To validate the Spanish version of the Uterine Fibroid Symptom and Quality of Life (UFS-QoL)questionnaire in women with uterine myomatosis, in order to assess severity of symptoms, and their impact on health-related quality of life.
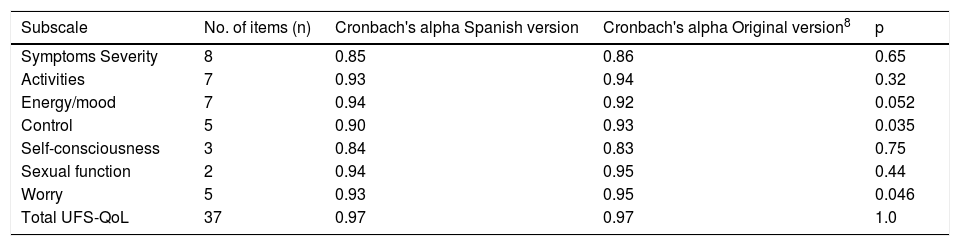
Materials and methodsThe participants were recruited in gynaecology clinics. The UFS-QoL questionnaire comprises 37 items, 8 of which assess severity of symptoms, and the remaining 29 assess health-related quality of life in 6 subscales. Internal consistency, concurrent and discriminant validity, test-retest reliability, and the scale’s sensitivity to change were evaluated.
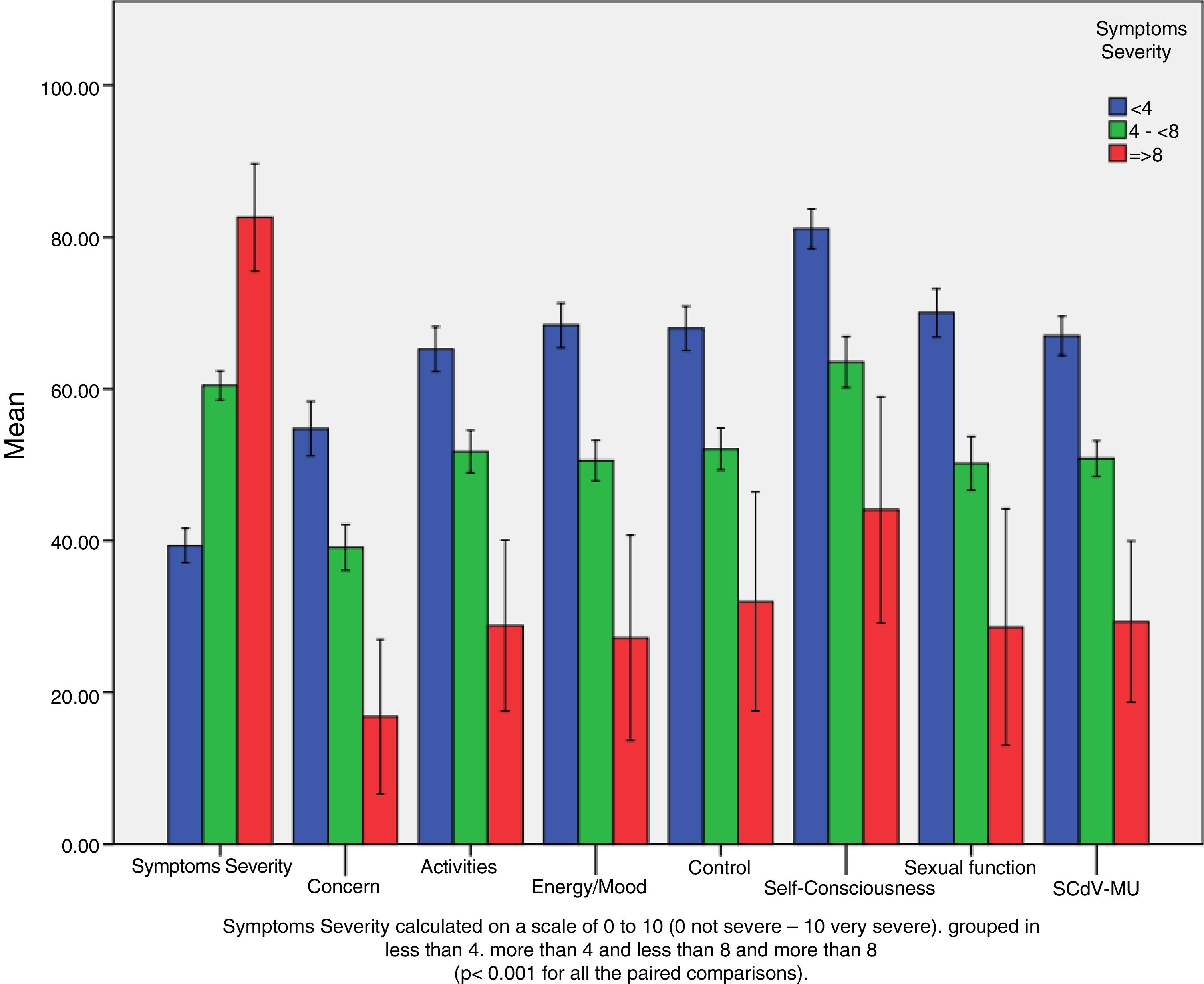
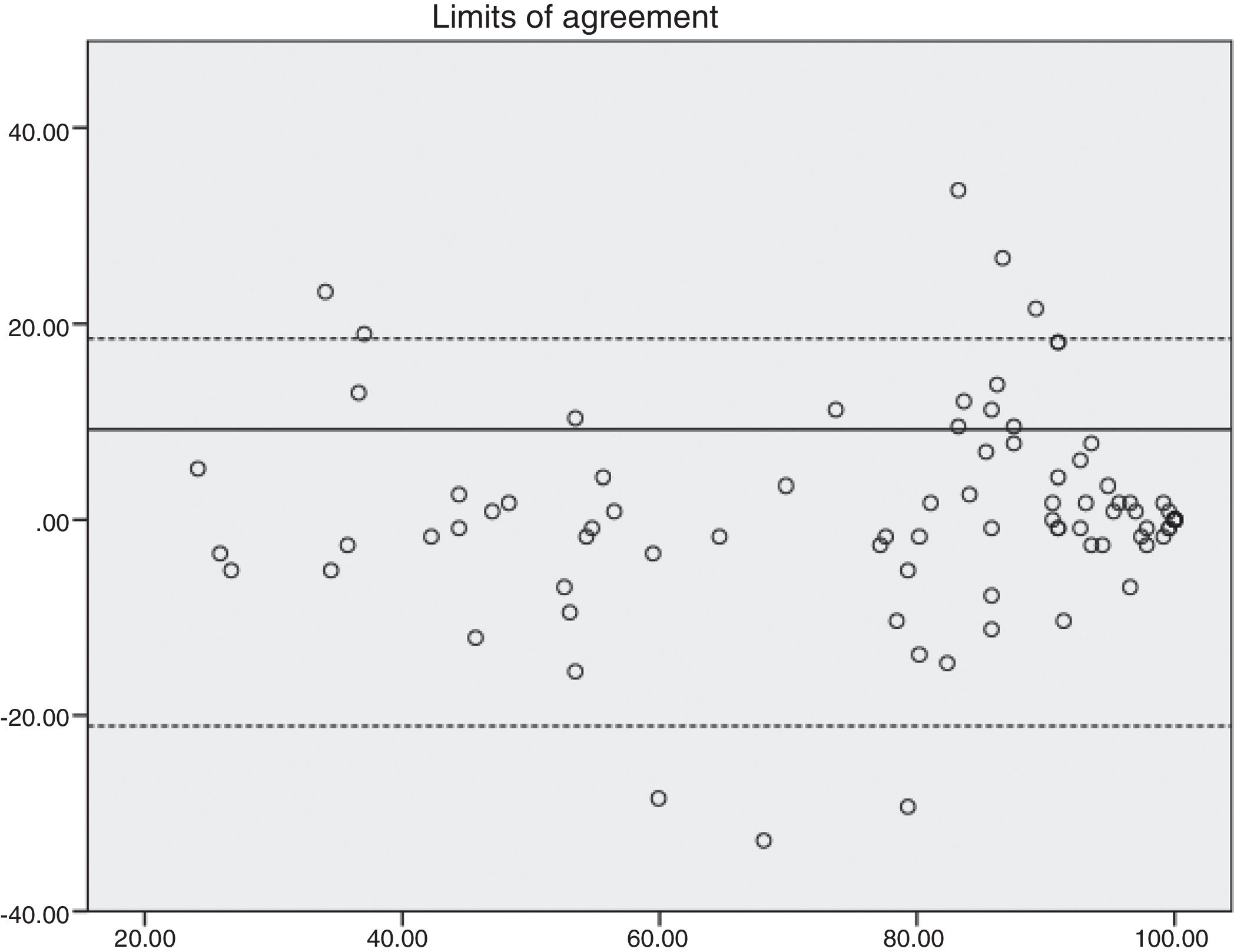
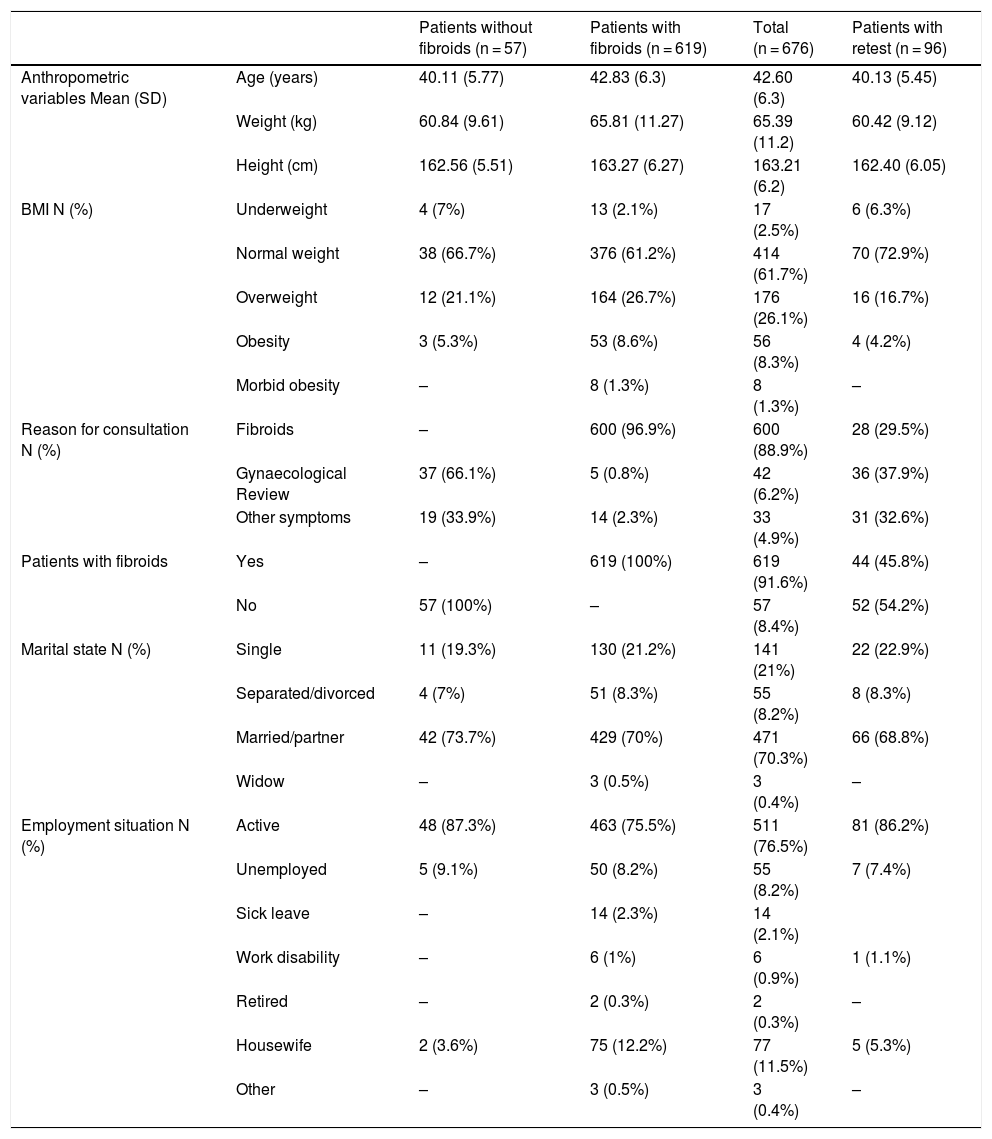
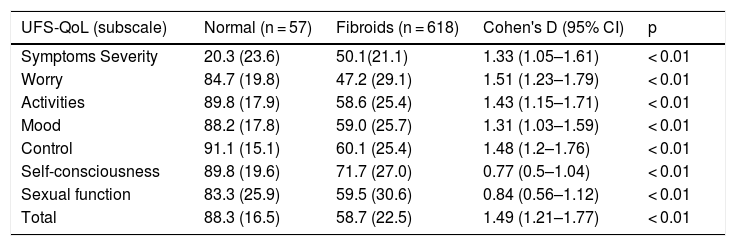
