Caudal regression syndrome (CRS) is a congenital malformation with a low incidence in the general population. The true pathogenesis is unknown although there is a clear relation with maternal diabetes. Prenatal diagnosis and imaging studies allow for reliable recognition and diagnosis. The physical exam and the diagnostic test necessary in the newborn period allow for the identification of likely complications and establishing a prognosis. We present a clinical case of a female neonate with a prenatal diagnosis of CRS, describing the workup and management of this patient.
Introduction
Caudal regression syndrome (CRS) is an infrequent disorder first described by Geoffroy Saint-Hilaire and Hohl in 1852, and in 1964 Duhmel coined the term "caudal regression syndrome".1-3 The CRS is a disorder caused by an anomaly of the distal spinal segments, and it extends to a wide range of anomalies like partial agenesis of the spinal cord, associated pelvic malformations, imperforate anus, genital malformations, cardiac anomalies, bilateral renal dysplasia or aplasia, pulmonary hypoplasia, and extreme external rotation with inferior joints fusion resulting in the most grave form in sirenomelia (mermaid syndrome). The CRS is also associated with femoral hypoplasia, deformed feet and lower extremity flexion contracture. Intelligence is preserved, in general.1,2,4 it affects between 0.1 and 0.25 out of every 10,000 pregnancies, with a male-female ratio of 2.7:1.3-5 At an embryonic level, it is believed that CRS is the result of defects in the induction of caudal elements in the embryo prior to the 28th day of gestation. The injury is produced in the posterior medial mesodermic axis causing the absence of development of the caudal mesoblastic yolk.4,6 The exact etiology is unknown; however, maternal diabetes, genetic predisposition and vascular hypoperfusion have been suggested as possible factors.4-7
Pre-gestational diabetes is without a doubt a teratogen, and there is good evidence that gestational diabetes may be implicated in the development of the most severe form of CRS.5
Pinter and reece proved that alterations induced by hyperglycemia in the closing of the neural tube include disordered cells, decrease of mitosis, and changes which indicate a premature maturation. The oxidative metabolism altered by maternal diabetes can cause an increase in the production of oxygen-free radicals in the developing embryo, which can be teratogenic.8 Between 16% and 22% of CRS cases are associated with maternal diabetes mellitus, which increases the risk of having a child with CRS by up to 400.3,4,7 Several cases of families with CRS have been reported, which suggests a possible genetic transmission with different possible transmission modes: X-linked dominant, multi-factorial polygenic, and autosomal dominant patterns with reduced penetrance and variable expressivity.7 The "vascular theft" theory was initially proposed by Kampmeier in 1927 and re-introduced in 1986 by Stevenson. Adra et al., considered Stevenson's "vascular theft" theory as a possible etiology of the CRS pathology.7 During the embryonic development stage the more caudal structures are separated from the cephalic elements such as the brain, the spine, and the spinal cord, hence the lack of cognitive alterations in this syndrome.4
