Post-COVID-19 syndrome is a series of chronic signs and symptoms that may appear after SARS-CoV-2 infection, including fatigue, dyspnoea, chest pain, palpitations, anxiety, depression, and joint and muscle pain. The purpose of this study was to review the controversies on post-COVID-19 syndrome, the frequency of neurological symptoms, and the potential pathophysiological mechanisms.
MethodsWe present a narrative review of studies published in PubMed since the beginning of the pandemic (January 2020–July 2021).
ResultsPatients with history of COVID-19 have been found to present persistent neurological symptoms, including cognitive complaints, memory and concentration problems, headache, anosmia, ageusia, vertigo, and insomnia. Post-COVID-19 syndrome is a heterogeneous disease that lacks a universally accepted definition, which may explain the great variability in the estimated prevalence (2.3%–85%) and symptom duration. The criteria differentiating post-COVID-19 syndrome from chronic fatigue syndrome or critical illness syndrome are ambiguous. Risk factors include older age, female sex, certain comorbidities, and greater number of symptoms in the acute phase. The pathophysiology of the syndrome is largely unknown, although it is probably multifactorial, including immunological mechanisms, neural network dysfunction, neurotransmitter alterations, persistent viral damage, and functional impairment.
ConclusionsPost-COVID-19 syndrome may present after mild or even asymptomatic SARS-CoV-2 infection, causing limitations in activities of daily living and in quality of life. Further research will clarify the origin and most appropriate management of these neurological alterations.
El término “síndrome post-COVID” se emplea para describir una serie de signos y síntomas crónicos que pueden surgir tras la infección por el virus SARS-CoV-2, como fatiga, disnea, dolor torácico, palpitaciones, ansiedad, depresión, dolores articulares y musculares entre otros. El objetivo es revisar las controversias asociadas al síndrome post-COVID-19, la frecuencia de los síntomas neurológicos y su posible fisiopatología.
MétodosRevisión narrativa crítica de los estudios publicados desde el inicio de la pandemia en pubmed (enero 2020 a julio 2021).
ResultadosSíntomas neurológicos persistentes (quejas cognitivas, problemas de memoria y concentración; cefalea, anosmia, ageusia, vértigo, insomnio, etc) se han descrito en personas que padecieron COVID-19. El síndrome post-COVID-19 no es una entidad homogénea y no tiene una definición universalmente aceptada, lo que explica la variación en las estimaciones sobre prevalencia (2,3%–85%) y duración de los síntomas. Los criterios que lo distinguen del síndrome de fatiga crónica o el síndrome del paciente crítico son ambiguos. Los factores de riesgo incluyen edad, sexo (mujer), comorbidades, y número de síntomas en la fase aguda. La fisiopatología es en gran medida desconocida, pero probablemente multifactorial, incluyendo mecanismos inmunológicos, disfunción de redes neuronales y alteración de neurotransmisores, daño viral persistente, y cuadros de origen funcional, entre otros.
ConclusionesLos síntomas post-COVID-19 pueden surgir tras padecer una infección leve o incluso asintomática, y causa limitaciones en las actividades de la vida diaria y calidad de vida. El progreso en la investigación nos ayudará a aclarar el origen y manejo de estas complejas alteraciones neurológicas.
As of 21 July 2021, the COVID-19 pandemic continues to expand, with over 192 million confirmed cases and 4.1 million deaths worldwide.1 Many survivors do not fully recover, presenting new or persistent symptoms and marked functional impairment lasting weeks or even months, regardless of the severity of the initial infection.2 These symptoms include neurological manifestations.3,4
Chronic symptoms are known to persist after the convalescence phase of infection with a wide range of viruses, including Epstein–Barr virus, coxsackievirus, dengue virus, Ebola virus, and other human coronaviruses.5 Post-infectious/immune aetiology is a possible cause of chronic fatigue syndrome, as well as other syndromes presenting with similar symptoms, such as post-treatment Lyme disease syndrome, when patients with history of Lyme disease present persistent, non-specific symptoms including fatigue, pain, or cognitive impairment following resolution of the infection.6 As in COVID-19, there is no evidence of an active infection; therefore, these patients do not benefit from prolonged antibiotic therapy. Similar symptoms have been observed in patients with history of severe sepsis or other viral infections. Advances in our understanding of the mechanisms involved in some of these symptoms may shed light on some other mechanisms.
Follow-up studies of survivors of the 2002 SARS-CoV outbreak demonstrate this problem: half of the survivors presented fatigue and sleep disorders 12 months after the initial infection.7
We review the controversies associated with post-COVID-19 syndrome, the frequency of the associated symptoms, and the possible pathophysiological mechanisms.
