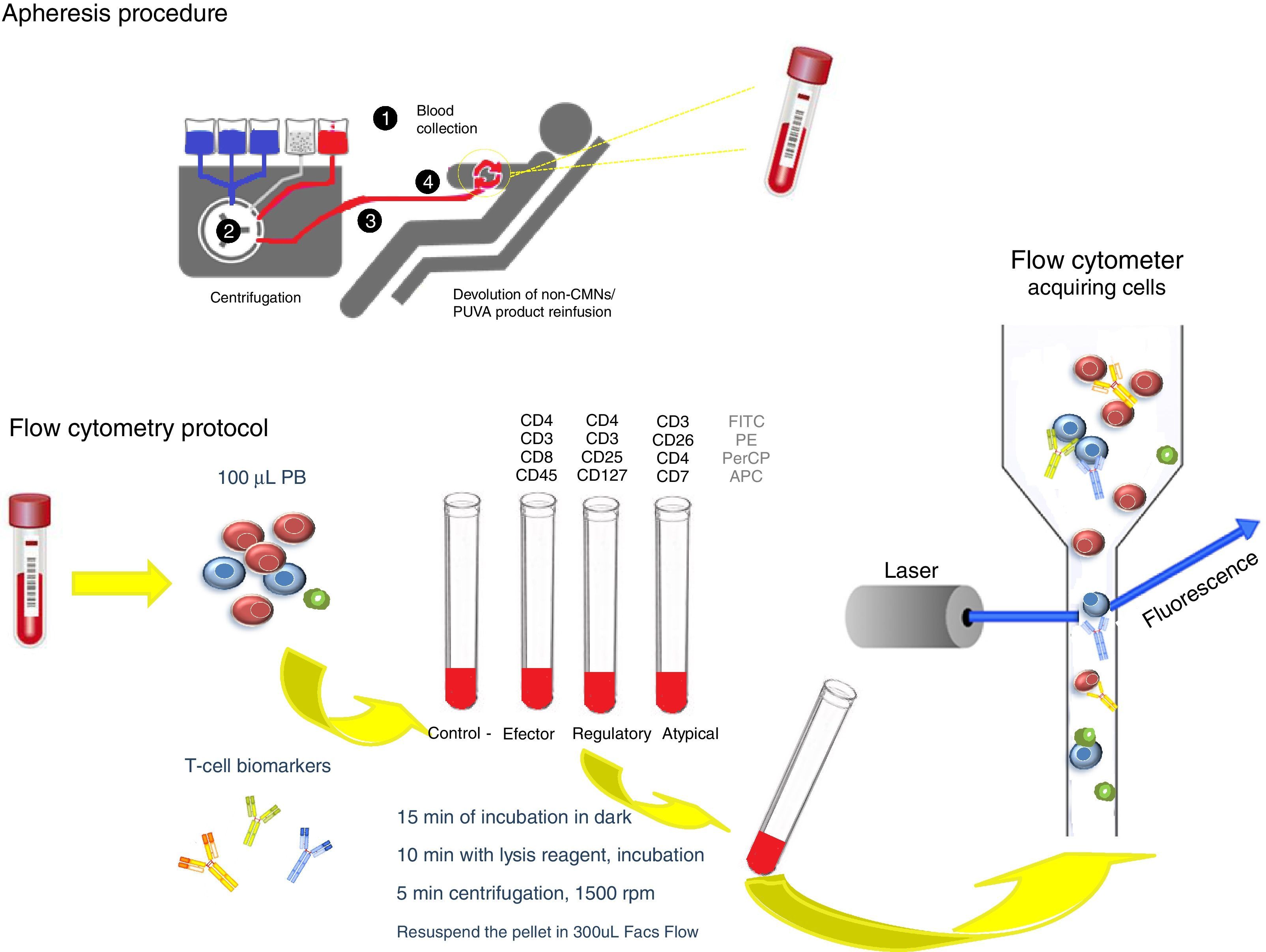
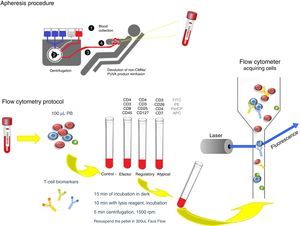
The extracorporeal photopheresis is indicated as immunomodulatory treatment in primary cutaneous T-cell lymphoma. There are no guidelines about the most suitable immunophenotypic panel to monitor and correlate clinical evolution and laboratory parameters.
In this study we characterize a patient with Sézary syndrome stage III, who performed 98 sessions of photopheresis; in the last 30, the expression of T-cells in peripheral blood was evaluated.
