Wristband devices used in the compression of the radial puncture site add cost to the procedure and have not been adequately compared with conventional compressive dressings. This study evaluated the effectiveness and safety of both forms of hemostasis in patients undergoing coronary angiography and/or percutaneous coronary intervention in daily practice.
MethodsA prospective, multicenter, nonrandomized study, which included consecutive patients who underwent procedures through radial access. The type of compression was at the interventionist's discretion and the availability of wristband devices. The main objective was to compare the patency of the radial artery on the 7th day after the procedure, measured by Doppler. Secondarily, the authors evaluated the occurrence of bleeding/hematoma at the puncture site during compression, after removal of the device and on the 7th day after the procedure.
ResultsThis study evaluated 528 patients, 416 using conventional compressive dressings and 112 using wristband devices. When the sheath was removed and soon after its removal, a higher incidence of bleeding in the conventional compressive dressings group was observed (13.4% vs. 0%; p < 0.001). All bleeding events were small (type I or type II) and did not require further actions. At 7 days, there were only small hematomas at the puncture site in 7.1% of cases that used the wristband device. There was no difference in the patency rates of the radial artery (3.8% vs. 7.1%; p = 0.20).
ConclusionsThe use of wristband devices for radial artery hemostasis did not result in higher rates of late arterial patency when compared to conventional compressive dressings.
Dispositivos dedicados à compressão do sítio de punção radial adicionam custo ao procedimento e não foram adequadamente comparados aos curativos compressivos. Avaliamos a efetividade e a segurança de ambas as formas de hemostasia em pacientes submetidos à cinecoronariografia e/ou intervenção coronária percutânea na prática diária.
MétodosEstudo prospectivo, multicêntrico e não randomizado, que incluiu pacientes consecutivamente submetidos a procedimentos por via radial. A modalidade de compressão ficou a critério do operador e da disponibilidade das pulseiras hemostáticas. O objetivo primário foi a comparação da patência da artéria radial no sétimo dia pós-procedimento, aferida por meio do Doppler. Secundariamente, avaliamos a ocorrência de hemorragia/hematoma no sítio de punção durante a compressão, após a retirada do dispositivo e no sétimo dia pós-procedimento.
ResultadosForam avaliados 528 pacientes, 416 que usaram o curativo compressivo e 112 que usaram a pulseira hemostática. Na fase da retirada do introdutor e logo após sua remoção, notou-se uma incidência maior de sangramento no grupo curativo compressivo (13,4% vs. 0%; p < 0,001). Todos os sangramentos foram pequenos (tipo I ou II) e não necessitaram medidas adicionais. Aos 7 dias, observou-se apenas formação de pequenos hematomas no sítio da punção em 7,1% dos casos que utilizaram a pulseira de compressão. Não houve diferença nas taxas de patência da artéria radial (3,8% vs. 7,1%; p = 0,20).
ConclusõesO uso de pulseira dedicada à hemostasia da artéria radial não resultou em maiores taxas de patência arterial tardia quando comparada ao curativo compressivo simples.
The use of radial access has become increasingly common in the contemporary scenario of percutaneous coronary intervention (PCI), given its superiority over the femoral access regarding the reduction of mortality, especially in acute coronary syndrome, as shown by several clinical trials.1–4 Additionally, this type of access is associated with lower rates of vascular complications/bleeding and promotes greater comfort for the patient.1,5–7
Aiming to make local hemostasis more practical after the use of radial approach, devices dedicated to puncture site compression have been recently developed. Although practical and effective, these devices add cost to the procedure and have not been adequately compared to the use of conventional compressive dressings, universally available and at a relatively low cost.
The present study aimed to compare the effectiveness and safety of both forms of hemostasis of the radial puncture site in patients undergoing coronary angiography and/or PCI in daily practice.
MethodsPopulation and study designThis is a prospective, multicenter, nonrandomized study including consecutive patients undergoing procedures through radial access. Patients older than 18 years undergoing elective diagnostic catheterization, elective PCI or cardiac catheterization, followed by percutaneous intervention (ad hoc), were included. The authors only excluded those patients with prior coronary artery bypass graft surgery and those who had cardiogenic shock at the time of examination.
