extremity, needed to exchange an 8-mm prosthesis for a 6-mm one; the final positioning was optimal (Figure 6). The second patient had a 5.7-mm PDA and a 16.8-mm aortic ampulla. A 12-mm prosthesis implant was placed and then replaced by an 18-mm implant. The first measurement of this ductus was 4mm, most likely due to spasm during the injection of contrast, which resulted in an inaccurate assessment and undersizing of the prosthesis.
– PDA type E measuring 3mm at the pulmonary extremity, in which an 8-mm Amplatzer® Vascular Plug II implant was placed and subsequently successfully replaced by a 6-mm one. In A, initial aortography in left view. In B, the angiography performed in the pulmonary trunk showing the 8-mm prosthesis with disc protrusion into the left pulmonary artery. In C, control aortography showing the 6-mm device well positioned within the ductus, with slight residual flow.
The third patient who required a device to be replaced had a 3.5-mm ductus arteriosus that was implanted in a 6.5 × 5mm Flipper coil, which embolised and needed to be recovered. An 8-mm AVP II device was later implanted, which resulted in complete closure of the defect.
All but one patient underwent post-procedure recovery in the catheterisation laboratory. The only patient whose recovery took place in the intensive care unit was a 9-month-old child who weighed 5kg; after a period of six hours, the child was transferred to the paediatric ward.
The echocardiographic study performed 24 hours after the procedure showed complete closure of flow through the ductus arteriosus in all cases, except in two patients, who had minimal residual flow. There was no increase in flow velocity in the aorta or pulmonary artery. Those patients with minimal residual flow still had less than six months of follow-up when this article was written.
DISCUSSIONThe present experience with the AVP II device showed good results, with high rates of PDA closure. Echocardiography performed 24 hours after the procedure revealed only intra-prosthesis residual flow with a low velocity. Due to the manufacturing characteristics of the device (with no tissue inside), immediate residual flow is usually observed and disappears in a short period of time (approximately 24 hours). The authors believe that total occlusion will be observed in all patients during the follow-up.
In one patient, the prosthesis was positioned via the arterial route without technical difficulties or vascular complications. This technique can be employed in simple cases to provide greater flexibility for the procedure. Since the AVP II is more flexible, it can pass through lower profile systems and can be used in lower-weight patients, such as the ten patients in this study.
Even when implanting devices that are smaller than the aortic ampulla (the mean aortic ampulla was 10mm and the prosthesis was 9.4mm), there was slight distal disc protrusion to the aorta in five patients, especially in small children. Because there was no local gradient, the prostheses were released. It is believed that the angiographic appearance should improve as the patients grow. Device replacement was necessary in three patients at the beginning of the study. At that point in time, the authors were still looking for an appropriate correlation between the size and anatomy of the PDA and the device being used. It is believed that, in addition to measurements of the aortic ampulla, the total length of both the ductus and the device should be taken into consideration so that there is no significant prolapse on the aortic side. Prolapse on the pulmonary side is unlikely because only one low profile disc is positioned on this side of the ductus.
There was only one unsuccessful case; the patient was small and had a large tubular ductus arteriosus. An attempt was made to implant a device approximately 30% larger than the diameter, treating the ductus as a vascular structure. Because the 8-mm prosthesis was not stable inside the ductus, a 10-mm implant was chosen. At first, this prosthesis showed satisfactory stability. However, after several minutes of observation, there was embolisation to the pulmonary artery. This episode exemplifies the difficulty of closing a tubular ductus that has great distensibility. The rescue with bioptome, introduced through a long 8F sheath, presented no technical difficulties. Despite this unsuccessful case, Schwartz et al.9 reported occlusion of a tubular ductus arteriosus with the AVP II in a series of 14 patients, including ductus types C, D, and E according to the Krichenko classification. Ng et al.8 also reported the successful closure of a tubular ductus arteriosus in two patients, one of whom was a young infant who was 3 months old. Despite these previous unfavourable experiences, the authors believe that a ductus with these anatomic features and size in low-weight patients can be closed successfully with the use of a more compact prosthesis. In such scenarios, the Amplatzer® Duct Occluder II (ADO II AS, St. Jude Medical – St. Paul, USA) may be useful because it has a nitinol mesh, which makes it more dense, and it is distributed in a single layer. Unfortunately, this prosthesis is not yet available for clinical use in Brazil.
Further studies with larger numbers of patients, different anatomic variations, and long-term results must be performed to assess the precise role of the AVP II in the percutaneous closure of a PDA.
CONCLUSIONSThe AVP II prosthesis was safe and effective for closure of a PDA in patients in this small series. The prosthesis can be applied to a large or small arterial ductus, and it can be used in small children and adults with a wide aortic ampulla. The AVP II should be considered as another tool for the percutaneous treatment of patent ductus arteriosus in selected cases.
CONFLICTS OF INTERESTCarlos Augusto Cardoso Pedra is a consultant and lecturer at St. Jude Medical Company in Brazil. The other authors declare no conflicts of interest.