The patient had exfoliative dermatitis (mainly in the lower limbs) and several analytical disorders: anemia, neutropenia and lymphocytosis; abnormal number of effector T-cells and a CD4/CD8 ratio higher than 10; atypical cells (CD7, CD26) with aberrant phenotype (characteristic of malignant T-cell clones); and regulatory T-cells indicating an immunotolerance state. The clinical evolution verified can be related with the therapeutic scheme adopted.
We propose a multiparameter flow cytometry approach to monitor patients with Sézary syndrome that realize photopheresis, including the aberrant cases.
Sézary syndrome (SS) is the most aggressive form of Mycosis fungoides, a cutaneous T-cell lymphoma (CTCL), classified as non-Hodgkin lymphoma. This disease derives from skin-homing memory T-cells and is characterized by exfoliative erythroderma, generalized lymphadenopathy, Sézary cells with cerebriform nuclei circulating in peripheral blood (PB) (≥1000/mm3) and poor quality of life. The diagnosis represents a challenge due to the similarity with other dermatologic diseases and should be based on criteria defined by the International Society for Cutaneous Lymphomas (ISCL) and the European Organization of Research and Treatment of Cancer (EORTC).1–3 After the diagnosis and the beginning of the treatment, it is important to monitorize the patient; however, there are no immunophenotyping standards to characterize the therapie's effectiveness. Extracorporeal photopheresis (ECP) is recommended as an immunomodulator treatment, offering better life quality for the patient. It is a personalized therapy, only available in specialized healthcare centers; normally performed as an adjuvant treatment, associated with protein inhibitors, steroids and chemotherapy. The ECP procedure is based on 8-methoxypsoralen effect (a photosensitizing agent), combined with UVA light, which is added to mononuclear cells collected by apheresis, and finally reinfused to the patient.4,5
We propose a multiparameter flow cytometry approach that can be used to monitorize Sézary syndrome patients performing ECP, including aberrant cases.
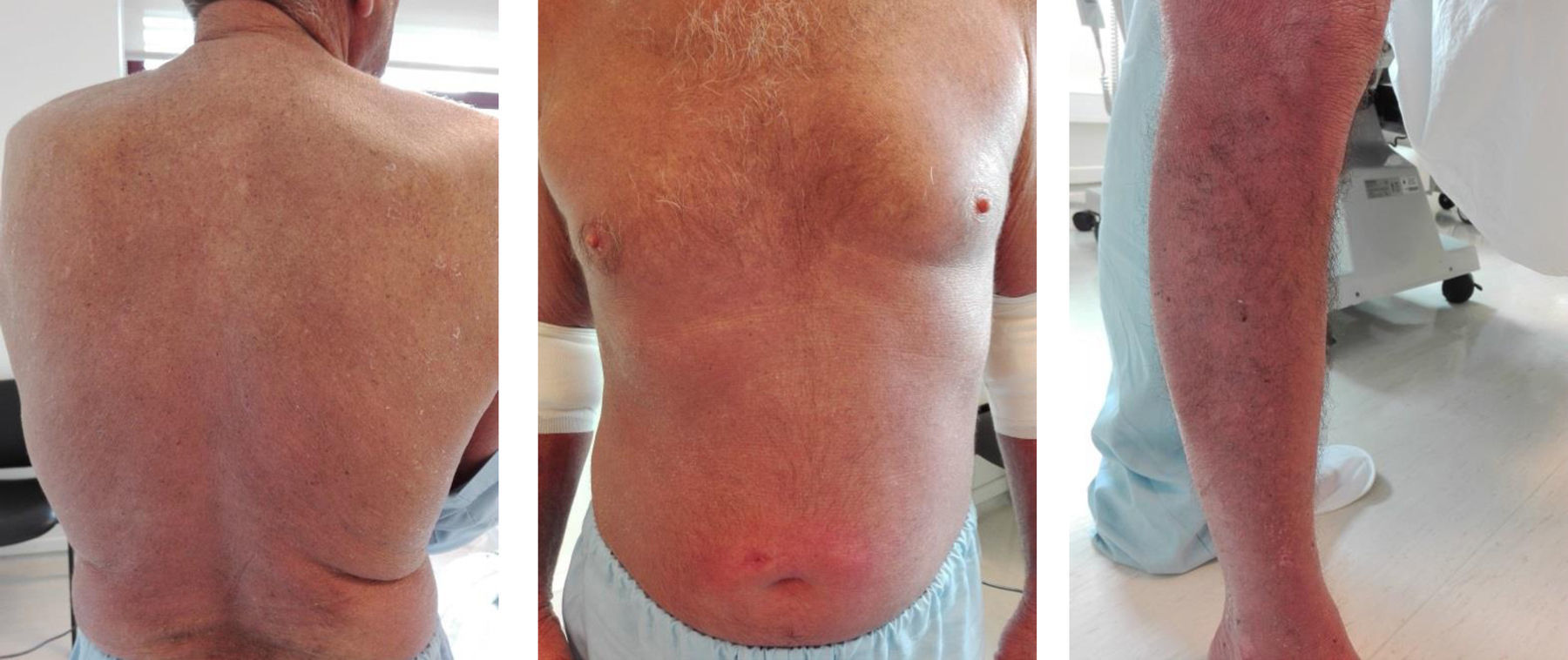
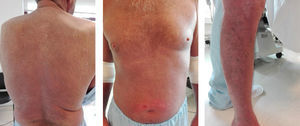