The use of dedicated wristband devices for hemostatic compression or compressive dressings was at the interventionist's discretion and the service's availability during the period of registry inclusion.
Radial punctureAfter the radial puncture and passage of the sheath, a solution containing 5,000 IU of unfractionated heparin, 40mg of isosorbide mononitrate, and 2mL of lidocaine was infused into the radial artery.
In cases of elective or ad hoc PCI, an additional dose of unfractionated heparin was administered, up to 100 IU/kg.
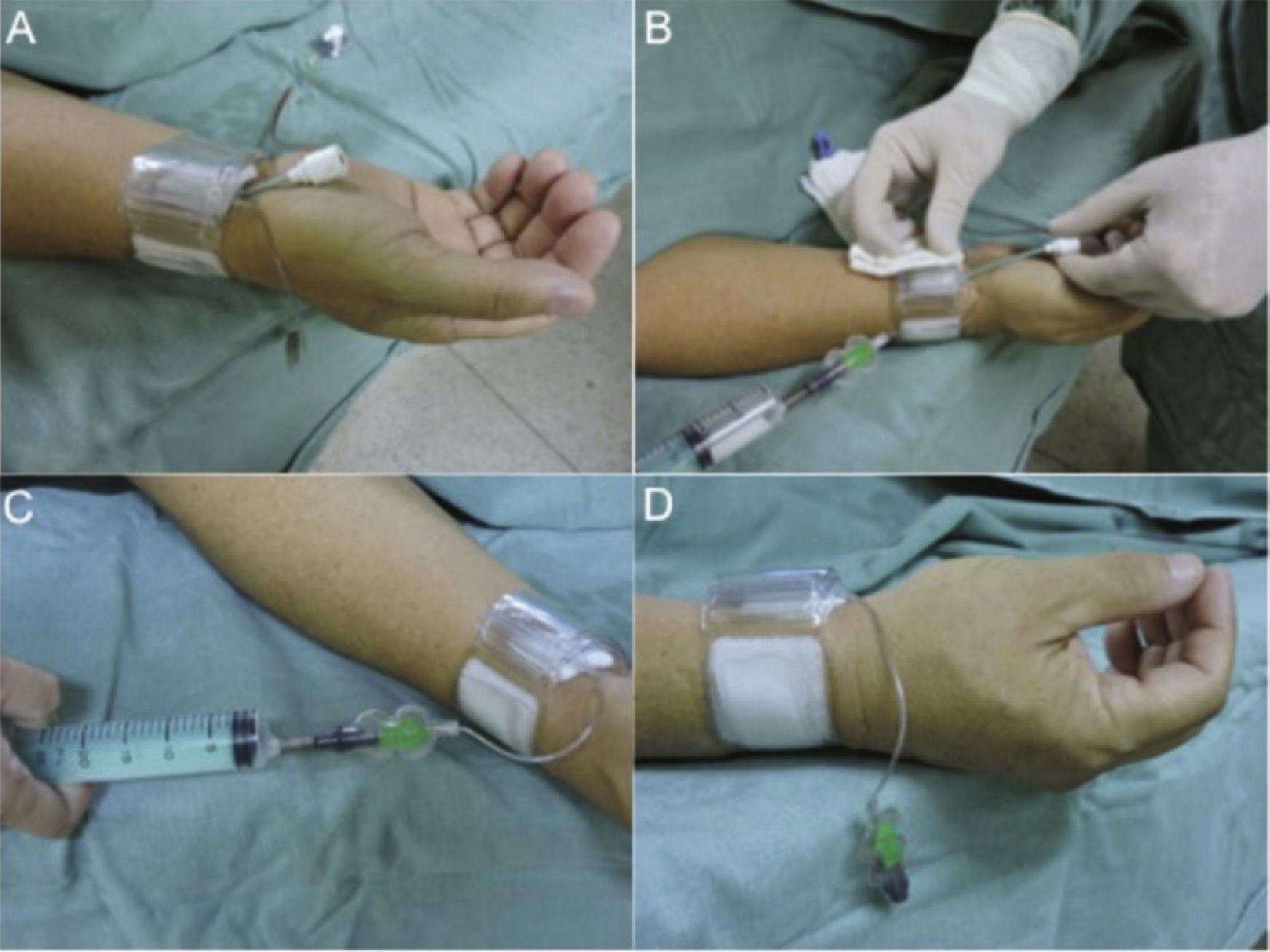
Radial compression technique after sheath removalWristband deviceTwo models of transradial compression wristband devices were used: the TR Band® compressive device (Terumo Medical, Tokyo, Japan) or the RadiStop® device (St. Jude Medical, St. Paul, USA). According to the manufacturer's recommendations, the authors positioned the compression point approximately 1cm above the puncture site with balloon inflation using 15mL of air, after which the sheath was submitted to traction and removed, and then deflation was started at each 3mL, observing whether or not bleeding occurred. If blood extravasation was observed, a new insufflation was performed with 3mL of air and the patient kept the limb at rest for 1 hour. After this period, decompression was performed, with 3mL of air being withdrawn every 30minutes until complete deflation (Fig. 1). Subsequently, a non-compressive dressing was maintained, followed by the recommendation to rest and return in 7 days.
