No formal indication currently exists for seizure prophylaxis in neurosurgical oncology patients. Neither have specific recommendations been made on the use of antiepileptic drugs (AED) in seizure-free patients with meningiomas scheduled for surgery. AEDs are generally prescribed on a discretionary basis, taking into consideration a range of clinical and radiological risk factors. We present a systematic review and meta-analysis exploring the effectiveness of antiepileptic prophylaxis in patients with meningioma and no history of seizures.
MethodsWe performed a systematic review of the PubMed/MEDLINE, Cochrane Central Register of Controlled Trials, Embase, and clinicaltrials.gov databases. Of a total of 4368 studies initially identified, 12 were selected for extraction of data and qualitative analysis. Based on the clinical data presented, we were only able to include 6 studies in the meta-analysis. We performed heterogeneity studies, calculated a combined odds ratio, evaluated publication bias, and conducted a sensitivity analysis.
ResultsAED prophylaxis in patients with meningioma and no history of seizures did not significantly reduce the incidence of post-operative seizures in comparison to controls (Mantel-Haenszel combined odds ratio, random effects model: 1.26 [95% confidence interval, 0.60-2.78]; 2041 patients). However, we are unable to establish a robust recommendation against this treatment due to the lack of prospective studies, the presence of selection bias in the studies reviewed, the likelihood of underestimation of seizure frequency during follow-up, and the strong influence of one study on the overall effect.
ConclusionsDespite the limitations of this review, the results of the meta-analysis do not support the routine use of seizure prophylaxis in patients with meningioma and no history of seizures.
En la actualidad, no existe una indicación formal de profilaxis anticomicial en neurocirugía oncológica. Tampoco existen recomendaciones específicas sobre el uso de fármacos antiepilépticos (FAE) en pacientes portadores de meningiomas y libres de crisis que van a ser intervenidos. En general, se prescriben FAE de forma discrecional, teniendo en cuenta diversos factores de riesgo clínico-radiológicos. Presentamos una revisión sistemática y meta-análisis sobre la efectividad de la profilaxis anticomicial en meningiomas sin historia previa de crisis.
MétodosSe realizó una búsqueda sistemática en las bases de datos PubMed/MEDLINE, Cochrane Central Register of Controlled trials, Embase y clinicaltrials.gov. De los 4.368 estudios inicialmente identificados, finalmente se incluyeron 12 para la extracción de datos y análisis cualitativo. Los datos clínicos permitieron incluir únicamente 6 estudios en el meta-análisis. Se realizaron estudios de heterogeneidad, cálculo de OR combinada, evaluación del sesgo de publicación y análisis de sensibilidad.
ResultadosLa profilaxis con FAE en meningiomas sin crisis previas no redujo de forma significativa la incidencia de crisis postoperatorias respecto a los controles (OR combinada de Mantle-Haenszel, efectos aleatorios, de 1,26, IC 95%, 0,60-2,78, sobre 2.041 pacientes). Sin embargo, la ausencia de estudios prospectivos, la presencia de sesgo de selección en los estudios, una probable infraestimación del número de crisis durante el seguimiento, y la influencia marcada de un estudio sobre el efecto global, impiden establecer una recomendación sólida en contra de la profilaxis anticomicial.
ConclusionesDentro de las limitaciones de esta revisión, los resultados del meta-análisis no apoyan el uso rutinario de la profilaxis antiepiléptica en pacientes con meningiomas sin historia previa de crisis.