Clinical caseWe describe a 56 years-old Caucasian male with SS stage III diagnosed in 2014 (according to TNMB classification of the ISCL/EORTC). The patient presented erythroderma, multiple palpable adenomegalies and lymphocytosis. He was initially treated with a combination of prednisolone, interferon-α (IFN-α) and ECP (bi-weekly); after, it was added systemic bexarotene. The pruritus was controlled with hydroxyzine and loratadine and the cutaneous lesions with local measures; a complex of iron, folic acid, vitamins D and B12 was also prescribed. Two years later (Fig. 1), we observe a positive clinical response with an improvement of the cutaneous exfoliation affecting mainly the back and the thorax without cracks or maceration; the dark-red color of skin also shows some decrease. However, he maintains itching specially in the face and lower limbs.
The PB was analyzed before and after each ECP by an automated hematology analyzers and through flow cytometry (FACSCanto II) using a biomarkers panel to characterize effector, regulatory and atypical T-cell populations. The protocol is schematically represented in Fig. 2. We also evaluated results obtained from a healthy control.
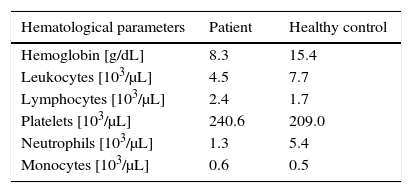
Results and discussionECP has a recognized long-term immunomodulatory effect. In that way we observe, 2 years after, some improvement in patient's skin and a gradual decrease of lymphocytosis; however, the low value of hemoglobin has no change was well as the neutropenia (mostly post-session) (Table 1).
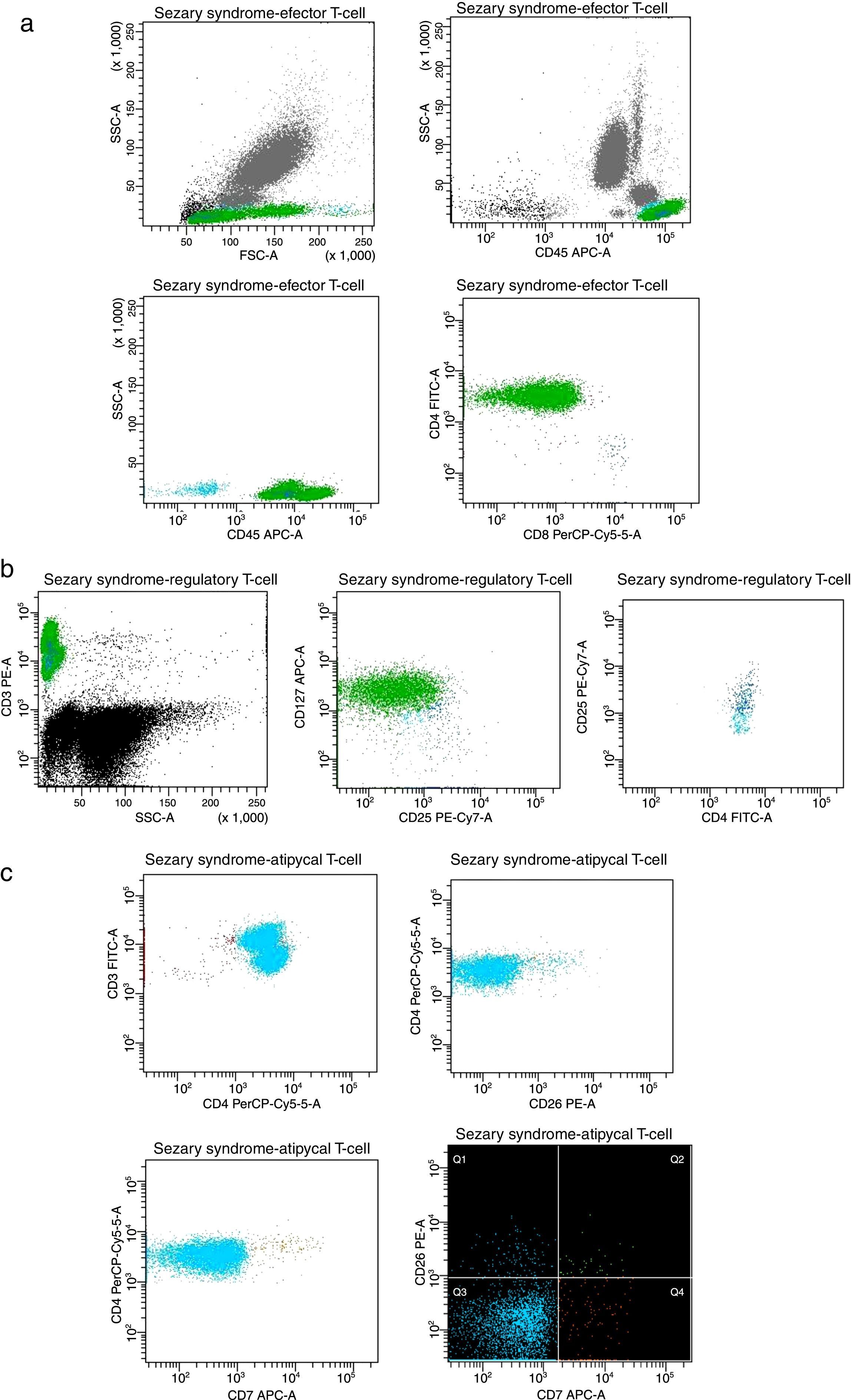
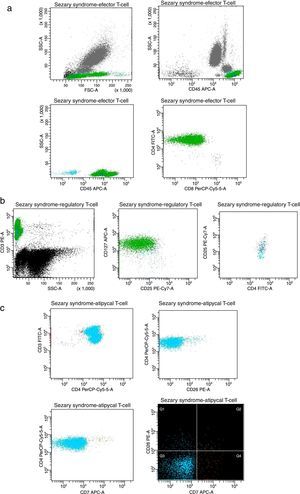
The circulating T-cells express an aberrant and stable immunophenotype over the ECP treatment, as represented in Fig. 3.
Plots of T-cell expression in peripheral blood from the studied patient after the last session. (A) Lymphocytes (cyan events), CD3+ effector T-cells showing CD4+ and CD8+ populations (green and navy, respectively). (B) Regulatory T-cells CD4+CD25+high showing phenotype CD25+CD127−low (navy). (C) Atypical cell clusters majority CD3+CD4+ (green) displaying CD7− (cyan) and CD26− (orange).
