To investigate the modulatory effects of different physical exercise modalities on connectivity of amygdala subregions and its association with pain symptoms in patients with knee osteoarthritis (KOA).
Methods140 patients with KOA were randomly allocated either to the Tai Chi, Baduanjin, Stationary cycling, or health education group and conducted a 12 week-long intervention in one of the four groups. The behavioral, magnetic resonance imaging (MRI), and blood data were collected at baseline and the end of the study.
ResultsCompared to the control group, all physical exercise modalities lead to significant increases in Knee Injury and Osteoarthritis Outcome Score (KOOS) pain score (pain relief) and serum Programmed Death-1 (PD-1) levels. Additionally, all physical exercise modalities resulted in decreased resting state functional connectivity (rsFC) of the basolateral amygdala (BA)-temporal pole and BA-medial prefrontal cortex (mPFC). The overlapping BA-temporal pole rsFC observed in both Tai Chi and Baduanjin groups was significantly associated with pain relief, while the BA-mPFC rsFC was significantly associated with PD-1 levels. In addition, we found increased fractional anisotropy (FA) values, a measurement of water diffusion anisotropy of tissue that responded to changes in brain microstructure, within the mind-body exercise groups' BA-temporal pole pathway. The average FA value of this pathway was positively correlated with KOOS pain score at baseline across all subjects.
ConclusionsOur findings suggest that physical exercise has the potential to modulate both functional and anatomical connectivity of the amygdala subregions, indicating a possible shared pathway for various physical exercise modalities.
Osteoarthritis (OA) is a prevalent joint disease and one of the most common symptomatic health conditions with a prevalence ranging from 12.3 % (self-report in the "Disability-Health" 2008 population-based survey in France) to 21.6 % (physician-diagnosed OA in the United States estimated by the 2003–2005 US National Health Interview Survey), and among all types of OA, knee osteoarthritis (KOA) has the highest incidence worldwide (Palazzo et al., 2016). The presence of KOA results in the manifestation of pain, swelling, stiffness, and limitations in joint mobility, thereby contributing significantly to functional impairment and disability. The presence of KOA incurs significant costs due to decreased productivity as well as the healthcare-related expenses such as knee replacement and medication associated with symptom management (Altman, 2010).
Pain is a common symptom among individuals with KOA, which can lead to physical limitations as well as increase the risk of all-cause mortality (Katz et al., 2021). However, traditional pharmacological treatments utilizing nonsteroidal anti-inflammatory drugs are associated with safety concerns and adverse effect (Costa et al., 2021). Prior research indicates that these medications do not halt progressive cartilage degradation nor promote healing within OA patients. Furthermore, long-term usage may cause renal toxicity, gastrointestinal distress, and heightened cardiovascular risk (Crofford, 2013; Hellio Le Graverand-Gastineau, 2010; Wong, 2019). Thus, the approach to treat KOA via pharmacological therapies is not highly recommended by the guidelines of the American College of Rheumatology (Hochberg et al., 2012).
Previous studies have indicated that physical exercise can exert antinociceptive effects by modulating pain perception and pain catastrophizing (Kolasinski et al., 2020). Thus, physical exercise including mind-body practices like Tai Chi, are strongly endorsed by various professional organizations for managing KOA (Katz et al., 2021; Kolasinski et al., 2020). Tai Chi is a popular mind-body exercise, and has exhibited comparable therapeutic effects in alleviating pain symptoms when compared to standard physiotherapy courses (Wang et al., 2016). For instance, Wang et al., found that both Tai Chi and physical therapy groups had improved the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain scores at 12 weeks and the two treatment groups did not significantly differ regarding the reported pain scores at 12 weeks. Furthermore, the authors observed that Tai Chi can lead significantly greater improvements in patient-reported outcomes related to physical function compared with other interventions such as balance training (Wang et al., 2016). Baduanjin, another popular mind-body exercise, has also demonstrated significant improvements as measured by the WOMAC (Ye et al., 2020). One potential explanation for these benefits is related to the biopsychosocial approach employed during mind-body exercises. Mind-body exercise interventions have the potential to enhance physical performance by reducing kinesiophobia and alleviating chronic pain and disability through mitigating other psychosocial factors such as anxiety, depression, and mental stress (Fernández-Rodríguez et al., 2022). Stationary cycling, a single general mode of physical exercise, does not specifically target the trunk muscles. The lack of substantial physiological training stimulus delivered to the trunk muscles during Stationary cycling supports the notion that psychological factors like catastrophizing may be one possible mechanism explaining its potential influence on pain perception (Marshall et al., 2013). Since it is still unclear which physical exercise program is most effective in treating KOA, it is important to explore the effects of different physical exercise regimen (Raposo et al., 2021). This exploration will aid in selecting a more appropriate intervention for patients and improving treatment fidelity.
Furthermore, previous studies have reported that different types and intensities of physical exercise elicit varying effects on pain ratings and pain-related brain activation patterns. The study conducted by Scheef demonstrated that walking, as opposed to running, resulted in a reduction of affective pain ratings. Exercise's impact on pain-related activation was observed in the periaqueductal gray, with similar trends seen in the pregenual anterior cingulate cortex and middle insular cortex after walking, but decreased or unchanged activity following running (Scheef et al., 2012). Schmitt and colleagues also reported that in trained male participants (exercising at least 3 times / week for 45 min for the past two years, aged 27.1 ± 4.0 years) low-intensity endurance exercise bouts led to increased resting-state functional connectivity (rs-FC) within brain networks associated with cognitive and attentional processing, while high-intensity endurance exercise increased rs-FC within affective brain networks and decreased rs-FC within the sensorimotor network (Schmitt et al., 2019).
Additionally, emotion regulation may be an important yet understudied risk factor for pain syndromes, pain-related disability, and psychological comorbidities of chronic pain (Koechlin et al., 2018). A previous study suggested that the amygdala, a complex almond-shaped structure located deep within the medial temporal lobe, is involved in modulating emotions such as anxiety, depression, reward processing, and pain perception (Allen et al., 2021). Pain is a common neurological disorder involving both sensory nociception and negative emotional components including anxiety and depression (Ge et al., 2022). The role of the amygdala regarding pain was initially proposed upon discovering a dedicated nociceptive pathway from the spinal cord to its central nucleus. The limbic amygdala plays a significant role in associating pain with emotions through converging inputs from nociceptive spinothalamic pathways and affective/cognitive corticolimbic pathways (Thompson & Neugebauer, 2017).
Interestingly, both mind-body exercises and physical exercises can alter amygdala-related brain networks, however, they do so differently. A neuroimaging study utilizing resting-state functional magnetic resonance imaging (fMRI) to investigate the effects of Tai Chi on brain connectivity in KOA patients revealed an increase in amygdala-medial prefrontal cortex connectivity after 8 weeks of intervention (Shen et al., 2022). Wan et al. reported that Baduanjin improves physical frailty in older adults with cognitive frailty, which may be associated with an increase in volume of the hippocampal-amygdala transition area (Wan et al., 2022). Additionally, a previous study suggests that voluntary running may contribute to fear extinction by deactivating gamma aminobutyric acid neurons in the central nucleus of the amygdala subdivisions and inducing plastic changes in amygdala subregions, leading to exercise-induced hypoalgesia in neuropathic pain model mice (Kami et al., 2020).
Based on cytoarchitectonic studies, the amygdala can be segmented into three main groups: the superficial amygdala (SFA), which is adjacent to the later basal group and involved with olfaction; the basolateral amygdala (BA), which is most involved in associative learning processes; and the centromedial amygdala (CMA), which plays a key role in generating behavioral responses (Beyeler et al., 2018; Wang et al., 2023). It has been reported that nociceptive pathways involve all three subregions of the amygdala. Nociceptive information inputs from spinal cord project to central nucleus through spino-parabrachio-amygdaloid pain pathway (Raver et al., 2020), while pain signals are also input from thalamus and cortex targeting lateral nuclei of amygdala. Moreover, basolateral amygdala projects prefrontal cortical areas suggesting its contribution towards cognitive aspects for modulation including memories and expectations for pain (Cardenas et al., 2019) .