Methodological difficultiesThere is some confusion around the terminology used to describe symptom persistence following COVID-19. Several terms have been used interchangeably in the literature (eg, post-COVID-19 syndrome, persistent COVID-19, ongoing COVID-19, long COVID-19), although the validity of these definitions has not been evaluated. The 2020 NICE guidelines on COVID-19 provide a definition for post-COVID-19 syndrome (Table 1), distinguishing it from the acute phase of the infection (COVID-19), with symptoms lasting up to 4 weeks, and ongoing symptomatic COVID-19, whose symptoms present between weeks 4 and 12 after infection.8 However, the term “post-COVID-19 syndrome” itself is ambiguous, since the natural history of the entity is unknown; therefore, the timing of symptom onset and persistence has been defined arbitrarily. Furthermore, new symptoms may appear after the initial infection.
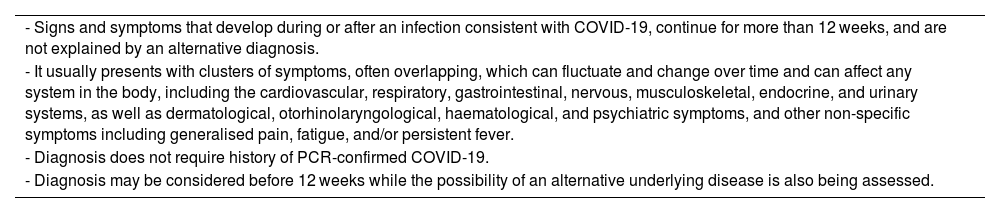
Post-COVID-19 syndrome. Definition and diagnostic criteria proposed by the British National Institute for Health and Care Excellence.
| - Signs and symptoms that develop during or after an infection consistent with COVID-19, continue for more than 12 weeks, and are not explained by an alternative diagnosis. |
| - It usually presents with clusters of symptoms, often overlapping, which can fluctuate and change over time and can affect any system in the body, including the cardiovascular, respiratory, gastrointestinal, nervous, musculoskeletal, endocrine, and urinary systems, as well as dermatological, otorhinolaryngological, haematological, and psychiatric symptoms, and other non-specific symptoms including generalised pain, fatigue, and/or persistent fever. |
| - Diagnosis does not require history of PCR-confirmed COVID-19. |
| - Diagnosis may be considered before 12 weeks while the possibility of an alternative underlying disease is also being assessed. |
Adapted from the National Institute for Health and Care Excellence guidelines.8
The United States Centers for Disease Control and Prevention distinguishes at least 3 post-COVID-19 conditions, persisting for more than 4 weeks after SARS-CoV-2 infection: (1) multiorgan effects of COVID-19; (2) effects of COVID-19 illness or hospitalisation (“post-intensive care syndrome” in the most severe cases); and (3) new or ongoing symptoms, which may affect both patients with history of mild or moderate COVID-19 and patients with more severe forms of the condition who required hospitalisation.9
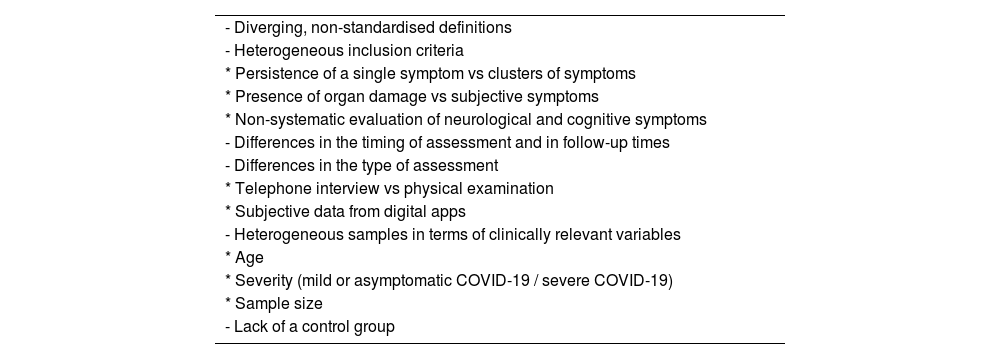
The design, methodology, and quality of the available studies into post-COVID-19 symptoms present marked differences, which makes it difficult to interpret and compare their results. Table 2 summarises the main methodological limitations (study population, definition of the syndrome, severity, associated symptoms, and follow-up times).10 The percentage of patients with persistent COVID-19 symptoms varies depending on the type of study and the moment of analysis: 27% at 60 days,11 13.7% at 84 days,12 37.7% at 12 weeks,13 53% at 4 months,14 46% at 6 months,15 and 34.7% at 7 months.16 One methodological problem of studies using patient-reported data is that the symptoms reported are common and not specific to COVID-19. Furthermore, absolute values should be interpreted with caution, since the real prevalence of these symptoms in the general population is unknown. (See Table 3.)