Epileptic seizures are frequent in the natural history of patients with intracranial meningiomas. Between 20% and 40% of patients present seizures at the time of diagnosis, and a further 20%-30% will develop seizures during the course of the disease.1,2 Epileptic seizures are an important cause of morbidity, negatively impact quality of life, may lead to neurocognitive impairment, and hinder or prevent the performance of some activities of daily living, such as driving.3–7
Larger lesion size, location in the frontoparietal or parasagittal regions or the convexity, and pronounced peritumoural brain oedema are known risk factors for preoperative epileptic seizures in patients with brain meningiomas.7–9 Furthermore, the risk of postoperative seizures is increased when seizures present prior to surgery, in the event of surgical complications, after partial resections, in tumours of higher histological grade, and in patients with recurrent or progressive tumours.1,3,7–9 In patients without history of seizures, location in the frontoparietal region or the convexity and midline shift are predisposing factors for postoperative seizures.3
Surgical resection of meningioma resolves seizures in the medium and long term in 60%-90% of patients with history of seizures.10–12 However, surgery itself causes de novo seizures in 12%-19% of patients without history of seizures, mostly in the first days or weeks after surgery.12 Therefore, it is important to establish the effectiveness of prophylaxis with antiepileptic drugs (AEDs) in patients without history of seizures. Some review articles, meta-analyses, and official guidelines recommend against the routine use of AEDs for seizure prophylaxis in patients who are scheduled to undergo surgery for meningioma; however, the authors acknowledge that the available evidence is not robust.1–3,8,9,13,14 Given the limited number of prospective studies and the lack of randomised trials on the subject, clinical practice is based on the discretionary prescription of these drugs, taking into account the above-mentioned risk factors for perioperative seizures.15
Two recent systematic reviews1,9 have analysed the role of seizure prophylaxis in general oncological neurosurgery; another review15 specifically assesses the effect of this treatment on meningiomas, although it does not provide a quantitative analysis due to the lack of data. In this study, we performed a systematic review of the literature on the effectiveness of seizure prophylaxis in patients with meningioma and no history of seizures.
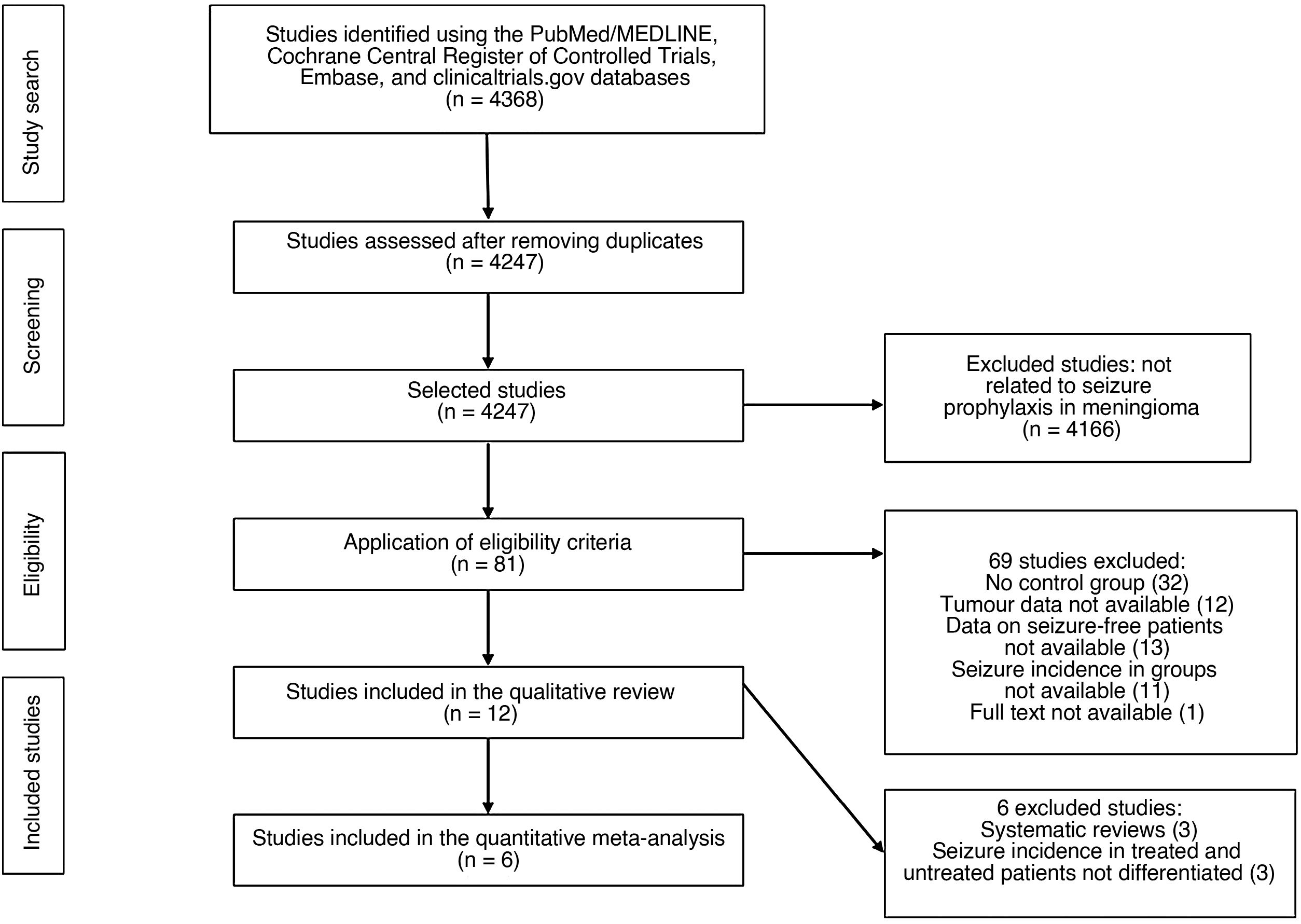
Material and methodsStudy design and literature search criteriaWe conducted a systematic literature review and meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria.16 The aim of our study was to evaluate the effectiveness of seizure prophylaxis in preventing postoperative seizures in patients with cranial meningioma and no history of seizures. The systematic literature search was independently performed by 2 authors (PDL and SOC) using the PubMed/MEDLINE, Cochrane Central Register of Controlled Trials, Embase, and clinicaltrials.gov databases on 16 March 2020. We used the following keywords: prophylaxis, prophylactic, prevention, preventive, AED, AEDs, seizures, epilepsy, epileptic fit, antiepileptic, anticonvulsant, phenytoin, levetiracetam, valproic, carbamazepine, gabapentin, meningioma, benign meningioma, atypical, anaplastic, malignant, grade II, grade II, grade III, brain tumor, extraaxial, craniotomy, resection, biopsy, surgery, postoperative, and perioperative. We used the necessary Boolean operators to combine the terms to perform as exhaustive and complete a search as possible. We reviewed the articles identified as well as the reference lists of the most relevant studies. In principle, we applied no limitations on language, type of publication, or study period. After eliminating duplicate studies, we evaluated the titles and abstracts in an initial screening (Fig. 1) to identify the studies specifically related to epileptic seizures in surgically-treated meningiomas. Discrepancies between 2 reviewers were resolved by consensus. Finally, we reviewed the full texts of 81 articles, to which we applied the inclusion criteria.
Inclusion criteria and data extractionWe included all studies reporting the use of any type of AEDs as seizure prophylaxis in patients with surgically-treated meningioma with no history of seizure. The quantitative study (meta-analysis) was performed with data from series exclusively including meningiomas and not from larger oncological series including meningiomas and other brain tumours. We included series in which at least 50% of patients presented no seizures before surgery and were treated with craniotomy as primary treatment. We excluded those studies not including a control group (placebo or no treatment), those comparing 2 or more AEDs, and studies not reporting the incidence of postoperative seizures. We also excluded those studies in which the incidence of seizures in patients receiving prophylaxis and in controls is not clearly distinguished. For the studies included in the final review, we recorded the type of study, year of publication, number of patients, medication used, treatment schedule and duration, incidence of early- and late-onset seizures, duration of follow-up, complications, general conclusions, and quality and limitations of the study.
Quality assessment and statistical analysisWe calculated the pooled odds ratio (OR) of the effectiveness of seizure prophylaxis vs controls, with 95% confidence intervals (95% CI). As no randomised studies were identified, we assessed the quality of the observational studies (prospective or retrospective) using the reviewed and validated version of the Methodological Index for Non-Randomized Studies (MINORS).17 To calculate combined risks, we used the Mantel-Haenszel random effects model. Heterogeneity between studies was assessed using the Galbraith plot and quantified with the Q statistic using the DerSimonian-Laird method. Publication bias was estimated using funnel plots and the Begg and Egger test. We performed a sensitivity study (repeated meta-analyses with the omission of one study per iteration) to detect the influence of each study on the overall estimation of the effect. We used the open access statistical package Epidat 3.1 (SERGAS, Servicio Gallego de Salud, Spain) for statistical analysis and plotting charts.