Placement of the hemostatic device: the device is placed approximately 1cm above the puncture site with balloon inflation using 15mL of air (A). The sheath is submitted to traction and withdrawn (B). Deflation starts at each 3mL, while observing the onset of bleeding (C). At this time, further inflation is performed with 3mL of air and the patient's limb is maintained at rest for 1 hour (D).
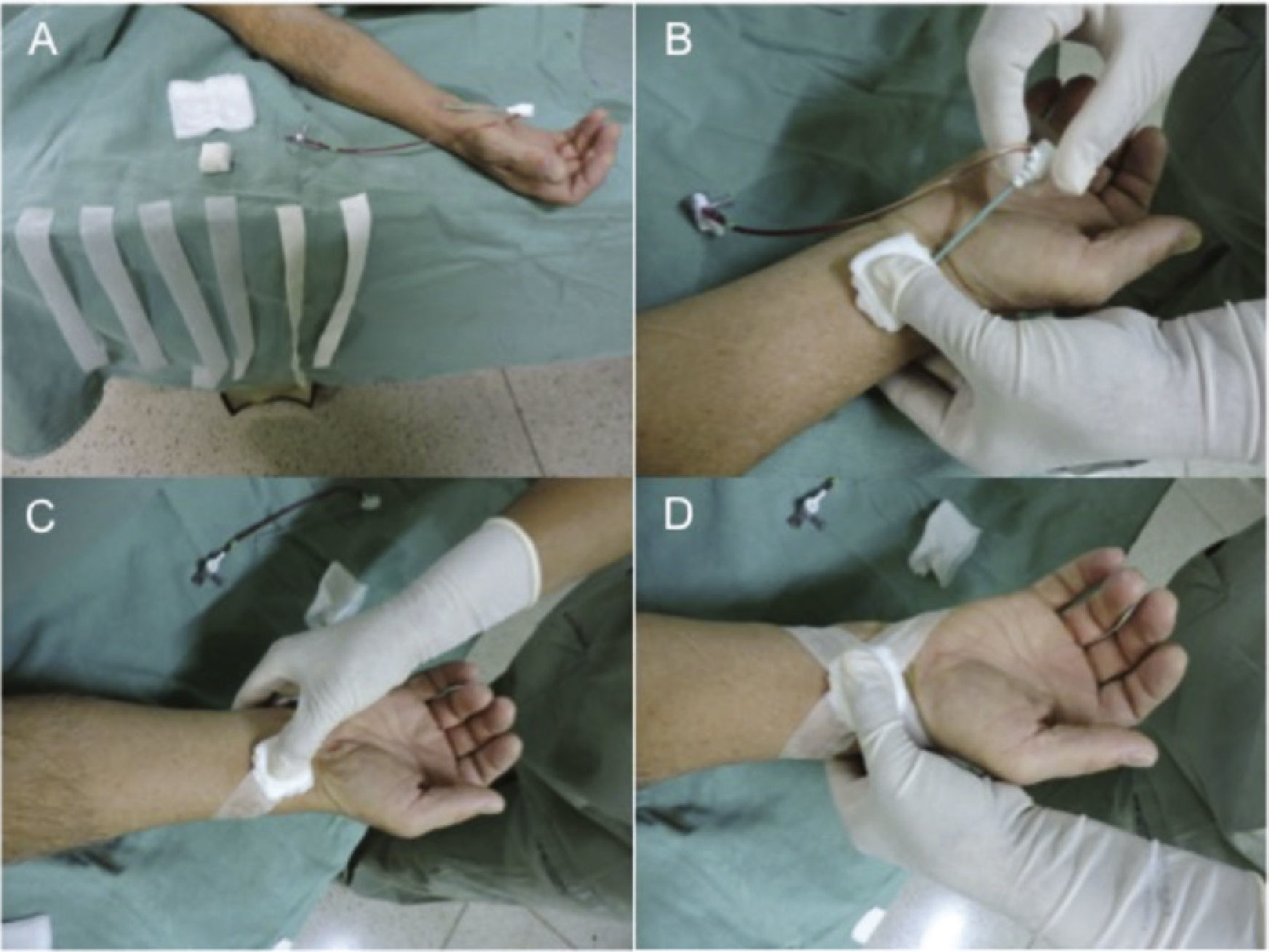
Hemostasis was initiated with the inflation of the sphygmomanometer cuff in the arm, at 200mmHg, followed by removal of the sheath. Then, a gauze pad and four strips of micropore tape (Fig. 2), in the shape of an “X”, were positioned. Over this first dressing, other gauze pads, previously folded into quadrangular shapes, were positioned and the compression dressing was performed with adhesive tape.
Placement of the conventional compressive dressing: externalization of the sheath is started after cuff inflation > 10mmHg of the patient's systolic blood pressure (A). Close to the sheath externalization, the dressing is positioned 1cm above the puncture site, applying manual pressure (B). Next, the first compression tape is placed (C), and then the dressing is completed in an “X” (D).
After that, the cuff was deflated and, if bleeding or distal cyanosis was observed, the dressing was remade. Similarly to compression using the wristband device, the patient rested for 1 hour with the compressive dressing. After this period, the compressive tape was removed and a simple dressing with micropore tape was maintained.
At the discharge, the patient received the same care and recommendations given to those who received compression wristband devices.
Study objectives and definitionsThe primary objective of this analysis was to compare the patency of the radial artery, assessed by Doppler ultrasound, 7 days after the procedure. Secondarily, the authors assessed the occurrence of bleeding/hematoma at the puncture site during compression, after removal of the dressing and on the 7th day after the procedure.