Methodological limitations of published studies into post-COVID-19 syndrome.
| - Diverging, non-standardised definitions | |
| - Heterogeneous inclusion criteria | |
| * Persistence of a single symptom vs clusters of symptoms | |
| * Presence of organ damage vs subjective symptoms | |
| * Non-systematic evaluation of neurological and cognitive symptoms | |
| - Differences in the timing of assessment and in follow-up times | |
| - Differences in the type of assessment | |
| * Telephone interview vs physical examination | |
| * Subjective data from digital apps | |
| - Heterogeneous samples in terms of clinically relevant variables | |
| * Age | |
| * Severity (mild or asymptomatic COVID-19 / severe COVID-19) | |
| * Sample size | |
| - Lack of a control group |
Proposed classification of the neurological symptoms of post-COVID-19 syndrome.
| 1. Headache |
| - Tension-type headache |
| - Chronic daily headache |
| - Other |
| 2. Cranial nerve involvement |
| - Anosmia/hyposmia |
| - Ageusia/dysgeusia |
| - Tinnitus |
| - Total/partial hearing loss |
| - Dizziness and vertigo |
| - Balance problems |
| - Dysphonia |
| 3. Musculoskeletal problems |
| - Muscle pain |
| - Muscle weakness |
| - Chronic fatigue |
| - Movement disorders |
| - Limb paraesthesia |
| - Neurogenic pain, lumbar pain, bone and joint pain |
| 4. Movement disorders |
| - Limb tremor |
| - Coordination problems |
| 5. Sleep disorders/ insomnia |
| 6. Autonomic nervous system alterations |
| - Impaired thermoregulation |
| - Orthostatic intolerance |
| - Exercise intolerance |
| - Postural orthostatic tachycardia |
| - Other |
| 7. Cognitive symptoms and “brain fog” |
| - Memory problems |
| - Attention deficit |
| - Difficulty concentrating |
| - Difficulty in decision-making |
| - Slower reaction times |
| - Language disorders |
| 8. Psychological and psychiatric symptoms |
| - Anxiety |
| - Depression |
| - Post-traumatic stress disorder |
| - Other |
Many patients with history of mild COVID-19 present recurrent or persistent symptoms. The COVID Symptom Study analysed symptoms reported by 4 million people in the United States, United Kingdom, and Sweden using a smartphone app; at least 10% of patients testing positive for SARS-CoV-2 presented symptoms lasting longer than 3 weeks.17
No universally accepted definition of post-COVID-19 syndrome has been established. Furthermore, as this syndrome is associated with a wide range of symptoms, some of which are non-specific (fatigue, muscle pain, difficulty concentrating, etc), some studies suggest making estimations based on patient-reported symptoms. The United Kingdom's Office for National Statistics conducted the Coronavirus (COVID-19) Infection Survey.12 On 1 July 2021, the Office for National Statistics estimated that 962,000 people in the United Kingdom had presented symptoms for longer than 4 weeks after infection that could not be explained by any other condition. Self-reported symptoms had an impact on activities of daily living in 634,000 respondents. Of an additional subsample of 20,000 individuals with PCR-confirmed SARS-CoV-2 infection, 13.7% presented symptoms lasting longer than 12 weeks, with higher prevalence among women (14.7% vs 12.7%).12
Post-COVID-19 symptoms may occur regardless of the severity of the infection. Some patients with post-COVID-19 syndrome were not hospitalised due to COVID-19 and had mild forms of the disease. Huang et al.11 studied the clinical histories of 178,971 patients from California who presented PCR-confirmed SARS-CoV-2 infection, identifying 1407 patients who had not been hospitalised but who presented new symptoms compatible with “persistent COVID-19.” The most frequent symptoms were palpitations, rhinitis, dysgeusia, insomnia, hyperhidrosis, anxiety, sore throat, and headache. Women accounted for 72% of this subgroup, and 27% reported persistent symptoms lasting over 60 days. Approximately 32% were asymptomatic in the acute phase, when diagnostic tests for SARS-CoV-2 infection were performed.
The REACT-2 study is a community-based study conducted in the United Kingdom evaluating the prevalence of 29 persistent COVID-19 symptoms in 508,707 individuals. The prevalence of self-reported COVID-19 was 19.2% (76,155 cases); 37.7% reported one or more symptoms persisting over 12 weeks. In this study, only one-third of patients with persistent symptoms at 12 weeks had history of severe COVID-19.13
Meta-analyses and systematic reviewsSeveral meta-analyses and systematic reviews have analysed the prevalence of post-COVID-19 symptoms. One methodological issue with these studies is that they include highly variable follow-up periods (from weeks to several months), and do not distinguish between patients with multiorgan sequelae and those with symptoms of recent onset.
Salamanna et al.2 analysed 145 studies into post-COVID-19 symptoms: 55% analysed specific systemic symptoms, 24.1% analysed neurological symptoms and olfactory disorders, and 20.7% analysed symptoms associated with altered pulmonary function.