ResultsReview of the studies selectedWe initially identified a total of 4 368 studies (Fig. 1). After removing duplicate studies, we screened 4247 studies by reviewing the title and abstract. We analysed the full texts of 81 studies meeting the eligibility criteria. In the quantitative study, we excluded those articles not strictly related to seizure prophylaxis in patients with meningioma and no history of seizures. Table 1 shows the main characteristics of the 12 studies selected for the qualitative study.1,3,7,15,18–26 Only 6 studies provided clinical data on the incidence of seizures in patients with and without prophylactic treatment, and were included in the meta-analysis.3,7,19–21,23
Main characteristics of the studies included in the systematic review.
| Author, year | Study design | Patients, use of AEDs | Findings: seizure incidence | Comments and limitations |
|---|---|---|---|---|
| Youngerman et al.,1 2020 | Retrospective review of patients with brain masses undergoing surgery, collected from the North American MarketScan database. 1 July 2009-30 June 2013 | 5895 intracranial masses; 4110 seizure-free patients; 1671 received prophylaxis with AED (40.7%). Prophylaxis was used in 61.9% of meningiomas. Levetiracetam was used in 78.5% and phenytoin in 20.5%. | Malignant lesions were more frequently treated with AEDs (42%-52%) than benign lesions (23%). A spike in duration of prophylaxis was observed at 30 days. Data on seizure incidence are not differentiated by treatment or control group. Does not mention benefits of prophylaxis or consensus guidelines. | Highly varied use of prophylaxis. Medication toxicity is not mentioned. Postoperative seizure treatment is not distinguished from prophylaxis. The study used data from a private insurance company. Suboptimal detection of postoperative seizures |
| Li et al.,19 2019 | Single-centre retrospective study. 2011-2012 | 778 supratentorial meningiomas; 678 patients with no history of seizures; 661 received prophylaxis with AEDs (97.5%). Type and duration of AED treatment not specified | 60/586 postoperative seizures in patients with no history of seizures: 55/515 receiving AEDs and 5/71 not receiving AEDs. Higher risk of preoperative seizures in patients with meningioma located in the motor cortex, oedema > 1 cm. Increased risk of postoperative seizure when diameter > 3.5 cm, seizures during admission, recurrence, or progression | 59% of patients with history of seizures and 87% of those with no history were seizure-free at 5 years. Prophylaxis was ineffective. Consider AEDs in cases of high risk. Probable seizure underestimation bias in the long term; recall bias. Treatment duration not specified |
| Islim et al.,3 2018 | Single-centre retrospective study. 2010-2015 | 283 meningiomas; 215 with no history of seizures; 19 receiving AEDs (8.1%), 196 not receiving AEDs. Phenytoin 48%, levetiracetam 26%. Highly variable duration of prophylaxis (1-1092 days) | Postoperative seizures in 5/19 patients receiving AEDs and 24/196 not receiving AEDs. Risk factors in previously seizure-free patients: frontal convexity meningioma and midline shift. Mean time until first seizure: 58 days | Prophylaxis with AEDs decreases the risk at 1 year by 40% (non-significant). Prophylaxis is recommended in patients with one or more risk factors. Variable type and duration of AED treatment. No adverse effects are reported. |
| Wang et al.,7 2018 | Single-centre retrospective study. Only surgically treated atypical and malignant meningiomas. June 2001-November 2009 | 102 meningiomas; 87 with no history of seizures; 51 receiving AEDs (58.6%), 36 not receiving AEDs. Duration of prophylaxis: 5-7 days | Of the patients receiving AEDs, 8/36 early-onset seizures and 13/36 late-onset seizures; no early-onset seizures in patients receiving prophylactic AEDs after surgery (0/51). Higher risk in cases of preoperative seizures, convexity or parasagittal location, oedema, no AED treatment, and recurrent lesions | Prophylaxis with AEDs prevented early-onset seizures, but not late-onset seizures. Greater impact on atypical than on malignant meningiomas. Radical resection increased the risk of postoperative seizures. Evident selection bias. Only clinically diagnosed seizures were considered. |
| Xue et al.,18 2018 | Single-centre retrospective study. Six years of follow-up. 2006-2008 | 113 meningiomas; 92 patients with no history of seizures; only 3 received prophylaxis with AEDs (3.3%). | 13/92 patients with de novo seizures after surgery (14%). | One-third of patients with history of seizure were seizure-free in the long-term, whereas 14% presented de novo seizures after surgery. Difference in seizure incidence between treated and untreated patients is not reported. Probable recall bias in the interviews |