There are 2 CRS groups: the first group is the most affected with the termination of the spinal cord above L1. The sacrum ends at S1, and in some cases, is absent. Patients in the second group display a less severe dysgenesis with a low implantation of the spinal cord and tethered by a thickened filum terminale or intraspinal lipoma.4
Case presentation
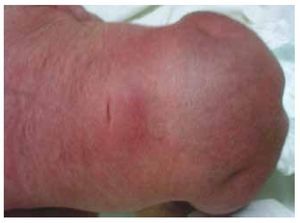
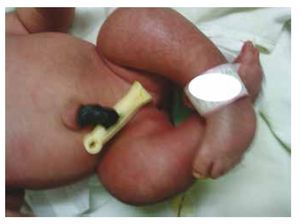
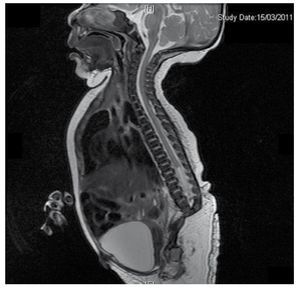
We present the case of a newborn girl from a 40-year-old mother with a medical history of untreated uterine fibroids, a background of irregular gynecology cycles, 3 pregnancies with 2 deliveries and cholecystectomy in 2008. She mentions an unplanned yet wanted pregnancy, with a high-risk pregnancy prenatal care. She attended over 15 prenatal consults. At the 18th week of gestation, compatible data with malformations of the neural tube with sacral agenesis was found, resulting in a CRS diagnosis. During the second trimester of pregnancy hyperglycemia was detected, therefore she was placed on insulin treatment. At 40 weeks of gestation, C-section was performed obtaining a healthy product, with an Apgar score of 8/9 with a gestational age of 39.6 weeks. At the physical exam we observed a weight of 3.44 kg (7.58 lbs), a length of 49 cm (19.2 in), a head circumference of 35 cm (13.77 in) and an abdominal circumference of 32 cm (12.59 in). During inspection we were able to observe an evident diabetic fetopathy, with abundant hair, and low implantation (Fig. 1). Normal anterior fontanelle (2 x 2 cm), round face, prominent cheekbones, horizontal palpebral fissures, short nasal bridge, round tip, thin lips, full palate, dysplasia of the auricular pavilions with hypertrichosis of the helix, short neck with a dorsal bulge, normal thorax, without murmurs during auscultation, the abdomen was soft without palpable masses or visceromegaly, linear spine with presence of a dimple on the skin at lumbosacral region (Fig. 2), no sacrum bone was palpable, normal position of the anus, permeable, with an absent tone. There was an evident shortening of the lower extremities and bilateral varus club foot (Fig. 3). X-rays were taken in order to see the bone malformations, and we were able to observe complete agenesis of the sacrum with a lumbar-iliac fusion. A brain and spine magnetic resonance imaging (MRI) (Fig. 4), reported an abrupt chord and lumbar termination at L3 level. After this level we are able to identify only amorphous masses with fat signal intensity, related to subcutaneous tissue. Short spinal cord, flat conus medullaris at T10 level, both iliac bones were hypoplastic, and fused at mid-level. We are not able to see the sacrum. It was possible to see both kidneys malrotated and hypoplastic, a prominent urinary bladder compatible with a neurogenic bladder. The brain image was reported as normal. An upper abdomen echography was requested in order to rule out other visceral anomalies finding a discrete discrepancy in the size of the left kidney, in addition to not finding its normal configuration, which suggested discrete hypoplasia as well as a possible malrotation. During their stay we performed multidisciplinary consultation, and cast splints were placed on both lower limbs. The patient remains in follow-up with Urology, Traumatology, Neurology and General Pediatrics, this in order to keep a close watch on all the risk factors and possible complications.
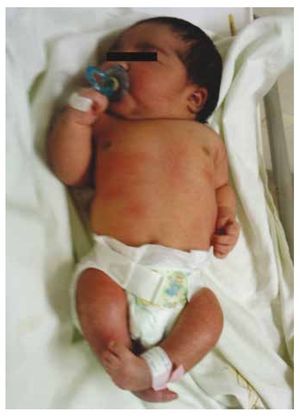
Figure 1 Newborn with diabetic fetopathy.
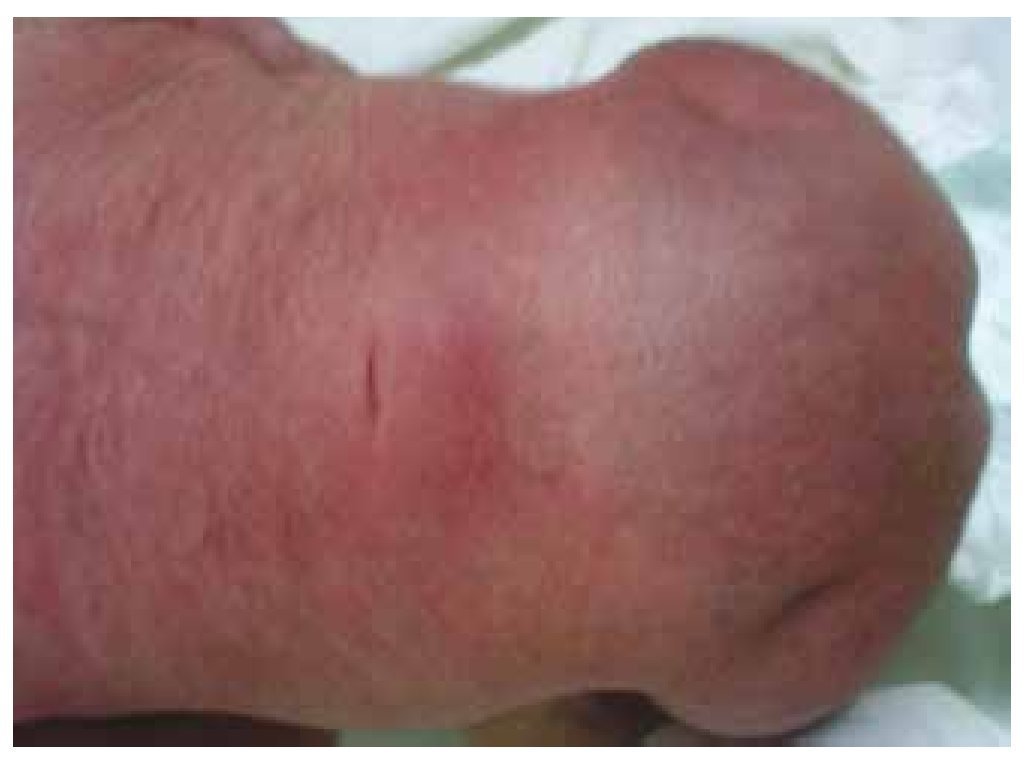
Figure 2 A dimple on the skin at the lumbosacral region.