The radial access was selected after the performance of the Allen test and, when needed, the Barbeau test was used for assessment of the palmar arch irrigation.
Arterial occlusion was defined as the absence of radial flow, assessed by Doppler, proximal to the puncture site on the 7th day after the procedure. The evaluation with Doppler ultrasound was performed by an experienced physician who was unaware of the type of compression used in each case.
The hematoma assessment during compression, removal of the dressing or device, and after 7 days was based on the classification of the EASY (Early Discharge After Transradial Stenting of Coronary Arteries) study:4 type I if < 5cm in diameter; type II if < 10cm diameter; type III if > 10cm, without reaching the elbow; type IV if the hematoma extended beyond the elbow; and type V if there was any hematoma with ischemic injury to the hand.
Statistical analysisCategorical variables were shown as absolute numbers and percentages and compared by the Chi-square test and, when indicated, by Fisher's exact test. Continuous variables were described as means and standard deviations, and compared by the Student's t-test. The Statistical Package for Social Sciences (SPSS) program, version 23.0 for Windows was used, and statistical significance was set at p < 0.05.
ResultsFrom March 2013 to June 2014, 1,290 procedures were performed, of which 1,150 used the radial approach (89.1%) in three Invasive Cardiology services coordinated by the same medical group. A total of 528 patients (45.9%) met the eligibility criteria and agreed to return for re-evaluation and Doppler ultrasound 1 week after the percutaneous procedure (416 in the compressive dressing group and 112 in the wristband device group), being the focus of this analysis.