| Carbamazepine was the most frequently used AED. | Preoperative seizure risk factors: diameter > 3.5 cm; postoperative seizure risk factors: diameter > 3.5 cm and history of seizures | |||
| Duration of AED treatment was not specified. | ||||
| Islim et al.,15 2017 | Systematic review with no quantitative meta-analysis. Review includes 11 studies. | 1143 patients with meningiomas and no history of seizures; 100% received prophylaxis in 10 studies. Overall, 776 received prophylaxis with AEDs (67.9%). Phenytoin, levetiracetam, valproic acid, etc. Only one study reported duration of AED treatment. | Early-onset postoperative seizures in 20/766 in patients receiving AEDs and 10/377 not receiving AEDs. Late-onset postoperative seizures in 52/766 patients receiving AEDs and 29/377 not receiving AEDs; non-significant differences | No meta-analysis due to lack of data. Routine prophylaxis is not recommended in patients with meningioma and no history of seizures. Treatment and control groups are not well-balanced. Only 2 studies provide data on seizure incidence in patients receiving AEDs vs those not receiving AEDs. |
| Skardelly et al.,20 2017 | Single-centre retrospective study. 2007-2012 | 634 meningiomas; 537 patients with no history of seizures; 23 received prophylaxis with AEDs (4.3%). Levetiracetam in 18 patients, other AEDs in 5 patients | Early-onset postoperative seizures in 3/23 in treated patients and 24/514 patients receiving no treatment. Higher risk of preoperative seizure: male sex, non–skull base location, tumour volume > 8 cm3 | Prophylaxis with AEDs did not reduce the incidence of early-onset postoperative seizures. No data on timing or duration of AED treatment. No protocol for postoperative seizure detection, leading to a probable underestimation |
| Wirsching et al.,21 2016 | Single-centre retrospective study. 2000-2013 | 779 meningiomas; 535 patients with no history of seizures; 244 received prophylaxis with AED (41.8%). Phenytoin was the most frequently prescribed AED. Follow-up of one year | Early-onset postoperative seizures in 18/244 treated patients and 9/291 patients receiving no treatment. Late-onset postoperative seizures in 48/244 and 29/291, respectively. Higher risk of postoperative seizures: in patients with preoperative seizures, surgical complications, younger age, and tumour progression | Of the patients with history of seizures, 59% were seizure-free after surgery. Of the patients with no history of seizures, 19.4% presented de novo seizures after surgery. Prophylaxis with AEDs was not effective. Patients with WHO grade II and III meningiomas who presented more complications received more AEDs. |
| Englot et al.22, 2016 | Systematic review and meta-analysis. The meta-analysis includes 6 studies. Publications from January 1980 and September 2014 | 39 observational studies, no clinical trials; 4709 supratentorial meningiomas, history of seizures in 29.2%; 1085 with no history of seizures; 402 received prophylaxis with AEDs (75.7%, 402/531). | Postoperative seizures in 55/402 patients receiving AEDs and 17/129 not receiving AEDs (data from 6 studies). Higher risk of preoperative seizures: male sex, oedema, non–skull base location, absence of headache. Higher risk of postoperative seizures: oedema, preoperative seizures | In patients with no history of seizures, 69.3% were seizure-free after surgery. De novo seizures in 12.3% of patients with no history of seizures. The timing and duration of AED treatment are not specified. Study selection bias. Recall bias and heterogeneity between studies. |
| Sughrue et al.,23 2011 | Single-centre retrospective study. Only convexity meningiomas. 1991-2009 | 180 patients with meningiomas and no history of seizures; 129 received prophylaxis with AEDs (71.7%). Duration of prophylaxis: 7 days after surgery. | None of the 129 patients receiving AEDs and 1/51 not receiving AEDs developed early-onset postoperative seizures. No late-onset seizures were identified. | No prophylaxis is recommended in convexity meningiomas due to the low incidence of postoperative seizures. Probable underestimation of subclinical seizures |
| Komotar et al.,24 2011 | Systematic review of studies between 1979 and 2010. Review includes 19 studies. | 19 studies including 698 supratentorial meningiomas. No patient had history of seizures. Prophylaxis with AEDs in 19 studies; 6 studies reported patients not receiving AEDs. | Early-onset postoperative seizures in 8/553 in patients receiving AEDs and 2/145 not receiving AEDs. Late-onset postoperative seizures in 42/475 of patients receiving AEDs and 13/145 not receiving AEDs | No significant differences in the incidence of early- or late-onset postoperative seizures were observed between patients receiving and not receiving AEDs. Tumour diameter is not provided in cohorts of patients not receiving AEDs. Wider resections in untreated patients. Recall bias. Considerable heterogeneity between studies |