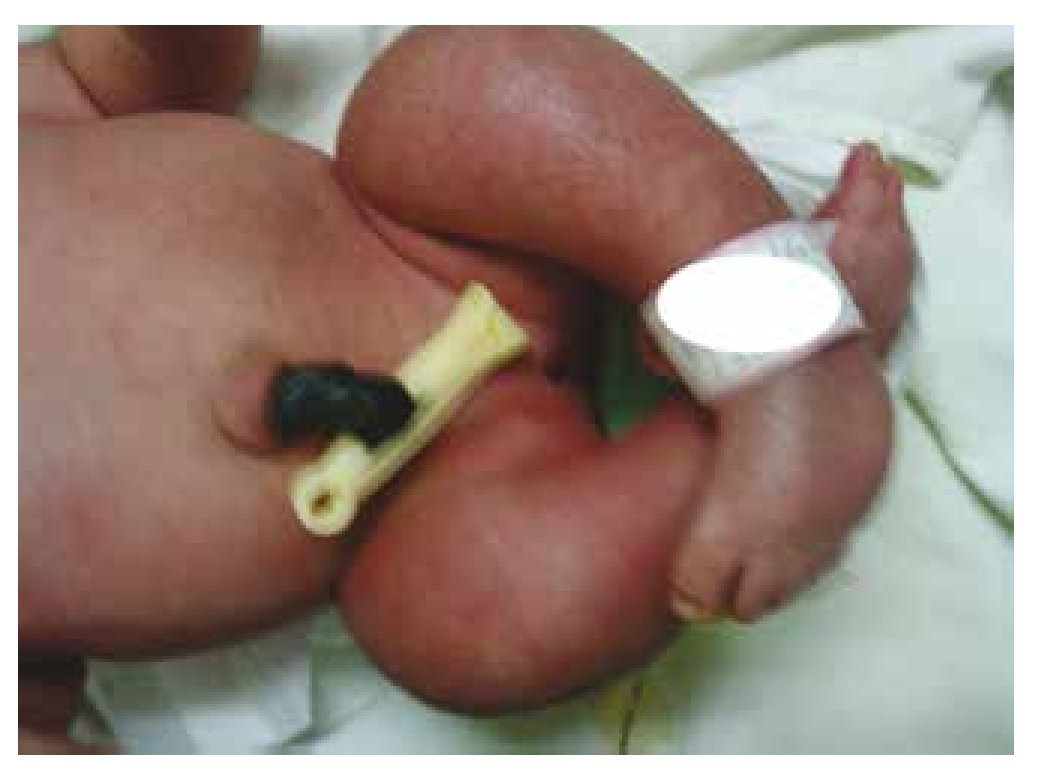
Figure 3 Equinovarus feet.
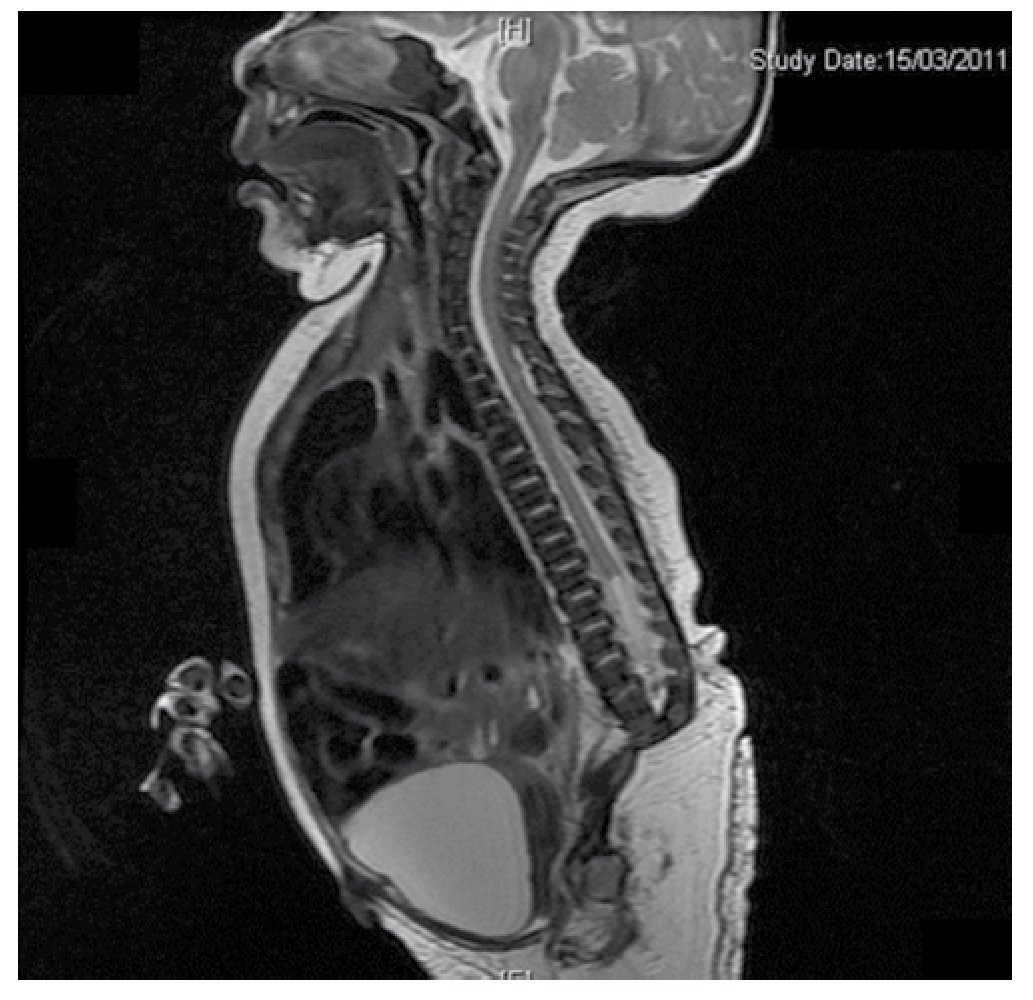
Figure 4 The lumbar spine magnetic resonance imaging (MRI) shows an abrupt chord and lumbar termination at the L3 level. Short spinal cord, flat conus medullaris at T10 level, hypoplastic iliac bones, fused at mid-level. No sacrum is seen.
Discussion
The CRS is a rare congenital malformation. Even though the specific etiologic factor is unknown, it is related to maternal diabetes, genetic predisposition and vascular hypoperfusion.3 Just as in the case presented, this alteration is characterized by agenesis of the sacrum involving iliac and lumbar vertebrae with their corresponding spinal segments and variable abnormalities in the lower limbs as well as in other organs. Fetal diagnosis tools allow early syndrome detection. Prenatal ultrasound is the most frequently used paraclinical instrument; a key element of prenatal ultrasound is the detailed evaluation of the spine and lower limbs; it also allows for a CRS diagnosis through the demonstration of an abrupt termination of the lumbar spine and hypoplastic lower limbs. When making a prenatal diagnosis, we must focus on discerning the degree of digenesis as well as the associated congenital anomalies with the purpose of establishing a prognosis and a timely plan of postnatal therapeutic interventions.3,5 renshav's classification, which was created in 1978, categorizes the syndrome in 4 degrees based on the severity of agenesis of the sacrum, and involvement of iliac and lumbar vertebrae.9 According to this classification, our patient belongs