A systematic review of 45 studies analysed the frequency of 84 persistent signs or symptoms after COVID-19 in 9751 patients (54% men). At least one persistent symptom was observed in 72.5% of patients; the most frequent were dyspnoea (36%), fatigue (40%), and sleep disorders/insomnia (29.4%).18
Another recent systematic review of 15 studies, including a total of 47,910 patients, analysed the prevalence of 55 specific symptoms in patients with history of COVID-19. Patients were aged between 18 and 87 years, and were followed up for 14–110 days. Approximately 80% of patients presented at least one symptom, the most common being fatigue (58%), headache (44%), attention disorders (27%), alopecia (25%), and dyspnoea (24%). Other frequent neurological disorders were ageusia (23%), anosmia (21%), memory complaints (16%), and tinnitus (15%).19
Descriptive studies into post-COVID-19 symptomsMany of the initial data on the prevalence of post-COVID-19 symptoms are from series of patients who were hospitalised due to COVID-19. According to the literature, 87% of patients hospitalised due to COVID-19 continue to present symptoms 60 days after disease onset.20 However, estimations for non-hospitalised patients range from 27% to 85%.11,21 Several studies have described symptom frequency at 2, 4, 6, and 8 months after acute SARS-CoV-2 infection.
Several studies of patients with mild and severe COVID-19 have described persistence of both systemic (fatigue, dyspnoea) and neurological symptoms (cognitive or psychiatric alterations).22–32 The post-COVID-19 neurological symptoms most frequently reported are headache, anosmia, ageusia, paraesthesia, muscle pain, sleep disorders, and cognitive complaints (memory, attention, and concentration problems), in addition to psychiatric symptoms (anxiety, depression, post-traumatic stress disorder). Asthenia and fatigue are also frequent and typically have a multifactorial origin, although their aetiology may also be musculoskeletal or neurological.
Many patients with persistent COVID-19 who were not hospitalised present fatigue and “brain fog,” which negatively affect cognitive function and quality of life. A prospective study into the first 100 patients with persistent COVID-19 treated at a neuro-COVID-19 clinic in the United States (mean age, 43 years; 70% women) and who had not previously been hospitalised found that the most common neurological symptoms lasting over 6 weeks were “brain fog” (81%), headache (68%), paraesthesia (numbness, tingling; 60%), dysgeusia (59%), anosmia (55%), and muscle pain (55%); 85% of patients reported fatigue.21 The most frequent comorbidities were anxiety/depression (42%) and autoimmune disease (16%). A total of 64% of patients recovered after a mean of 5 months, although some patients continued to present symptoms 9 months after onset.
In a study conducted in Spain, including 274 patients with post-COVID-19 symptoms, 12% presented neurological symptoms 10–14 weeks after the initial infection.28 Carvalho-Schneider et al.22 evaluated 150 individuals with history of mild COVID-19 2 months after onset; two-thirds of patients reported symptoms, with the most frequent being asthenia and fatigue (40%), dyspnoea (30%), and anosmia/ageusia (23%). In a longitudinal study of consecutive patients with PCR-confirmed SARS-CoV-2 infection, 53% of the 180 non-hospitalised patients presented at least one persistent symptom after a mean follow-up period of 4 months.14
Case–control studiesData on the frequency of the most relevant specific symptoms (whether neurological or systemic) and prognosis are reported in case–control studies.
In a prospective study of 606 patients with COVID-19 who presented new neurological symptoms during hospitalisation, 395 survivors were compared against a control group of patients with COVID-19 who did not present neurological symptoms. In this study, 196 patients with neurological symptoms and 186 controls were followed up for 6 months. Compared to controls, patients with neurological symptoms presented more severe functional dependence at 6 months and a lower probability of returning to work.33
The COMMUNITY study, conducted in Stockholm, Sweden, gathered data on post-COVID-19 symptoms in healthcare workers at 2, 4, and 8 months after mild COVID-19. The study compared symptom frequency in 323 SARS-CoV-2–positive patients and 1072 SARS-CoV-2–negative individuals, who prospectively reported their symptoms using a smartphone app. In that sample, 26% of patients (vs 9% of controls) reported functional symptoms at least 2 months post-COVID-19, and 15% (vs 2% of controls) reported symptoms at 8 months. The most frequent neurological symptoms at 8 months were anosmia (9% vs 0.1%), fatigue (4% vs 1.5%), ageusia (3.7% vs 0.1%), sleep disorders (2.2% vs 0.8%), headache (1.5% vs 1%), muscle pain (0.6% vs 0.4%), concentration problems (0.6% vs 0.2%), and memory complaints (0.3% vs 0.2%). Anosmia persisted for over 2 months in 14.6% of patients, and for 4 months in 10.8%.27
Common neurological symptoms: Specific studiesSeveral studies report that headache is a common neurological post-COVID-19 symptom. The COVERSAN study evaluated a prospective cohort of 201 patients with mild COVID-19 and a mean age of 45 years, with one-third of participants being healthcare professionals; at 4 months, the study reports a prevalence of 83% for headache, 87% for muscle pain, and 98% for fatigue.24