Over the past three decades, extensive research has been conducted on the involvement of various brain regions in pain experienced by arthritis patients, facilitated by advancements in functional and structural neuroimaging techniques. fMRI is employed for short-term evoked pain examination and treatment evaluation, while morphological brain assessment provides evidence for long-term structural changes associated with chronic pain (Harvey et al., 2012). Notably, white matter alterations may play a role in processing the affective component of pain among older adults without dementia (Oosterman et al., 2006). Anatomically, white matter establishes both intra-cortical and intra-subcortical connections as well as interconnections between cortical and subcortical regions, thereby playing a crucial role in frontal functioning that can be integrated into the pain system. For instance, white matter may contribute to pain inhibitory processes mediated by the prefrontal cortex (Oosterman et al., 2006).
Furthermore, Stillman et al. proposed that physical exercise-induced effects on a specific outcome may be mediated by different levels of analysis ranging from cellular and molecular level (level 1) to brain systems and behavioral levels (levels 2 and 3) (Stillman et al., 2016). It is plausible that changes in cellular and molecular pathways induced by physical exercise initiate macroscopic brain (level 2) and behavioral (level 3) modifications influencing overall behavioral function. Discussing mechanisms across multiple levels of analysis may stimulate comprehensive exploration of systems leading to a better understanding of how physical exercise influences KOA pain symptoms.
Immune responses along with inflammatory processes have been identified as pivotal components contributing to KOA pathogenesis development progression (Klinedinst et al., 2022; Lopes et al., 2017; Ruan et al., 2021; Simão et al., 2014; Yang et al., 2021), which could potentially serve as therapeutic targets for treating KOA (Li et al., 2016). The study demonstrated elevated levels of the proinflammatory mediator IFN-γ in the synovial fluid and / or peripheral blood of patients with OA compared to control (Drvar et al., 2022; Woodell‐May & Sommerfeld, 2020). Increased levels of IFN-γ mRNA and protein expression were observed in T-cell and NK-cell subsets known to enhance the immune response (Gotthardt et al., 2019; Griffin & Scanzello, 2019). It is well established that Programmed Death Receptor-1 (PD-1) molecules are expressed on follicular helper T cells' surface, serving as negative regulators for their activity. A significantly higher percentage of PD-1+CXCR5+CD4+ cells was identified in OA patients compared to healthy controls (Shan et al., 2017) which suggests that PD-1-expressing follicular helper T cells may act as negative regulators for both the number and functionality of these cells, thereby minimizing collateral damage caused by the immune response. Physical exercise mobilizes PD-1+ CD8+ lymphocytes, leading to an increase in circulating PD-L1 levels which represents one aspect of the anti-inflammatory response to exercise (Wadley et al., 2020). T cell immunoglobulin domain and mucin domain 3 (TIM-3), a member of the immunoglobulin superfamily, plays a critical role in downregulating T cell responses and is associated with increased susceptibility to KOA (Chen et al., 2015). Brain-derived neurotrophic factor (BDNF), a member of the neurotrophic growth factor family, is also involved in inflammatory cytokines and inflammatory responses (Lai et al., 2021). Joint cells can produce BDNF induced by inflammation (Gowler et al., 2020). BDNF exerts pro- and / or anti-inflammatory effects partly through modulation of cytokines related to inflammation (Fernandes et al., 2013; Klein et al., 2012; Papathanassoglou et al., 2015). Previous studies have found elevated plasma BDNF levels in KOA patients which positively correlate with self-reported pain (Simão et al., 2014). Converging lines of evidence suggest the involvement of BDNF in regulating physical exercise-induced changes in inflammatory status (Papathanassoglou et al., 2015; Puts et al., 2023).
In summary, this study aims to investigate how different physical exercise modalities modulate connectivity of amygdala subregions, levels of immune and inflammatory serum biomarkers and their association with clinical outcomes. We hypothesize that compared to the health education control group, all three intervention groups will be associated with a reduced level of knee pain which will be accompanied by changes in specific subregions of the amygdala pathway and alterations in serum immune and inflammatory markers.
Methods and patientsThe current study was registered with the Clinical Trial Registry under the identifier ChiCTR-IOR-16,009,308. A detailed account of this investigation has been previously documented in our previous studies (J. Liu, Chen, Chen et al., 2019a, 2019b).
Prior to registering the clinical trial, we conducted a sample size calculation using G-power software (http://www.gpower.hhu.de/) to investigate the effects of different intervention types on pain relief. We performed an F test with four intervention groups, setting a statistical power of 0.95, effect size of 0.4 and significance level at 0.05 (two-sided), resulting in a recommended sample size of 112 participants. To account for potential loss to follow-up, we included 140 participants (35 per group as registered) in our study.
A total of 227 older adults were screened, and 140 individuals meeting the following criteria were enrolled in Fuzhou City, Fujian, China: 1. aged between 40 and 70 years old, right-handedness, diagnosed with KOA, body mass index (BMI) ≤ 30 Kg/2; 2. Brief Pain Inventory (BPI) scale score greater than 2; 3. Kellgren-Lawrence Scale confirmed grade 2 or 3 KOA through X-ray examination (Holzer et al., 2015), and Beck Depression Inventory-II (BDI-II) score < 14 as consistent with our previous studies (Chen et al., 2014; J. Liu et al., 2019a). The diagnosis of KOA was made by a rheumatologist at the Affiliated Rehabilitation Hospital of Fujian University of Traditional Chinese Medicine, and written informed consent was obtained from all participants. The present study (ethical approval number: 2015KY-017–01) received approval from the Medical Ethics Committee of the hospital.
Exclusion criteria included recent knee surgery within six months or corticosteroid injection three months; knee pain attributed to rheumatic or inflammatory diseases; primary or secondary muscle disease (e.g., certain muscular dystrophies, the aging-induced loss of muscle mass and strength, et.al.,); mental disorders (e.g., obsessive-compulsive disorder, affective disorders, schizophrenia/psychosis, et.al.,); MRI contraindications such as dentures, porcelain teeth, and pacemakers; and bleeding disorders.
Participants were randomly assigned to one of four groups (Tai Chi group, Baduanjin group, Stationary cycling group, healthy education control group) using the IBM SPSS Statistics software (https://www.ibm.com/support/pages/how-cite-ibm-spss-statistics-or-earlier-versions -SPSS, IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.), with 35 individuals in each group. Due to the non-pharmacological nature of this intervention trial, blinding participants and exercise coaches was not feasible. Therefore, two types of blind codes were used for outcome assessors and data statisticians instead. An independent research manager oversaw random allocation sequence and assignment of blind codes. After completion of data analysis, the true meaning behind each code will be revealed (Zheng et al., 2016). Demographic data was collected at the onset of the study, while clinical outcomes/serum biomarkers and MRI/functional MRI/Diffusion tensor imaging (DTI) data were obtained both at baseline and after completion of the program.
The observation period for KOA patients in this study lasted for 12 weeks, during which they underwent five days of physical training once a week (from the Monday to the Friday on consecutive days, consisting of a 10 -minutes warm-up, 30 min- of physical exercise, 10 min of breathing techniques, and 10 min of relaxation in Tai Chi and Baduanjin group; as well as five-minute breaks depending on subjects' conditions during Stationary cycling training), or one session per week focused on health education (also lasting for 60 min). Three staff members monitored the physical training sessions, which were led by professional instructors with over five years’ teaching experience.
The Tai Chi group participants received training in the "eight section Tai Chi", which is a modified version of the Yang-style 24-form Tai Chi. The Baduanjin exercise consists of ten postures and follows the "Health Qigong Baduanjin Standard" established by the State Sports General Administration in 2003 (including preparation and concluding postures) Health Qigong Management Center of General Administration of Sport of China, 2003). The Stationary cycling training was conducted under the guidance of rehabilitation professionals. Participants in the healthy education control group received weekly sessions of basic health education, including fundamental knowledge on managing KOA and other relevant health information, for a duration of 12 weeks (60 min per session). Additionally, subjects were instructed to maintain their existing physical activity routines.
The program was individualized for each participant by estimating the individual exercise intensity which was operationalized via heart rate and set to 70–75 % of their target exercise heart rates (Salacinski, 2012). To determine the maximum heart rate, a specific formula was used: HRmax=(220-age) ± 15 % (Fox et al., 1971). Heart rate monitors (Mio Sport SD, America) were provided to ensure adherence to the exercise guidelines. In order to reduce knee stress in the Tai Chi and Baduanjin groups, all 90° knee-flexor joint stances were modified to exceed 90°. Additionally, participants were instructed to maintain their usual physical activity habits throughout the study (Wang et al., 2014).
Outcome measuresThe primary clinical outcome in the present study is the pain subscore of Knee injury and Osteoarthritis Outcome Scores (KOOS) (Roos & Toksvig-Larsen, 2003). Furthermore, we utilized KOOS symptoms, KOOS daily living, KOOS sport, and KOOS quality of life as our secondary outcome measures.