| Chozick et al.,25 1996 | Single-centre retrospective study. January 1980-November 1992 | 158 supratentorial meningiomas; 95 patients with no history of seizures; 63 with history of seizures. All patients with history of seizures received AEDs before surgery. Overall, 88.9% of patients with history of seizures were seizure-free in the long term. | Postoperative seizures in 24/63 and 8/95 of the patients with and without history of seizures, respectively. Of the patients with history of seizures, 40% were seizure-free after surgery. Higher risk of postoperative seizures in patients with preoperative seizures and parietal tumour location | Surgery positively influences the incidence of postoperative seizure. Patients with de novo seizures presented more atypical meningiomas, permanent postoperative sequelae, subtotal resections, recurrences, and parietal location. |
AED: antiepileptic drug; WHO: World Health Organization.
All series included supratentorial meningiomas in diverse locations that were treated with craniotomy (patients treated with stereotactic biopsy were not included). No randomised trials or prospective series were identified. Nine articles were single-centre retrospective series1,3,7,19–21,23–25 and 3 were systematic reviews.15,22,24 Overall, series included a median of 634 patients (range, 102-4709) and a high percentage (68.7%-100%) of seizure-free patients with meningioma, with the exception of the extensive systematic review by Englot et al.22 The percentage of seizure-free patients who received prophylaxis with AEDs was variable, accounting for only 3.3%, 4.3%, and 8.1% of patients in 3 series, and 41.8% to 97.5% in the remaining studies.
The most frequently used AEDs were levetiracetam and phenytoin. Duration of prophylaxis was highly variable; it was not reported in all studies, but was mainly limited to the initial postoperative period. Six studies reported the incidence of postoperative seizures in treated and untreated patients.3,7,19–21,23 We observed a high degree of consensus regarding the preoperative risk factors for seizures and the risk factors for de novo seizures after surgery. The conclusions of almost all studies mentioned limitations regarding the possibility of extrapolating results, due to the retrospective methodologies followed and the possible underestimation of the incidence of postoperative seizures during follow-up.
No study recommended routine seizure prophylaxis in seizure-free patients with meningioma, generally citing a relatively low incidence of postoperative seizures.15,20,21,23,24 However, several authors agree on the need to consider several risk factors when prescribing AEDs on a discretionary and personalised basis, especially in cases of meningioma in the frontoparietal convexity, pronounced perilesional oedema, partial resection, tumours of higher histological grade, perioperative complications, and recurrent tumours.3,7,18–20.Most studies identified preoperative seizures as an important risk factor for postoperative seizures and agreed on the effectiveness of surgery to decrease the probability of seizure persistence in the long term.15,24,25 The studies included in the meta-analysis did not specifically analyse the possible morbidity or toxicity associated with the medication.
Seizure prophylaxis in patients with meningioma and no history of seizuresWe calculated the risk of postoperative seizures in patients treated with seizure prophylaxis vs controls in a sample of 2041 patients from 6 studies. Seizure prophylaxis did not significantly decrease the incidence of postoperative seizures in patients with meningioma and no history of seizures (Mantel-Haenszel pooled OR [random effects] of 1.26; 95% CI, 0.60-2.78). Fig. 2 shows individual and pooled ORs for the studies analysed. We observed some degree of heterogeneity between studies, although this was not statistically significant (DerSimonian-Laird method, Q = 11.43; P = .043). Variance between studies amounted to 0.47, and the percentage of total variance attributable to variance between studies was 58%. The Galbraith plot (showing the precision of each study, that is, inverse standard error vs standardised effect size) revealed that only one study significantly contributed to the overall heterogeneity (Fig. 3).