Presence of headache during the acute phase of the infection is associated with a higher prevalence of headache, fatigue, and other chronic post-COVID-19 symptoms. In another case–control study, 205 patients with headache and 410 patients without headache were evaluated 7 months after discharge with a telephone interview. Presence of headache during the acute phase of the infection was associated with history of migraine and with persistent tension-type headache.34
Anosmia and ageusia, which are characteristic of the acute phase, may persist during the chronic phase, as shown in a prospective, multicentre study of 138 patients with COVID-19 and evaluated at 2 months; 5.8% of patients presented moderate-to-severe olfactory alterations and 4.3% presented dysgeusia.35 In another prospective study of 183 patients with mild symptomatic COVID-19, 18.6% of patients presented olfactory or gustatory alterations at 2 months.36 According to results on the Brief Smell Identification Test, the prevalence of olfactory disorders at 3 months after onset of mild COVID-19 is estimated at 14.3%.37 Hyposmia lasting longer than 6 months has been described in 15% of cases.38
Hearing problems and vestibular and balance disorders are also frequent. In a retrospective study of 48 patients with history of mild COVID-19, 8.3% reported hearing loss at one month of follow-up, 4.2% reported tinnitus, 8.3% dizziness, 2% spinning vertigo, 2% dynamic imbalance, and 6.3% static imbalance.39
Cognitive symptoms are more severe and more frequent in patients requiring hospitalisation, although percentages vary depending on the study. An Italian study describes cognitive complaints at 1–3 months in 17% of a sample of 105 patients discharged after pneumonia due to COVID-19.30 Garriges et al.25 evaluated 120 patients who had been admitted to a COVID-19 unit; at 3 months, 55% presented fatigue, 34% reported memory problems, and 28% reported difficulty concentrating. Halpin et al.26 compared the prevalence of post-COVID-19 symptoms in patients admitted to the intensive care unit against those admitted to a hospital ward; attention and memory disorders were found to be more frequent in patients requiring intensive care (52% vs 33.8%). Cognitive symptoms may persist for up to 4 months after discharge. In the COMEBAC study, which included data collected through a telephone survey, 21% of respondents reported new cognitive problems at 4 months after discharge; however, cognitive alterations were detected in 38% of the 174 patients evaluated in person.31
The frequency of sleep disorders varies between studies. In a study of 119 patients admitted due to COVID-19-related pneumonia, 57% of patients presented sleep disorders at 2 months after discharge.23 Another study reports sleep disorders at 3 months in 31% of patients admitted to COVID-19 units.25 However, different studies report diverging results regarding the frequency of sleep problems; a longitudinal study conducted in Wuhan, China, which included 538 individuals, reported sleep disorders in 18% of patients at 3 months after discharge.32
Symptom clustersIn the REACT-2 study, 14.8% of individuals reported at least 3 symptoms lasting over 12 weeks.13 The study describes 2 different clusters of symptoms persisting for over 3 months: (1) tiredness associated with muscle pain, sleep disorders, and dyspnoea (n = 15,799), which was particularly frequent in patients with history of mild COVID-19; and (2) respiratory symptoms, fatigue, and chest pain (n = 4441), which was more frequent in patients with history of severe COVID-19. The prevalence of many neurological symptoms was higher in patients with the “respiratory” cluster: headache (15.3%, vs 13.9% of patients with the “tiredness” cluster), muscle pain (23.7% vs 16.7%), paraesthesia (7.9% vs 5.1%), hoarse voice (9.1% vs 5%), dizziness/vertigo (12.3% vs 7.5%), and limb weakness (12.5% vs 7.7%). The exception was olfactory (9.6% vs 15.5%) and gustatory alterations (9.3% vs 12.5%), which were more common in patients with the “tiredness” symptom cluster.13 The sensitivity of this symptom pattern must be assessed in prospective studies.
Sudre et al.40 identified 2 patterns of symptoms at 28 days after onset of COVID-19: the first includes fatigue, headache, dyspnoea, persistent cough, and anosmia, and the second includes systemic and gastrointestinal symptoms and fever. Huang et al.11 described the presence of 5 different sets of specific symptoms lasting over 60 days: cough + chest pain, cough + dyspnoea, anxiety + tachycardia, abdominal pain + nausea, and lumbar pain + joint pain. In the COVERSAN study, 42% of the cohort reported 10 or more symptoms at 4 months.24
Risk factorsAccording to data from the United Kingdom Coronavirus Infection Survey, the prevalence of post-COVID-19 symptoms was higher among patients aged 35–69 years, in women, in people living in more deprived areas, and among those with previous comorbidities or disability.12
Patients presenting persistent symptoms are more frequently women and middle-aged, and present history of anxiety and depression (42%) before COVID-19. The REACT-2 study described higher prevalence of post-COVID-19 symptoms in individuals with low socioeconomic levels (51% vs 29%) and living in more deprived areas. Persistence of symptoms beyond 12 weeks was more frequent in women (OR = 1.51; 95% CI, 1.46–1.55); prevalence increased in line with age, with an increment of 3.5% per decade of life.13 However, other studies suggest that prevalence is higher in middle-aged individuals.12 After adjusting for age and sex, persistent symptoms were also associated with overweight and obesity, smoking, and previous hospitalisation due to COVID-19.