Serum BDNF, IFN-γ, PD-1, and Tim3 levels analysisIn this study, to investigate the correlation between the immune response and pain symptom change of KOA, the serum BDNF, IFN-γ, PD-1, and Tim3 levels were measured using Enzyme-Linked Immunosorbent Assay (ELISA) (ELISA kits from the Huamei biological engineering co. LTD, Wuhan, China) according to the manufacturer's instructions and previous studies (Hui et al., 2016; Yanaba et al., 2016). The participants were asked to fast for 12 h before the blood samples were drawn which is necessary to ensure the precision and reliability of the measurements obtained from their blood samples. To minimize potential variations caused by circadian rhythms, the blood collection process was conducted in the morning. This meticulous attention to detail guaranteed that our results would not be influenced by natural fluctuations in these biomarkers throughout the day. Once collected, the blood samples (3 ml) were meticulously handled and processed. They were allowed to clot at room temperature for 15 min before being centrifuged at 3000 × g for 10 min. This step effectively separated the serum from other components of blood such as red and white blood cells. Subsequently, the resulting serum was promptly stored at −80 °C to maintain its stability until further analysis. By preserving it at this low temperature, we could prevent degradation or alteration of these crucial biomarkers over time. Upon completion of our study or intervention programs/treatments administered on participants, we once again employed ELISA kits according to manufacturer's instructions to measure BDNF, IFN-γ, PD-1, and TIM-3 levels in each sample. These measurements provided valuable insights into immune function and neurotrophic factors associated with various physiological processes. To accurately quantify these biomarkers during analysis, an ELISA reader (Bio Tek Model ELX800) was utilized. By measuring absorbance at a wavelength of 450 nm, we could reliably determine both standard and test sample concentrations. Overall, this standardized protocol ensured precise measurement of serum BDNF, IFN-γ, PD-1, and TIM-3 levels before and after administration of intervention programs.
MRI data acquisitionThe MRI data were acquired using a 3.0T magnetic resonance scanner (General Electric, Milwaukee, WI, USA) equipped with an eight-channel phased-array head coil at two separate time points - the beginning and end of the study. The participants were instructed to keep their eyes closed and remain motionless throughout the scanning session.
For resting state functional MRI, the following parameters were used: repetition time (TR) = 2100 ms, echo time (TE) =30 ms, flip angle = 90°, voxel size = 3.125 mm × 3.125 mm × 3.6 mm, acquisition of 42 axial slices with a field of view (FOV) of 200 mm × 200 mm and phase encoding steps of 230. T1-weighted images were collected using the following parameters: flip angle=15°, slice thickness=1 mm, FOV=240 mm, and acquisition of 160 slices. Diffusion-weighted images were acquired using echo planar imaging (EPI) with a total of 32 diffusion directions (0 s/mm2 b-factors and 1000s/mm2). The acquisition parameters for diffusion-weighted imaging included TR=14,000 ms, TE=87.3 ms, an acquisition matrix size of 256 × 256, and voxel size of 0.9 × 0.9 × 1 mm.
Data analysisThe analysis of clinical dataClinical outcomes were analyzed using IBM SPSS Statistics software https://www.ibm.com/support/pages/how-cite-ibm-spss-statistics-or-earlier-versions-spss. The data were tested for normality using the Shapiro-Wilk test. Two-way repeated measures ANOVA was employed to compare the effects between the groups and different time points of the exercise mode regarding KOOS scores and serum indicator levels if the data were normally distributed (each group in pre or post intervention). If any group in pre or post intervention was not normally distributed data, the ANCOVA group analysis was performed to test the group effects (the changes between post- and pre-intervention as dependent variable and the changes data should be normally distributed, changes=post-intervention minus pre intervention) with age (years) and gender as covariates. When there were significant group and time interactions (Two-way repeated measures ANOVA) or group effects (ANCOVA group analysis) for above indexes, ANCOVA analysis was performed to compare between group changes difference with age (years) and gender as covariates. Baseline characteristics of the subjects among groups were compared using one-way ANOVA and Chi-square tests. Nonparametric Kruskal–Walli's test was utilized to compare group differences for non-normally distributed data (i.e., the changes or the baseline characteristics is non- normally distributed data). The statistical significance was determined at a two-sided test with a significance level of P < 0.05. Effect sizes were classified as small (0.2–0.5), medium (0.5–0.8), or large (>0.8) (Cohen, 2013).
Seed to voxel functional connectivity analysisThe seed-to-voxel correlational analyses were conducted using the CONN toolbox v17 in MATLAB, following a similar approach as described in our previous studies (http://www.nitrc.org/projects/conn) (Egorova et al., 2015; Liu et al., 2016; Ni et al., 2016; Song et al., 2017). Three amygdala subregions (left and right separately) were selected as regions of interest. These subregions included the centromere subdivision (CMA; left: 56 mm3, right: 47 mm3), basolateral subdivision (BA; left: 196 mm3, right: 218 mm3), and superficial subdivision (SFA; Left: 111 mm3, right: 113 mm3) based on the stereotaxic, probabilistic maps of cytoarchitectonic boundaries (Amunts et al., 2005; Roy et al., 2009). The amygdala subregion ROIs used in this study were extracted using FSL's Juelich histological atlas.
The functional images underwent preprocessing steps, including slice-time correction, realignment, coregistration with the subjects' respective structural images, normalization, and smoothing using a 6 mm full-width half-maximum kernel (FWHM). Temporal confounding factors were removed by segmenting gray matter, white matter, and cerebrospinal fluid (CSF) regions. Band-pass filtering was applied within a frequency window of 0.01–0.089 Hz to extract relevant signals. To eliminate correlations caused by head motion and artifacts, we identified outlier time points in the motion parameters and global signal intensity using ART (https://www.nitrc.org/projects/artifact_detect). Images were considered outliers if the composite movement from a preceding image exceeded 0.5 mm or if the global mean intensity deviated more than three standard deviations from the mean image intensity. The temporal time series of head motion matrix outliers were also entered as first-level covariates to control for potential confounds related to head motion. Seed-to-voxel connectivity analyses were performed by extracting BOLD time course from each seed and computing Pearson's correlation coefficients between that time course and all other voxels in the brain after Fisher transformation into "Z" scores for analysis purposes. ANOVA was used to explore group differences among four groups while pair T-tests were used to examine within-group effects. The main effects and between-group effects analyses were adjusted for age and gender as covariates, ensuring the statistical control of these variables.
For the analysis of exercise main effect, a voxel-wise threshold of P < 0.005 uncorrected with a minimum cluster size of 15 voxels was applied. In within- and between-group difference data analysis, a voxel-wise threshold of P < 0.005 uncorrected with cluster-level correction for false discovery rate (FDR) at P < 0.05 was utilized.
The data processing of DTIThe DTI data processing was performed using programs from the FMRIB Software Library (FSL) version 5.10 (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki). The processing of DTI data involved a series of steps, including format conversion, eddy correction to address distortions and motion artifacts in the dataset, tissue removal for enhanced registration accuracy, diffusion tensor estimation using the least square dispersion method to calculate FA values, spatial registration employing indirect linear registration techniques, and deterministic tracking. Deterministic fiber tracking was employed to acquire comprehensive images of the entire bundle of white matter fibers in the subjects.
To investigate the contribution of white matter changes, specifically focusing on the FA value, to pain relief following exercise training in patients with KOA, we defined the BA as the seed region using a mask extracted based on FSL's Juelich histological atlas for both left and right hemispheres. Additionally, we identified the temporal pole as our target brain region utilizing AAL brain atlases for both left and right hemispheres. An exclusion mask was also created in AAL brain atlases to isolate only direct pathways from BA to temporal pole. Multiple regression analysis adjusted for age and gender at baseline across all subjects was employed to examine the association between mean FA values of the BA-temporal pole pathway and KOOS pain scores.
Voxel-wise statistical analysis of FA was performed using permutation-based inference for nonparametric statistical thresholding (5000 permutations) with FSL's "randomize" tool, incorporating threshold-free cluster enhancement (TFCE), and two-sample t-tests with age and gender as covariates. Regions of interest were defined by the BA-temporal pole pathway that was tracked. Multiple comparisons were corrected using a false discovery rate (FDR) correction at a significance level of p < 0.05 with a minimum cluster size of 40 contiguous voxels.