The lymphocyte CD3+ population represents more than 60% of the total number of leucocytes (Fig. 3A). The effector T-cells display an abnormal proportion, around 99% are CD4+ and only 1% CD8+, resulting in a high CD4/CD8 ratio (>10, with 88% >100).8–10 A high fraction (80%) of activated regulatory T-cells (Treg) CD4+CD25high are also detected (Fig. 3B), which is similar to the healthy control.3,11,12 In the last twenty sessions, we have observed a slight increase of CD8+ associated with Treg cells reduction, so the CD4/CD8 ratio also decreases. We speculate that this immunotolerance state is promoted by the downregulation of circulating cells, induced by ECP treatment and/or IFN-α action.6
We investigated the presence of Sézary cells using CD7 and CD26 biomarkers. Considering the thresholds in subsets recommended by the ISCL/EORTC (CD4+CD7− >40% and CD4+C26− >30%), we detect >1000 neoplastic cells/μL.3,7 The atypical T-cells exhibit an aberrant arrangement which is often expressed by malignant T-cell clones (Fig. 3C). An overexpression of CD3+CD4+CD7− (98%) and CD3+CD4+CD26− (95%) was demonstrated, without evidence of loss or alteration of these T-cell antigens.8,9 However, the high expression of biomarkers typically related to the severity of Sézary's disease suggest that more studies should be performed to corroborate these insights, especially to better understand the Sézary typical cells relevance.
The multiparameter flow cytometry assay can be considered as an advanced tool during the follow-up of Sézary disease, including the aberrant phenotypes. According to the best of our knowledge, PB analysis should be performed after the second session, once there is no significant difference between pre- and post-assay. Based on the tumor-associated immunophenotype identified, we suggest the use of CD3, CD4, CD7, CD8, CD25, CD26, CD45, CD127 biomarkers to monitorize circulating neoplastic T-cells and assess the ECP response in these patients with LTCL.
Conflicts of interestThe authors declare no conflicts of interest.
We are grateful for the support of Instituto Português de Oncologia do Porto Francisco Gentil, EPE, Liga Portuguesa Contra o Cancro – Núcleo Regional do Norte and professional team of Serviço de Terapia Celular.