The mean age of these individuals was 58 ± 9 years, most of whom were males (78.3%). In the wristband device group, there was a higher incidence of patients with ST-elevation myocardial infarction (3.8% vs. 14.2%). Most used non-hydrophilic 6 F sheaths in both cohorts, with no statistical difference between them (Table 1).
Basal characteristics of the studied population.
| Characteristics | Compressive dressing group (n = 416) | Wristband device group (n = 112) | p-value |
|---|---|---|---|
| Age, years | 59 ± 22 | 57 ± 13 | 0.36 |
| Male gender, n (%) | 321 (77.3) | 89 (79.4) | 0.69 |
| Arterial hypertension, n (%) | 328 (78.8) | 88 (78.5) | > 0.99 |
| Diabetes mellitus, n (%) | 144 (34.6) | 64 (57.1) | < 0.001 |
| Smoking, n (%) | 152 (36.5) | 48 (42.8) | 0.26 |
| Clinical presentation, n (%) | < 0.001 | ||
| Stable angina/silent ischemia | 304 (73.1) | 80 (71.6) | |
| NSTE-ACS | 96 (23.1) | 16 (14.2) | |
| STEMI | 16 (3.8) | 16 (14.2) | |
| Percutaneous coronary intervention, n (%) | 184 (44.2) | 72 (64.2) | < 0.001 |
| Sheath type, n (%) | 0.75 | ||
| 6 F | 392 (94.2) | 104 (92.8) | |
| 5 F | 24 (5.7) | 8 (7.1) | |
| Adjunct medication, n (%) | |||
| Heparin | 192 (46.1) | 64 (57.1) | 0.06 |
| Acetylsalicylic acid | 128 (30.7) | 24 (21.4) | 0.07 |
| Dual antiplatelet therapy | 72 (64.2) | 0.66 | |
| Glycoprotein IIb/IIIa inhibitors | 16 (3.8) | 8 (7.1) | 0.22 |
NSTE-ACS: non-ST-elevation acute coronary syndrome; STEMI: ST-elevation myocardial infarction.
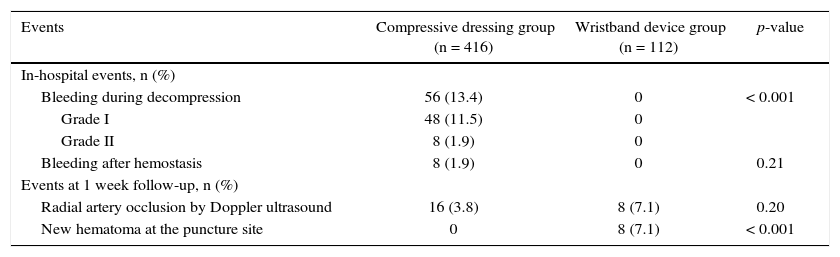
There was a higher occurrence of bleeding during and after the withdrawal of the sheath in the group using the compressive dressing (13.4% vs. 0; p < 0.001). All hematomas were classified as type I or II according to the EASY classification. Of note, 1 week after the percutaneous procedure there was no bleeding or hematoma formation in the compressive dressing group, whereas in the wristband device group there were eight cases (7.1%) of small local hematoma, none of which required intervention (Table 2).
Vascular and bleeding events.
| Events | Compressive dressing group (n = 416) | Wristband device group (n = 112) | p-value |
|---|---|---|---|
| In-hospital events, n (%) | |||
| Bleeding during decompression | 56 (13.4) | 0 | < 0.001 |
| Grade I | 48 (11.5) | 0 | |
| Grade II | 8 (1.9) | 0 | |
| Bleeding after hemostasis | 8 (1.9) | 0 | 0.21 |
| Events at 1 week follow-up, n (%) | |||
| Radial artery occlusion by Doppler ultrasound | 16 (3.8) | 8 (7.1) | 0.20 |
| New hematoma at the puncture site | 0 | 8 (7.1) | < 0.001 |
Regarding the primary outcome of this analysis, at the end of the first week after the percutaneous procedure, there was no significant difference in radial occlusion rate by Doppler ultrasound in both groups, although numerically a two-fold higher occlusion rate was observed in the group using the wristband device (3.8% vs. 7.1%; p = 0.20). Notably, all the occlusion cases in the group using the dedicated device occurred in patients with late formation of local hematoma.