Correlations analysis between brain and behaviorTo investigate the association between changes in rsFC among the three physical exercise groups, KOOS pain scores, and serum biomarker levels (specifically PD-1 and IFN-γ, two significant biomarkers that exhibited changes following the physical training; please refer to the results section), we computed the average z-values of significantly overlapping rsFC clusters (including the temporal pole, medial prefrontal cortex [mPFC], right dorsolateral prefrontal cortex [DLPFC], bilateral mPFC/left anterior cingulate cortex [ACC], and left middle frontal gyrus) in each group. Subsequently, a multiple regression analysis was conducted with age and gender as covariates. Additionally, we performed a correlation analysis between the average FA value in the BA-temporal pole pathway and its corresponding KOOS pain score while controlling for age and gender.
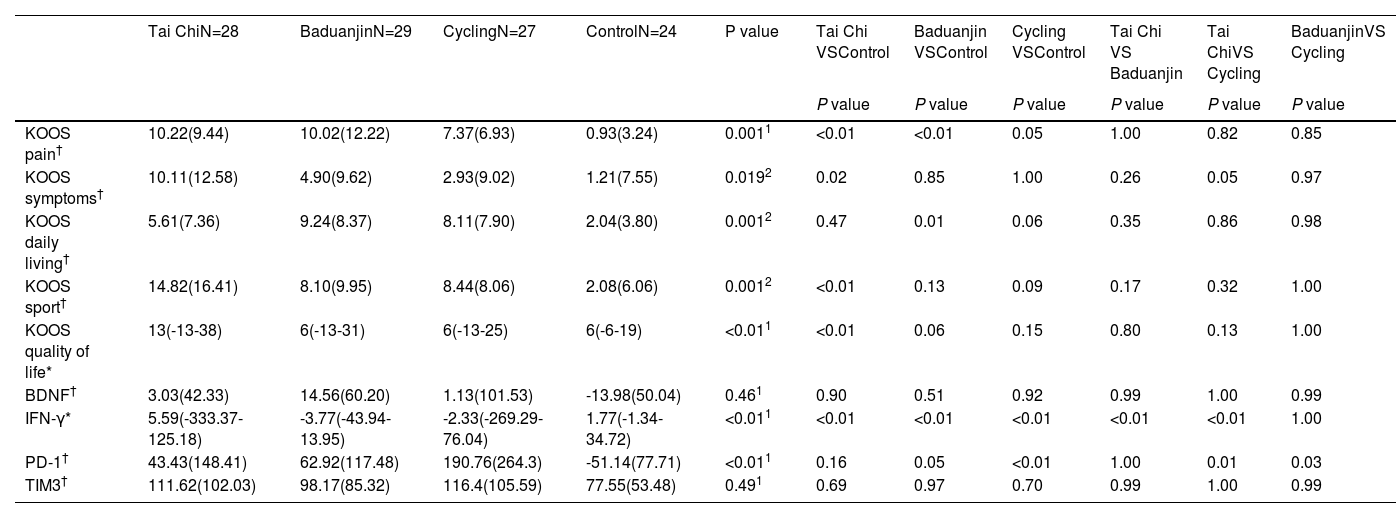
ResultsResults of demographic and clinical data analysisIn relation to the primary clinical outcome, a statistically significant difference was found among the four groups (P < 0.01) in the pain subscore change of KOOS. Subsequent post-hoc analysis revealed that Tai Chi, Baduanjin, and Stationary cycling groups exhibited significantly greater improvements in KOOS pain scores compared to the control group (i.e., pain relief, Table 1). Further exploratory analysis revealed significant differences in various subscores of KOOS, including symptoms, daily living, sport, and quality of life (P < 0.05), as well as changes in serum levels of IFN-γ (P < 0.01) and PD-1 (P < 0.01) (Table 1). No statistically significant group difference was observed among the groups for the terms of demographic and baseline clinical characteristics (Supplementary Table 1 and Supplementary Table 2). Results of the normal distribution test for each group regarding baseline or post-intervention or changes were shown in Supplementary Table 3/4/5.
Comparison of KOOS sub-scores and serum biomarker changes (post minus pre-treatment) among the four groups.
| Tai ChiN=28 | BaduanjinN=29 | CyclingN=27 | ControlN=24 | P value | Tai Chi VSControl | Baduanjin VSControl | Cycling VSControl | Tai Chi VS Baduanjin | Tai ChiVS Cycling | BaduanjinVS Cycling | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P value | P value | P value | P value | P value | P value | ||||||
| KOOS pain† | 10.22(9.44) | 10.02(12.22) | 7.37(6.93) | 0.93(3.24) | 0.0011 | <0.01 | <0.01 | 0.05 | 1.00 | 0.82 | 0.85 |
| KOOS symptoms† | 10.11(12.58) | 4.90(9.62) | 2.93(9.02) | 1.21(7.55) | 0.0192 | 0.02 | 0.85 | 1.00 | 0.26 | 0.05 | 0.97 |
| KOOS daily living† | 5.61(7.36) | 9.24(8.37) | 8.11(7.90) | 2.04(3.80) | 0.0012 | 0.47 | 0.01 | 0.06 | 0.35 | 0.86 | 0.98 |
| KOOS sport† | 14.82(16.41) | 8.10(9.95) | 8.44(8.06) | 2.08(6.06) | 0.0012 | <0.01 | 0.13 | 0.09 | 0.17 | 0.32 | 1.00 |
| KOOS quality of life* | 13(-13-38) | 6(-13-31) | 6(-13-25) | 6(-6-19) | <0.011 | <0.01 | 0.06 | 0.15 | 0.80 | 0.13 | 1.00 |
| BDNF† | 3.03(42.33) | 14.56(60.20) | 1.13(101.53) | -13.98(50.04) | 0.461 | 0.90 | 0.51 | 0.92 | 0.99 | 1.00 | 0.99 |
| IFN-γ* | 5.59(-333.37-125.18) | -3.77(-43.94-13.95) | -2.33(-269.29-76.04) | 1.77(-1.34-34.72) | <0.011 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 1.00 |
| PD-1† | 43.43(148.41) | 62.92(117.48) | 190.76(264.3) | -51.14(77.71) | <0.011 | 0.16 | 0.05 | <0.01 | 1.00 | 0.01 | 0.03 |
| TIM3† | 111.62(102.03) | 98.17(85.32) | 116.4(105.59) | 77.55(53.48) | 0.491 | 0.69 | 0.97 | 0.70 | 0.99 | 1.00 | 0.99 |
We used mean (SD) if the measurements were normally distributed (†), median (min-max) if the measurements were not normally distributed (*).
Six average head movement parameters were extracted using CONN software, and a one-way ANOVA was performed. The results showed no statistically significant differences among the four groups (P = 0.86). Additionally, Supplementary Table 6 presents the exercise-induced main effects, and Supplementary Table 7 displays within-group effects on amygdala subfields as seed regions.
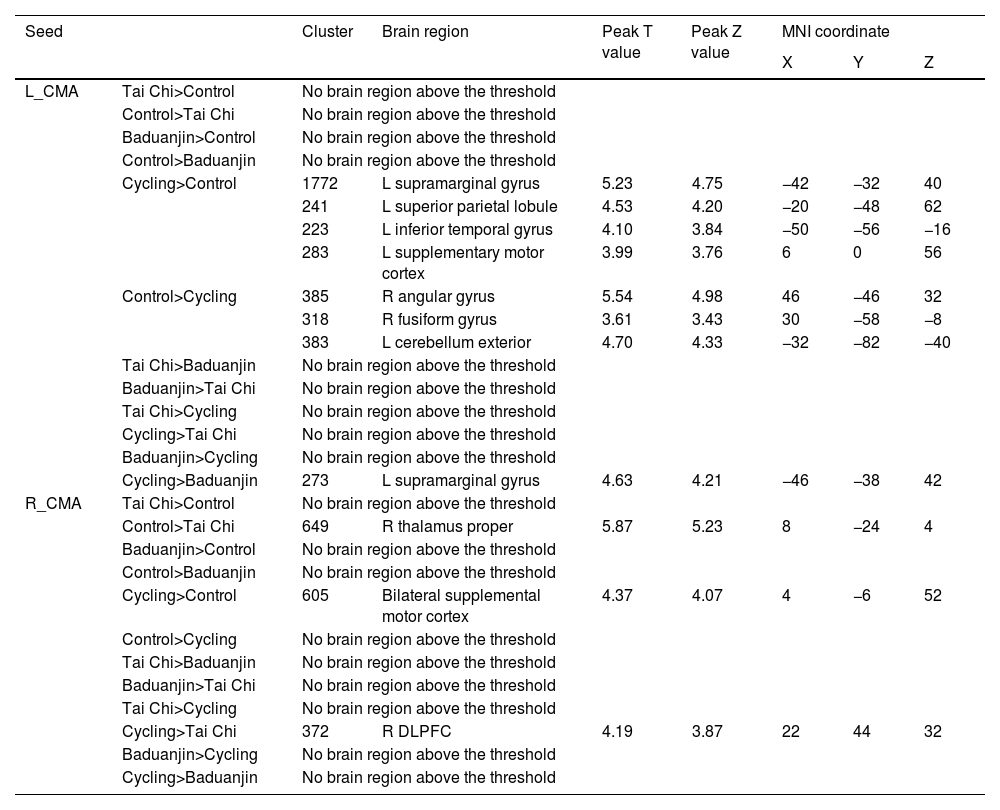
Between-group affects results on rsFCFunctional connectivity using CMA as seedsUsing the left CMA as a seed, we observed significantly increased rsFC between the cycling group and the control group with the left supramarginal gyrus, left superior parietal lobule, and left supplementary gyrus. Conversely, decreased rsFC was found with the right angular gyrus/fusiform gyrus and left cerebellum exterior in the cycling group compared to the control group. There was significantly increased rsFC between the left CMA and the left supramarginal gyrus in cycling group when compared to Baduanjin group.