Galbraith plot showing the precision of the studies with regard to the standardised effect. The result reported by Wang et al.7 falls outside the confidence interval, which suggests a relevant contribution to the heterogeneity between studies.
No randomised trial was included in the review. Retrospective observational studies presented a median score of 17 on the MINORS scale (range, 13-20 out of a possible maximum score of 24 for comparative studies). The funnel plot did not reveal any significant publication bias (Fig. 4). Begg and Egger test results (0.37, P = .70 and –1.24, P = .28, respectively) also suggested an absence of significant publication bias. However, the sensitivity analysis (Fig. 5) showed that the overall effect was substantially influenced by the strong effect reported in the study by Wang et al.7 After eliminating that study (which reported a trend opposite to the overall effect), the pooled OR for the 5 remaining studies amounted to 1.84 (95% CI, 1.12-3.01, for a sample of 1954 patients; Q = 2.34; P = .67; Begg test: Z = 0.24, P = .80; Egger test: t = –0.66, P = .55), which suggests that AEDs have a counterproductive effect on the onset of postoperative seizures.
The sensitivity analysis (repeated meta-analyses omitting one study per iteration) shows that the study by Wang et al.7 causes a pronounced influence contrary to the overall effect.
The findings of this systematic review support the common idea that seizure prophylaxis in seizure-free patients with meningioma is not systematically indicated, and underscore the importance of some preoperative risk factors in the development of postoperative seizures. In fact, the results of the meta-analysis suggest that AEDs have a null or even negative impact on the prevention of seizures. This paradoxical effect may partly be explained by the retrospective approach of the studies included, which leads to 2 types of bias. Firstly, there is a selection bias, acknowledged by the authors of several of the studies included, in which there was a tendency to preferentially prescribe prophylaxis to more severe patients. In all cohorts, patients presenting greater risk of postoperative seizures received more AEDs, that is, patients with larger tumours, located in the frontal or parietal cortex, and of higher histological grade. Secondly, almost all studies included among their limitations the possibility of having underestimated the incidence of seizures over the postoperative follow-up.
Our quantitative study was based on series exclusively including patients with surgically-treated meningiomas, rather than larger oncological series including other types of primary or secondary brain tumours, in which the overall effect of prophylaxis may have been masked by the influence of treatment of other tumour types. Some authors have questioned the validity of meta-analyses exclusively based on retrospective series, despite the adequate selection and inclusion criteria and statistical analyses used.26 The overall quality of the studies included in our meta-analysis may be considered moderate, according to the mean score reported above. However, the sensitivity and publication bias studies revealed that one study7 had a significant influence to the contrary of the combined overall effect. We should highlight that this study only included grade II (atypical) and grade III (malignant) meningiomas, subgroups that seem to benefit more from prophylactic treatment than benign meningiomas, which are much more frequent. In fact, when this study was removed from the analysis, the pooled OR became statistically significant, suggesting ineffectiveness of the preventive antiepileptic treatment. Likewise, some authors suggest that assessing publication bias is also questionable when fewer than 10 studies are included in the analysis.9
Therefore, this review supports the daily practice of prescribing AEDs on a discretionary basis based on the judgement of the neurosurgeon, neurologist, or oncologist responsible for the patient. Considering that the presence of postoperative seizures is associated with relevant physical, emotional, and legal implications, it is necessary to find a balance in each individual case between the possible deleterious effect of recurrent seizures and the toxicity and cost of AEDs. Pending clarification of this issue in future studies, we must take into account several clinical and radiological risk factors. Furthermore, as the AEDs used are generally well-tolerated and relatively affordable, some authors support systematically prescribing seizure prophylaxis to all patients, despite the lack of robust supporting evidence.8,27
Patients with meningiomas measuring less than 3-3.5 cm in diameter and extensive cortical expression present a very significant risk of postoperative seizures, according to several studies and systematic reviews.3,19–21 Before anatomical pathology findings are obtained, some radiological characteristics suggest more aggressive biological behaviours and, therefore, an increased risk of postoperative seizures. These radiological findings are: large tumour size, irregular or non-spherical tumour shape, absence of dural tail, heterogeneous contrast uptake, evident bone or extraosseous involvement, intratumoural cystic changes, perilesional brain oedema, decreased apparent diffusion coefficient, and increased cerebral blood volume in specific MRI sequences.28,29