DiscussionThe main finding of this analysis is that hemostasis following procedures using radial approach can be equally obtained with the use of compressive dressings or dedicated compression wristband devices, with no harmful effects on late patency of the approached artery. It is noteworthy that the incidence of bleeding/hematoma in the initial phase of sheath withdrawal is slightly higher with the use of compressive dressings, but without prolonging in-hospital stay and/or generating additional procedures.
Maintaining radial patency is important not only for upper limb vascularization but also for the maintenance of access for future percutaneous interventions. Several measures influence the radial artery patency, from technical issues related to puncture and vessel canalization, as well as issues related to the material used (hydrophilic sheaths, dedicated catheters etc.) and adjunctive pharmacology.8–10 Studies have shown that the routine administration of 5,000 IU of heparin could reduce the rate of radial artery occlusion by up to ten times.11 This measure was used in all patients from both cohorts of this study.
Another crucial point in the late maintenance of radial patency is related to sheath withdrawal and the subsequent compression. More recent studies have shown that maintaining some antegrade blood flow during radial hemostasis (patent hemostasis) could help to reduce the occurrence of thrombosis in this access.7 Although in theory the use of dedicated devices promotes better control of vascular patency during hemostasis, there is a scarcity of studies evaluating the different types of compression devices and even compression dressings, often used in this service's routine.12 It is noteworthy that in the authors’ experience although there was no statistical difference, numerically the use of hemostatic devices resulted in rates of radial artery occlusion twice as high in the assessment with Doppler ultrasound.
Another factor to be considered when deciding on the type of hemostasis to be adopted is related to the occurrence of bleeding complications, including local hematomas. It is important to note that, unlike the femoral artery approach, bleeding events in the radial approach are in general not very significant, with no impact on mortality, but are related to the patient's comfort.7,13–15 In this analysis, the most frequent occurrence of bleeding and hematoma was found during and right after sheath withdrawal in the group using a compressive dressing. There is no doubt that this is related to the greater difficulty of adjusting the correct level of compression with this type of dressing. However, as also mentioned, the bleeding was small, did not require additional measures to stabilize the patient, and did not result in increased length of hospital stay. The authors did not perform an evaluation of patient comfort and pain in either group, which does not allow for assessing the impact of this complication on the patient's perception regarding the safety and comfort of the procedure.
Finally, although this study does not provide a cost-effectiveness analysis, it leads to reflection on the issues of expenses related to percutaneous intervention and the incorporation of new devices into this practice. Although the cost of the compression devices varies per manufacturer and according to the sales agreement with different hospitals, in Brazil these devices cost 20 times more than the conventional compressive dressings with gauze pads (R$ 50.00 vs. R$ 2.50). Considering that, in the period of this evaluation, 112 dedicated devices were used and that the other stages of the percutaneous procedure were similar in both groups, the use of these devices generated an additional expense of R$ 5,600.00, with which the authors could perform compressive dressings in another 2,240 patients undergoing procedures by radial approach.
LimitationsThe main limitation of the study is the fact that it was not randomized, which may have introduced bias in the compared populations. The non-availability of procedure variables (re-use of radial approach, number of puncture attempts, duration of procedure) may have influenced the results. Also, the lack of application of a questionnaire on patient comfort during the sheath withdrawal period prevents comparison of the compression types in this regard.
ConclusionsThe use of conventional compressive dressings to perform hemostasis after percutaneous procedures through the radial approach showed to be equivalent to the use of wristband hemostatic devices in relation to the late patency of the access artery, although their use was associated with slightly higher rates of bleeding. Hemorrhagic complications by radial access were relatively rare and minor, with no impact on the short-term evolution of both cohorts.
Funding sourcesNone.
Conflicts of interestThe authors declare no conflicts of interest.
Peer review under the responsibility of Sociedade Brasileira de Hemodinâmica e Cardiologia Intervencionista.