When using the right CMA as a seed, we discovered that compared to control group, there was a significant decrease rsFC within right thalamus proper in Tai Chi group while an increase of bilateral SMA was noted in cycling group. Furthermore, when comparing with Tai Chi group, there existed significantly increased rsFC between right CMA and right DLPFC in cycling group (Table 2).
The rsFC results using CMA as seed.
L: left; R: right; mPFC: medial prefrontal gyrus; CMA: centro-medial amygdala.
The comparison is between post minus pre intervention across different groups.
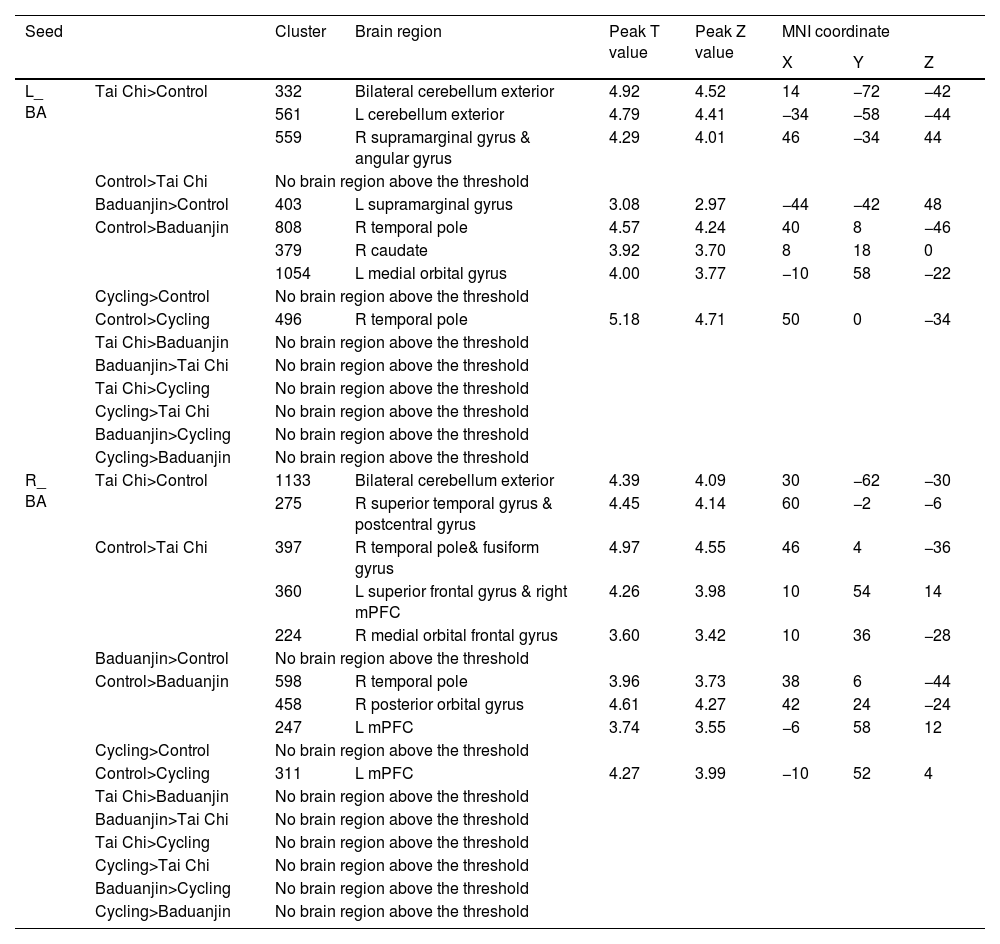
Using the left BA as a seed, we observed significantly increased rsFC in the bilateral cerebellum exterior and right supramarginal/angular gyrus in the Tai Chi group compared to the control group. Additionally, there was increased rsFC in the left supramarginal gyrus in the Baduanjin group. Conversely, decreased rsFC was found in the right temporal pole, right caudate, and left medial orbital gyrus in the Baduanjin group, as well as reduced rsFC in the right temporal pole gyrus in the cycling group (Table 3).
The rsFC results using BA as seed.
L: left; R: right; mPFC: medial prefrontal gyrus; BA: basolateral amygdala;.
The comparison is between post minus pre intervention across different groups.
Utilizing the right BA as a seed region for analysis revealed a significant augmentation of rsFC within both bilateral cerebellar exterior regions and within the right supramarginal/angular gyrus among participants practicing Tai Chi when compared to controls. Furthermore, enhanced rsFC was observed specifically within the left supramarginal gyrus among individuals engaged in Baduanjin exercises. In contrast, diminished rsFC was evident within various brain areas including: right temporal pole; right caudate; and left medial orbital gyrus for those practicing Baduanjin exercises; similarly reduced rsFC was noted within only right temporal pole gyrus for individuals involved in cycling activities (Table 3).
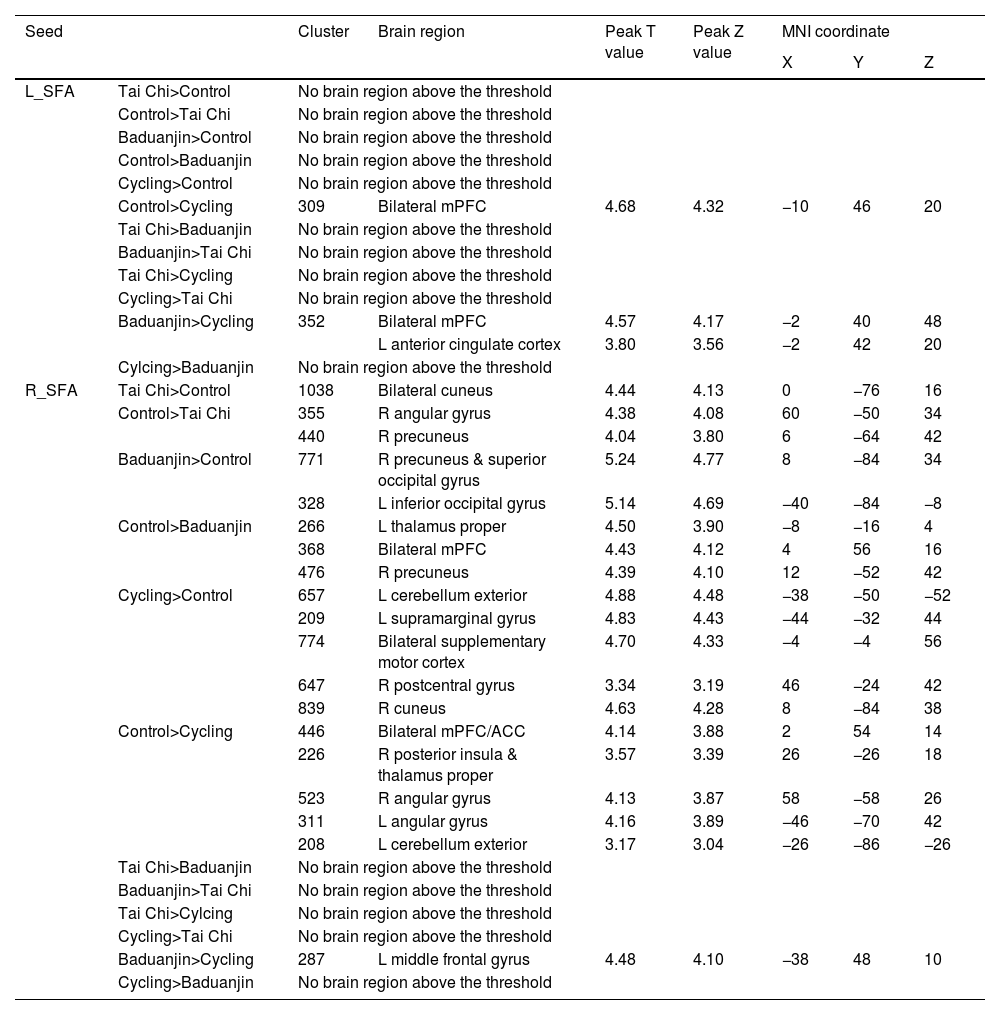
Functional connectivity using SFA as seedsUtilizing the left SFA as a seed, our findings indicate that the cycling group exhibits significantly decreased rsFC in both bilateral mPFC regions compared to the control group and decreased rsFC in both bilateral mPFC regions and left ACC compared to the Baduanjin group (Table 4).