to grade iV (complete agenesis of the sacrum with iliac-bones fusion). This group is associated with an even worse prognosis with a greater neurological impact and multisystemic sequels, mainly at a renal level. This case is a clear example of the wide range of alterations that can affect the growing fetus as a result of uncontrolled maternal diabetes during pregnancy. Because of the high correlation between this defect and the diabetic mother, and its development during the early stages of pregnancy, it is imperative to have a preventative strategy which includes strict glycemic control prior to the embryonic organogenesis period, or even before in high risk patients. Proper guidance and pregestational genetic exams are also important. Treatment is a challenge for the doctor as well as for the parents, and it demands a multidisciplinary approach involving a pediatrician, a pediatric surgeon, an orthopedic surgeon, a physical therapist and an urologist depending on the severity. Given the fact that the primary pathology is irreversible, treatment is just supportive, with the sole purpose of accomplishing a life as normal as possible.
Conflicts of interest
The authors have no conflicts of interest to declare.
Funding
No financial support was provided.
Received: September 2013;
Accepted: January 2014
* Corresponding author:
Service of Pediatric Neurology, Department of Neurology,
"Dr. José Eleuterio González" University Hospital,
Faculty of Medicine, Universidad Autónoma de Nuevo León, Monterrey, N. L., Mexico.
Telephone: (044) 818 016 9944.
E-mail address: kazaamba@hotmail.com (M. A. Duncan).