Persistent post-COVID-19 symptoms are correlated with the severity of acute COVID-19: higher prevalence of these symptoms is reported in individuals who were hospitalised due to COVID-19.20,41 Data from the COVID Symptom Study app suggest that the number of symptoms in the acute phase (more than 5 symptoms during the first week; OR = 3.53; 95% CI, 2.76–4.5), older age (18% in individuals aged 18–49 years vs 22% in those older than 70), and female sex are associated with higher risk of presenting chronic post-COVID-19 symptoms.40
PathophysiologyAt the time of writing (July 2021), a year and a half after the onset of the pandemic, huge amounts of clinical data on SARS-CoV-2 infection are now available. This has enabled us to identify the main neurological manifestations of acute infection, affecting both the central and the peripheral nervous systems; these may appear early or late in the course of the disease, and may be mild or severe. Thus, during the acute phase of the infection, the most frequent and least severe symptoms are anosmia, headache, muscle pain, and instability/dizziness.42–45 The rarest and most severe manifestations include encephalopathy, syncope, epileptic seizures, and stroke.42–45 Other neurological complications have been described in isolated cases and in small patient series, including Guillain–Barré syndrome and its variants, acute disseminated encephalomyelitis, and cranial nerve involvement.42,46 Furthermore, alterations may appear at later stages; this is the case in 20%–70% of cases, according to series from the United Kingdom and Germany.47
This vast body of clinical data stands in contrast with the relatively limited knowledge of the pathogenic mechanisms, in part due to the lack of basic information and animal models. However, we do have information on other similar viruses, and the increasing amount of data from autopsies and other studies is shedding light on the underlying mechanisms causing neurological symptoms. Deeper understanding of this issue will enable a more rational, effective, and personalised approach to management, improving short- and long-term prognosis and preventing sequelae.
The following section focuses on the mechanisms of neurological invasion and damage during the acute phase of SARS-CoV-2 infection and post-infection manifestations, as well as on the underlying mechanisms of persistent COVID-19.
Can SARS-CoV-2 access the central nervous system?SARS-CoV-2 may enter the central nervous system (CNS) through different pathways. The virus is primed by the TMPRSS2 membrane protein and binds to its receptor (ACE2) via the spike protein, enabling it to enter cells.48 In the nervous system, this receptor is mainly found in brain vessels and the olfactory epithelium, and to a much lesser extent in some parenchymal nervous cells.49
There is controversy around the level of evidence of the CNS invasion routes used by the virus:
(1) Olfactory route
Airborne transmission enables the virus to access the olfactory epithelium, which contains ACE2 receptors; at this location, and exploiting the proximity of olfactory nervous tissue, it enters nerve endings through the cribriform plate and follows the axonal pathway of the olfactory tract, reaching the cerebral cortex and respiratory and cardiovascular control centres in the brainstem.50
(2) Haematogenous route
The marked release of inflammatory mediators, including cytokines, during acute infection may lead to increased blood–brain barrier (BBB) permeability, allowing the virus to enter the brain.51 Circumventricular organs, located around the third and fourth ventricles and lacking a BBB, may constitute another route of entry. This is also true of the choroid plexus, which seems to be consistently infected by SARS-CoV-2, and therefore represents another route of access to the CNS.52
Pericytes, the cells that connect the endothelium with astroglia, can also be infected by SARS-CoV-2, and may be a location for viral replication, promoting nervous system invasion and viral dissemination to contiguous astrocytes and other parenchymal cells.53
(3) Gastrointestinal route
Some studies suggest that SARS-CoV-2 may reach the nervous system by infecting the gastrointestinal system, since ACE2 is present in enterocytes.46 From this location, the virus would reach the terminal branches of the vagus nerve, subsequently travelling by retrograde transport to the brainstem; both the nucleus of the solitary tract and the dorsal nucleus of the vagus nerve seem to express ACE2 receptors.54
Is damage direct or indirect?The first question that arises regarding the neurological manifestations of COVID-19 is whether damage is caused by direct viral infection of nervous structures or rather by other indirect mechanisms triggered by the infection.
- a)
Direct damage
The current evidence suggests that direct viral damage is not the main mechanism. Firstly, PCR testing of CSF samples from patients with neurological symptoms very rarely detects viral genetic material. Analysis of genetic material from the frontal cortex of deceased patients with COVID-19 and controls shows absence of the virus in the brain. However, cell alterations in the brain parenchyma suggest that the choroid plexus detects peripheral inflammation and relays it to the brain, where T cell infiltration is observed. The brain displays microglial and astroglial changes similar to those observed in several neurodegenerative diseases, as well as alterations in synaptic transmission in neurons involved in cognitive function, which may explain the neurological involvement associated with COVID-19.53
Secondly, the great majority of CSF samples analysed did not display signs of inflammation (pleocytosis, increased protein levels, etc).