The rsFC results using SFA as seed.
L: left; R: right; mPFC: medial prefrontal cortex; ACC: anterior cingulate cortex; SFA: superficial amygdala;.
The comparison is between post minus pre intervention across different groups.
When using the right SFA as a seed, we observed significantly increased rsFC in both bilateral cuneus regions and decreased rsFC in right angular gyrus and right precuneus areas within the Tai Chi group when compared to controls. The Baduanjin group displayed significant increases of rsFC in right precuneus, right superior occipital gyrus, left inferior occipital gyrus, cerebellum exterior, left supramarginal gyrus, bilateral SMA, and right postcentral gyrus while exhibiting decreases of rsFC in left thalamus proper, bilateral mPFCs and right precuneus when contrasted with controls. In comparison with the participants in the cycling group, those practicing Baduanjin demonstrated significantly increased rsFC within the left middle frontal gyrus (Table 4).
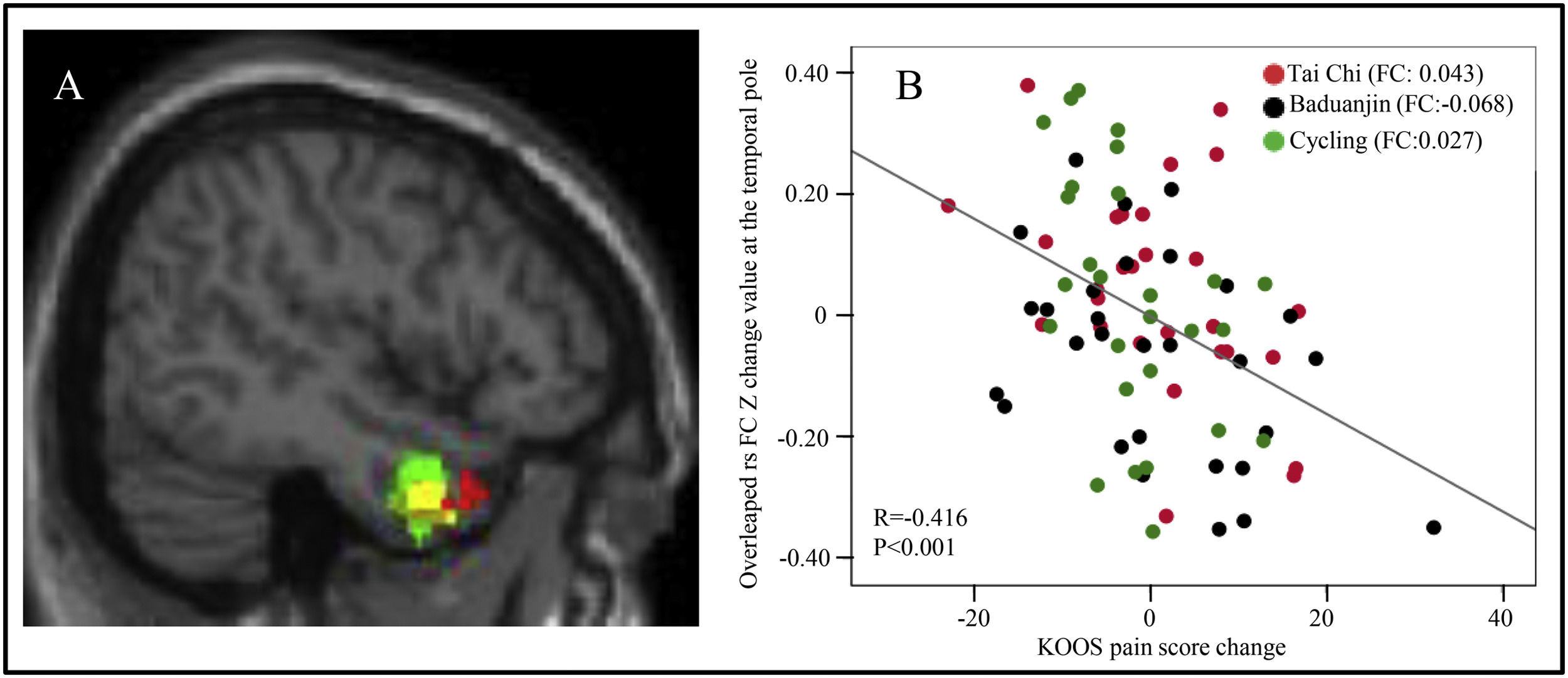
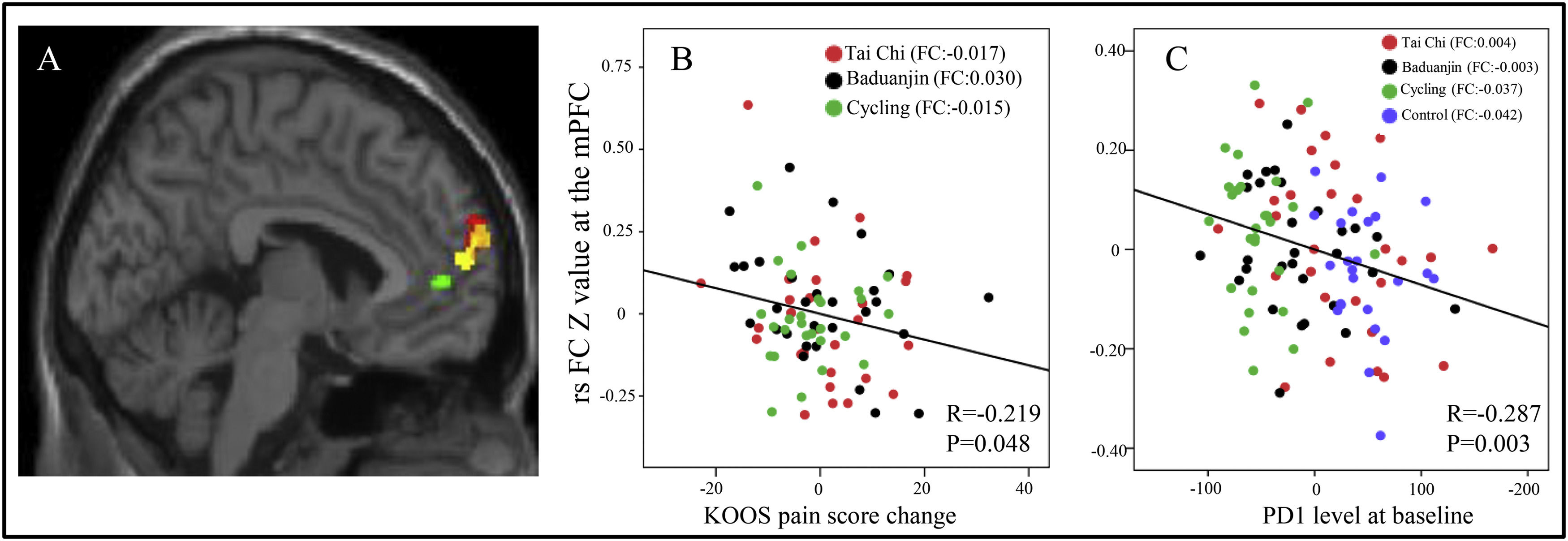
The overlapped rsFCs in between-group comparisonsInterestingly, we observed overlapping rsFC in the following contrasts: 1. between the right temporal pole and right BA in both the Tai Chi group and Baduanjin group compared to the control group, and 2. between the right temporal pole and left BA in the cycling group compared to the control group (Fig. 1A). Furthermore, we identified overlapping rsFC between the left mPFC and right BA in both the Tai Chi and Baduanjin groups compared to the control group (Fig. 2A).