The presence of preoperative seizures is a known risk factor for postoperative seizures,3 and seizure prophylaxis in patients with history of seizures is a reasonable and widely used option. After surgery, histological grade, degree of resection, and biological behaviour of the possible tumour remnants determine the need for retreatment and the use of AEDs. According to the extensive series by Islim et al.3, frontal or parietal cortical location and presence of pronounced midline shift are associated with greater incidence of postoperative seizures in patients with no history of seizures; the AEDs administered to these patients seem to decrease seizure incidence by approximately 40% in the first year after surgery. Regarding the timing and duration of prophylactic treatment, there are no standardised protocols, and some authors recommend discontinuing medication early in the absence of early-onset seizures, whereas others prefer maintaining AEDs in the long term, and even for life.3,7,15,23 We should bear in mind the cost and adverse events associated with medication. In general, levetiracetam seems more favourable than phenytoin or other AEDs in terms of toxicity and cost.30 Further studies are needed to assess the effect of new AEDs, such as lacosamide, as compared to levetiracetam in this context.
In our opinion, the issue raised in this meta-analysis may be subject to study in a preferentially multi-centre, blinded, randomised controlled trial. In principle, we do not consider there to be any ethical constraint preventing the performance of such a trial; furthermore, there are many potentially eligible patients, histological diagnosis is properly standardised, clinical and radiological follow-up is routinely performed in all cases, and the prospective evaluation of seizure incidence is perfectly viable. Ideally, such a study would stratify according to possible confounding factors as tumour location, histological grade, degree of resection, presence of perilesional oedema, presence of surgical complications, and the type and dose of the AEDs used. This would imply recruiting a relatively large number of participants, and therefore collaboration between centres would be advisable. To minimise bias, it would be necessary before starting the trial to perfectly define the event seizure, who should report the event (the patient, physician, nurse, family member), and what time window should be considered before starting the trial.
As is the case of gliomas31 or ependymomas,32 future classifications of brain tumours are likely to include molecular parameters for the diagnosis and stratification of meningiomas, according to the various genetic mutations and epigenetic profiles already described in the literature. These factors are correlated with the biological aggressiveness of tumours and may influence the prescription and use of preventive AEDs. Currently, DNA methylation analysis seems to have simplified the current 15 histological subtypes, divided into 3 histological degrees, to only 6 methylation classes that better correlate with the prognosis and biological behaviours than anatomical pathology findings.33 Such molecular tests will probably become important risk indicators of the biological behaviour of meningiomas, to be considered when deciding on a possible prophylactic treatment for seizures.
This systematic review shares several limitations with the studies included. We used a relatively small number of studies in the combined calculations; we could not include any prospective study; all the studies included are retrospective with a probable recall bias; most of the authors identify a possible underestimation bias regarding the number of postoperative seizures; and several types of medication, therapeutic schedules, and doses are used. However, histologically confirmed diagnosis of meningioma was established for all patients, and all cohorts included comparable percentages of patients receiving treatment.
ConclusionsThe findings of this systematic review do not support the routine use of seizure prophylaxis in patients with meningioma and no history of seizures. Cumulative data from several retrospective series do not support the routine prescription of preventive AEDs, although several methodological issues and biases prevent us from issuing a strong recommendation against this treatment. It is necessary to weigh effectiveness of AEDs against their toxicity, taking into account several clinical and radiological factors that increase the risk of postoperative seizures. The efficacy of seizure prophylaxis in this context may be addressed in a randomised controlled trial considering potential clinical, radiological, and, eventually, genetic confounding factors.
Conflicts of interestThe authors have no conflicts of interest to declare.
This study has received no funding of any kind.
No specific patient data were used in this study.