Thirdly, brain MRI studies frequently yield normal or nonspecific findings, with no signs of viral encephalitis; however, in some cases, olfactory cortex alterations have been observed in patients with anosmia,55 while other patients have displayed signs of acute disseminated encephalomyelitis56 or cytotoxic lesions to the corpus callosum,57 which may also appear in other types of meningoencephalitis.58 Leptomeningeal enhancement59 and nonspecific cortical alterations60 have been detected in patients admitted to the intensive care unit. Post-mortem brain MRI studies do not reveal changes suggestive of encephalitis.61
Finally, anatomical pathology studies of post-mortem brain tissue samples do not reveal signs of encephalitis; alterations are mainly secondary to brain hypoxia and ischaemia. In a study of 43 brain autopsies of patients who died due to COVID-19, Matschke et al.62 observed mild neuropathological changes, mainly brainstem inflammation, with no evidence of direct damage by the virus, and also detected viral protein in the vascular endothelium, but not in neurons or glia. Another study analysed tissue samples from the frontal cortex of patients who had died due to COVID-19 and from controls, finding no viral genetic material in the brain.
In some cases, however, viral particles or viral genome have been detected in the CNS. Presence of SARS-CoV-2 in the CNS causes a local response mediated by HLA-DR+ microglia; this response is correlated with presence of inflammatory mediators in the CSF.50
- b)
Indirect damage
Given the lack of evident direct damage, we must reflect on the role of indirect mechanisms associated with SARS-CoV-2 infection, which are varied and complex.
Hypoxia secondary to respiratory failure damages the most sensitive brain regions, such as the hippocampus and cerebellum. Viral invasion of CNS endothelial cells results in neutrophil and macrophage activation, thrombin production, and complement activation, promoting thrombus formation; this phenomenon has consistently been seen in cases of stroke associated with COVID-19.51
Furthermore, there is growing evidence of the release of cytokines in patients with COVID-19, which may lead to cytokine release syndrome and even to the “cytokine storm” phenomenon.63 These entities are systemic inflammatory syndromes characterised by marked release of inflammatory mediators and caused by medications, infections, neoplasms, and autoimmune or genetic disorders. The COVID-19 pandemic has given rise to a renewed interest in these inflammatory phenomena, which are sometimes difficult to distinguish from a strong inflammatory response, and may occasionally be fatal. COVID-19 is associated with marked release of cytokines and inflammatory mediators, including Il-1β, IL-2, IL-4, IL-6, IL-10, IL-12, CXCL8, CXCL10, IFN-γ, macrophage inflammatory protein, VEGF, and TNF-α.64,65 One study suggests that different serum and CSF cytokine profiles are associated with different manifestations.64 For instance, encephalopathy was associated with increased serum levels of IL-6, a predictor of poor prognosis. CSF IL-2 levels were also elevated in these patients. Thus, encephalopathy seems to be of multifactorial origin, including immune-mediated processes. Parainfectious processes associated with COVID-19, however, were associated with high serum levels of IL-6, CXCL8, and CXCL10.64
The release of these proinflammatory substances increases BBB permeability, which in turn promotes the transport of cytokines into the CNS, leading to microglial and astrocytic activation. Activated microglia release other inflammatory mediators, including interleukins, complement factors, TNF-α, glutamate, and quinolinic acid. This leads to increased glutamate levels and NMDA receptor expression, resulting in excitotoxicity and neuronal loss, which may cause memory and learning problems, hallucinations, and neuroplasticity alterations. In fact, microglial and astroglial changes are similar to those observed in several neurodegenerative diseases; these patients also display impaired synaptic transmission in neurons involved in cognitive function, which may at least partially explain the neurological manifestations of the infection.53 A study of eight patients with encephalopathy and COVID-19 detected SARS-CoV-2 antibodies in the CSF, which suggests intrathecal synthesis or BBB rupture; this in turn may allow cytokines and inflammatory mediators to enter the nervous system, promoting neuroinflammation and neurodegeneration.66 Cytokine release has also been shown to play a role in the pathogenesis of COVID-19-related headache, due to the activation of nociceptive neurons.
Post-infectious neurological damageSimilarly to other viral infections, SARS-CoV-2 infection has been associated with a wide range of neurological complications that manifest late in the course of the disease and that are probably mediated by immunological mechanisms. These manifestations involve the central and peripheral nervous systems, and mainly include acute disseminated encephalomyelitis and Guillain–Barré syndrome and its variants. In these patients, the virus is not detected in the CSF; this supports the hypothesis of a post-infectious immune-mediated mechanism, rather than direct viral damage. In fact, antiganglioside antibodies have been detected in some patients with peripheral nervous system involvement following COVID-19, which may be explained by cross-reactivity to SARS-CoV-2 spike glycoproteins.67
Persistent COVID-19Persistent COVID-19 symptoms may present in 30%–80% of patients.20,41,68 Some data suggest a correlation between severity of the acute pulmonary disease and presence of persistent symptoms; this association is more marked among women.11
In a series of 120 healthcare workers with history of moderate-to-severe COVID-19 4 months previously, no significant differences were observed in cognitive assessment results as compared against a control group of individuals with no history of COVID-19.69 However, patients did present higher levels of stress, anxiety, and depression. In contrast, and as an example of the divergent findings, Woo et al.47 found moderate cognitive impairment in 14 of 18 young patients who had presented COVID-19 a median of 85 days previously, as compared to 10 healthy individuals.