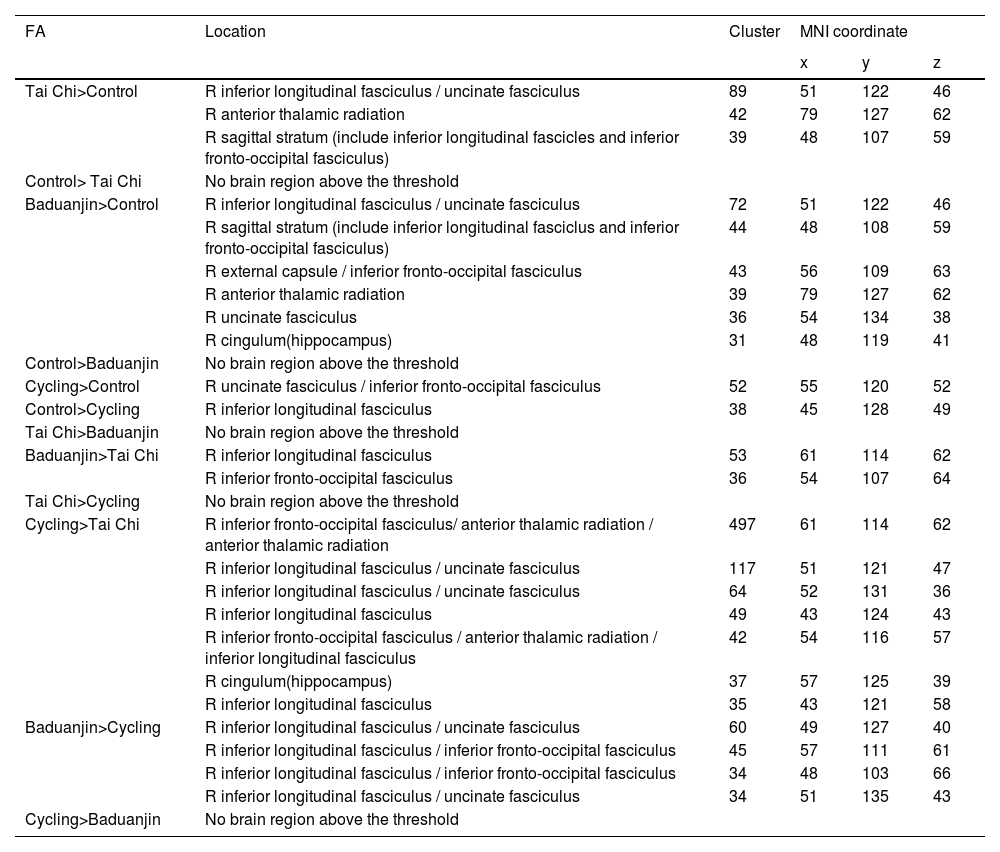
A: The overlapped rsFC change (post-intervention minus pre-intervention) among the four groups using the BA as seed. Red: compared to the control group, decreased rsFC between right BA and right temporal pole in the Tai Chi group; Yellow, compared to the control group, decreased rsFC between right BA and right temporal pole in the Baduanjin group; Green, compared to the control group, decreased rsFC between left BA and right temporal pole in the cycling group. B: Scatter plots indicate the association between KOOS pain score change and mean z values change in the right temporal pole cluster across all exercise groups adjusted for age and gender. FC: the mean change in rsFC values, adjusted age and gender.
A: The overlapped rsFC change (post-intervention minus pre-intervention) among the four groups using the BA as seed. Red: compared to the control group, decreased rsFC between right BA and left mPFC in the Tai Chi group; Yellow, compared to the control group, decreased rsFC between right BA and left mPFC in the Baduanjin group; Green, compared to the control group, decreased rsFC between right BA and left mPFC in the cycling group. B: Scatter plots indicate the association between KOOS pain score change and mean z values in the left mPFC cluster (cycling group vs control group) across subjects of all treatment groups adjusted for age and gender. FC: the mean change in rsFC values, adjusted age and gender. C: Scatter plots indicate the association between serum PD-1 level and mean z values in the left mPFC cluster (cycling group vs control group) across all subjects adjusted for age and gender at baseline. FC: the mean rsFC values at baseline, adjusted age and gender.
Additionally, our function MRI results revealed significantly decreased rsFC in the Tai Chi group between the right CMA and the right DLPFC compared to the cycling group, increased rsFC in the Baduanjin group between the left SFA and bilateral mPFC/left ACC compared to the cycling group and increased rsFC in the Baduanjin group between right SFA and left middle frontal gyrus compared to the cycling group. These findings suggest that exercise may modulate KOA through its effects on prefrontal cortical regions.
The correlation results between the significant rsFCs and the pain scores/serum PD-1 levelsWe found there was a significant negative association between rsFC z values changes postintervention minus preintervention) at the overlapped cluster in the temporal pole (Fig. 1A) and the corresponding KOOS pain score (P < 0.001, r = −0.416) across all exercise groups adjusted age and gender (Fig. 1B). Furthermore, a significant negative association was identified between rsFC z values in the mPFC cluster (Fig. 2A) and corresponding KOOS pain change scores (post-intervention minus pre-intervention) (P = 0.048, r = −0.219) across all physical exercise groups adjusted age and gender (Fig. 2B), as well as a significant negative correlation between rsFC z values in the mPFC cluster and corresponding PD-1 levels across all subjects at baseline adjusted age and gender (P = 0.003, r = −0.287) (Fig. 2C).
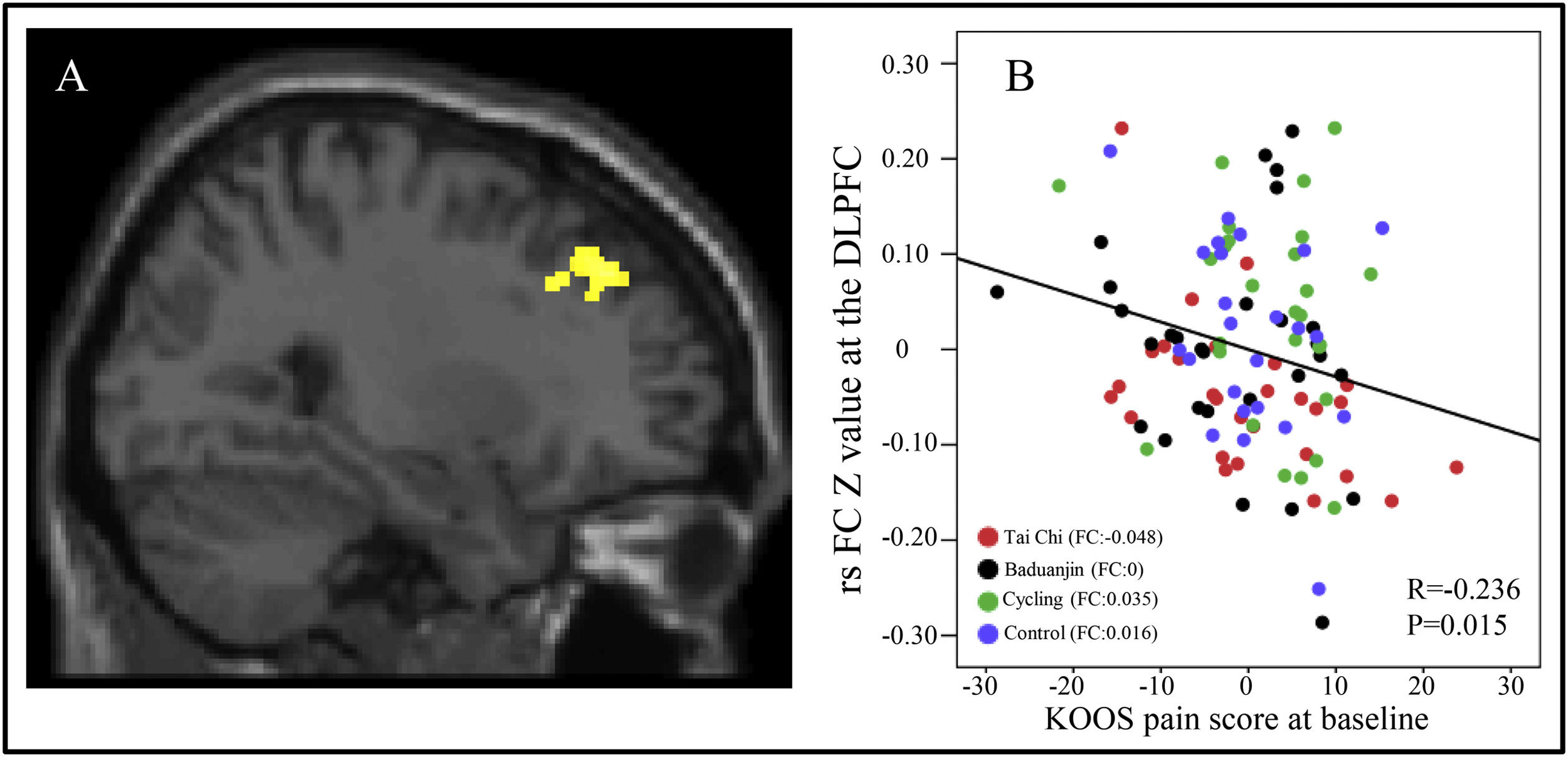
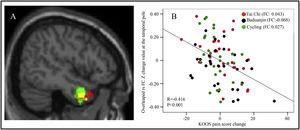
To investigate the correlation between three altered rsFCs in the three exercise groups and the KOOS pain score, we extracted average z-values of clusters located in the right DLPFC, bilateral mPFC/left ACC, and left middle frontal gyrus from all four groups at baseline. Subsequently, a multiple regression analysis was conducted with age and gender as covariates. Our findings revealed a significant negative association between rsFC z-values at the right DLPFC and corresponding KOOS pain scores (P = 0.015, r = −0.236) across all groups at baseline after Bonferroni's correction for multiple comparisons (0.05/3) (Fig. 3A and B).