ResultsA total of 619 patients with uterine myomatosis, and 57 women without myomatosis, took part in the study. Cronbach’s alpha was 0.97, and the test-retest reliability was 0.90 for the overall scale. TheUFS-QoL not only discriminated between patients and healthy controls but also between patients with different degrees of uterine myomatosis. The scale responded to changes after treatment with an effect size of 1.2.
ConclusionsThe Spanish version of the UFs-QoL questionnaire, used in a sample of the Spanish population, proved a valid and reliable tool to differentiate patients with uterine myomatosis and different grades of symptoms, and to evaluate the impact of the severity of these symptoms on health-related quality of life. In addition, the UFs-QoL proved sensitive to the changes generated by myomatosis treatment.
Validar la versión española del Síntomas y Calidad de Vida en los Miomas Uterinos (SCdV-MU ) en mujeres con miomatosis uterina para evaluar la gravedad de los síntomas y su impacto en la calidad de vida relacionada con la salud.
Materiales y métodosLas participantes fueron reclutadas en consultas de ginecología. El Cuestionario SCdV-MU consta de 37 ítems, 8 de los cuales evalúan la gravedad de los síntomas, mientras que los 29 restantes evalúan la calidad de vida relacionada con la salud (CVRS) en 6 subescalas. Se determinaron la consistencia interna, la validez concurrente y discriminante, la fiabilidad test-retest y la sensibilidad al cambio de la escala.
ResultadosUn total de 619 pacientes con miomatosis uterina y 57 mujeres sin miomatosis participaron en el estudio. El coeficiente alfa de Cronbach fue de 0.97 y la fiabilidad test-retest de 0.90 para la escala global. El Cuestionario SCdV-MU no solo discriminó entre pacientes y controles normales sino también entre pacientes con distintos grados de miomatosis uterina. La escala respondió a los cambios tras el tratamiento, con un tamaño de efecto de 1.2.
ConclusionesLa versión española del Cuestionario SCdV-MU, administrada en una muestra de la población española, ha demostrado ser una herramienta válida y fiable para diferenciar las pacientes con miomatosis uterina con diferentes grados de síntomas y valorar el impacto de la gravedad de estos síntomas en la calidad de vida relacionada con la salud. Además, el UFS-QoL ha demostrado ser sensible a los cambios generados por el tratamiento de la miomatosis.