The mechanisms operating at this stage are even less well understood than those of the acute phase, and are probably multifactorial, including possible direct effects of viral infection, immune response, systemic inflammation, neurotransmitter alterations, corticosteroid treatment, hospitalisation at the intensive care unit, and social isolation.68 Although the profile of serum cytokines in the acute phase is correlated with prognosis, less information is available on this stage of the infection.70
Furthermore, there is evidence suggesting that COVID-19 may cause autonomic nervous system dysfunction. The symptoms of persistent COVID-19 include palpitations, gastrointestinal problems, orthostatic hypotension, and postural orthostatic tachycardia syndrome.71 It has been hypothesised that virus or proinflammatory molecules accessing the brain through circumventricular organs and the area postrema may affect the autonomic control centres located in the brainstem that regulate blood pressure and respiration. Furthermore, the cytokine storm associated with COVID-19 is in part caused by activation of the sympathetic nervous system, whereas parasympathetic nervous system activation may have an anti-inflammatory effect; this opens new doors for the treatment of these patients. It has also been suggested that the presence of autoantibodies targeting autonomic receptors may be triggered by viral infection, as described in postural orthostatic tachycardia syndrome.72
Metabolic dysfunction of several neural networks due to direct and indirect viral damage may contribute to the pathogenesis of these patients' symptoms. In a study of 35 patients with symptoms persisting for over 3 weeks, PET revealed hypometabolism in the olfactory region and its connections in the temporal lobe (hippocampus and amygdala), brainstem, and cerebellum.73 Symptoms included fatigue, olfactory alterations, memory problems, pain, and insomnia. A study of two patients with persistent COVID-19 and “brain fog” revealed hypometabolism in the cingulate gyrus, which is involved in emotions, memory, depression, and decision-making; alterations in this area may be responsible for the symptoms reported by these patients.74 Another study of four patients older than 60 years with cognitive complaints or seizures presenting within 2 weeks of COVID-19 onset detected alterations in frontal and cerebellar metabolism.75 As symptoms improved with immune therapy (IV immunoglobulins or pulse corticosteroids), the authors suggest an immune-mediated mechanism, particularly in the light of the fact that CSF analysis results were normal, including negative PCR results for SARS-CoV-2. However, the study did not include a control group, and hypometabolism may have been caused by other factors, such as depression. In two patients, the study of neurodegenerative markers in the CSF detected decreased levels of β-amyloid (1–42), as occurs in Alzheimer disease.75
A longitudinal study of seven patients with acute encephalopathy and COVID-19 who underwent 18F-FDG PET studies revealed a pattern of hypometabolism in an extensive cerebral network including the frontal cortex, anterior cingulate, insula, and caudate nucleus.76 Patients predominantly presented cognitive and behavioural frontal disorders. Brain MRI studies detected no alterations. At 6 months, patients had improved clinically, but cognitive and emotional disorders persisted; PET studies continued to detect alterations in the same structures. This functional impairment is very likely to contribute to persistence of cognitive symptoms in patients with history of COVID-19, particularly in severe cases and those associated with encephalopathy.
Fatigue, defined as decreased physical and mental performance, may be caused by a wide range of factors, including psychological (anxiety, depression, sleep disorders), central (neurotransmission alterations, inflammation), and peripheral factors (functional or structural muscle alterations)77; however, the correlation between COVID-19 and fatigue is yet to be confirmed, as are the hypotheses of the autoimmune reaction and the viral reservoir, among others.
In some cases, both in patients with COVID-19 and in individuals vaccinated against the virus, functional neurological disorders have been diagnosed in individuals with highly suggestive symptoms, including abnormal irregular movements and gait alterations, with no evidence of underlying neurological dysfunction.78–80 This is a complex entity that raises concerns among the general population; effective communication is needed to reduce fears regarding vaccination.81
ConclusionsCommunity-based studies may be more representative of the severity of the disease in the general population than hospital-based registries. Post-COVID-19 syndrome is associated with a wide range of clinical manifestations. Studies of patient-reported data include common, non-specific symptoms; therefore, the prevalence of persistent symptoms may have been overestimated.
Numerous systemic, neurological, and cognitive symptoms have been detected in a high percentage of patients with history of COVID-19, several weeks or months after disease onset. Many of these are chronic symptoms, requiring long-term treatment and rehabilitation therapy. Inability to return to work and disability associated with post-COVID-19 syndrome have a huge socioeconomic impact.
Though they are not well understood, the mechanisms underlying these symptoms are probably multifactorial, including exaggerated inflammatory response, neurotransmission alterations, persistent viral damage, and functional alterations. Future research will help to determine the most appropriate management for these complex patients.