A: The decreased rsFC in Tai Chi group between right CMA and right DLFPC compared to the cycling group. B: Scatter plots indicate the association between KOOS pain score and mean z values in the right DLPFC cluster across all subjects adjusted for age and gender at baseline. FC: the mean rsFC values at baseline, adjusted age and gender.
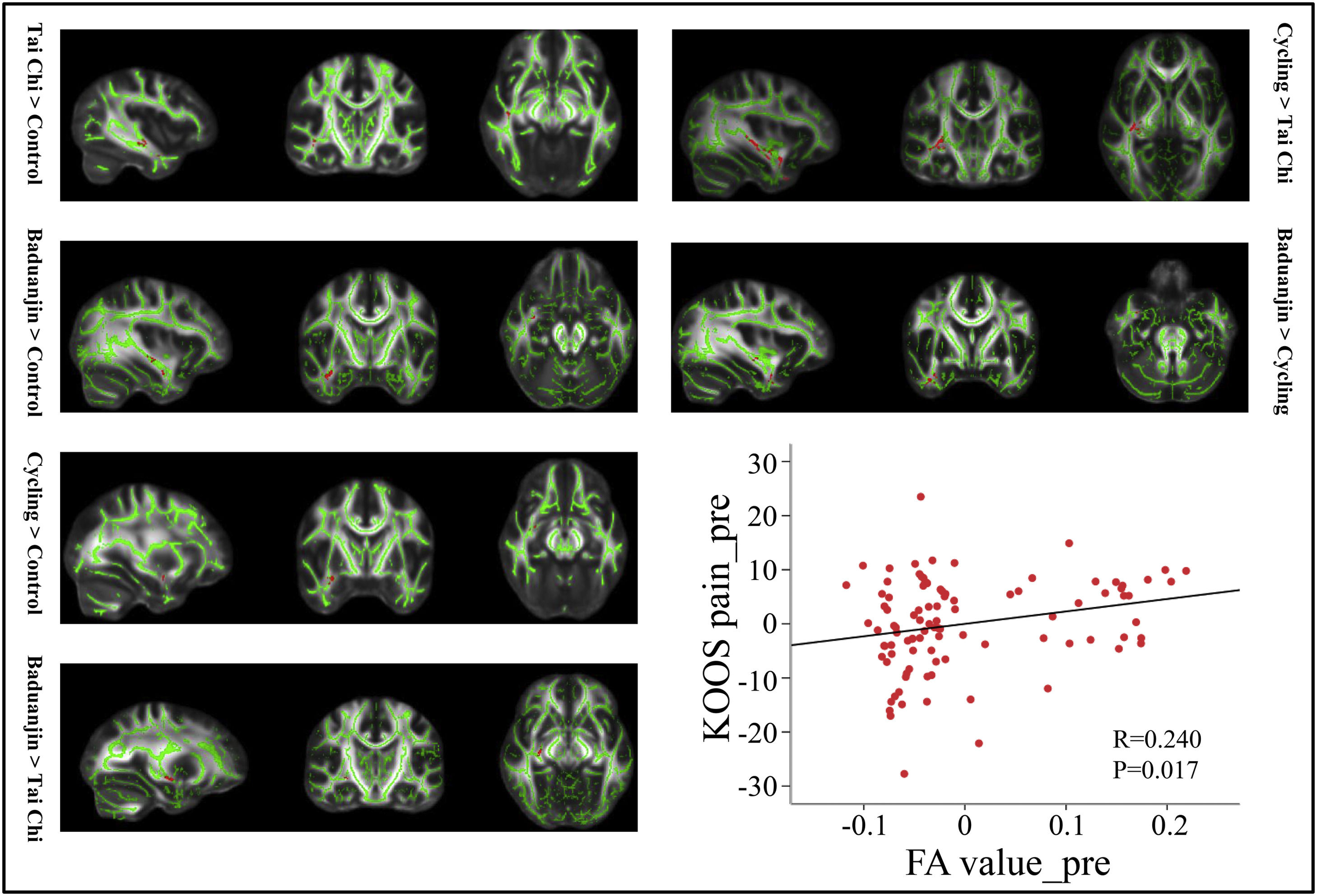
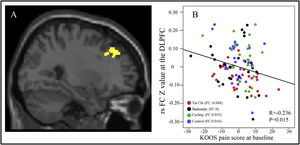
Voxel-wise data analysis was presented in Table 5. Specifically, we found that both Tai Chi and Baduanjin are associated with increased FA value in the pathway connecting BA to temporal pole. Furthermore, we observed a significant positive correlation between the average FA value and corresponding KOOS pain score at baseline across all subjects, after adjusting for age and gender (P = 0.017, r = 0.240) (Fig 4).
The DTI results (FA value).
The comparison is between post minus pre intervention across different groups.
In this study, we investigated the potential of 12-week mind-body exercises (Tai Chi and Baduanjin) and physical exercise (cycling) in alleviating KOA-related pain syndrome and explored their underlying mechanisms. Our findings revealed that all three physical exercise modalities significantly relieved pain compared to the control group. Moreover, all three exercise modalities decreased rsFC of BA-temporal pole and BA-mPFC and exhibited significant modulation of serum PD-1 level and serum IFN-γ level when compared to the control group. These results suggest that engaging in different physical exercise modalities may relieve KOA-related pain syndrome through a common pathway while maintaining their own characteristics in sub-regions of amygdala (especially in right BA and CMA subregions).
Different physical exercise modalities relieve pain in KOAPhysical exercise modalities constitute a fundamental therapeutic approach for KOA. Previous systematic reviews and meta-analyses have consistently demonstrated the efficacy of diverse exercise modalities in effectively alleviating pain among KOA patients, which aligns with the findings of our present study (Guo et al., 2022; Raposo et al., 2021; Yang et al., 2021). Furthermore, although no significant between-group difference was observed, our current study shows that mind-body exercises may elicit a non-significant greater improvement in KOOS pain scores compared to physical exercise, which is line in a previous finding (Russell & Arcuri, 2015). The mind-body exercise combines slow physical movements with deep, controlled breathing exercises and relaxation techniques. These benefits can be explained by the implementation of a biopsychosocial approach during mind-body exercises, which have shown potential in improving physical performance by alleviating kinesiophobia as well as enhancing management of chronic pain and disability through reduction of other psychosocial factors such as anxiety, depression, and work-related mental stress (Fernández-Rodríguez et al., 2022) .
Different physical exercise modalities exert distinct effects on alleviating pain symptoms in KOA by modulating the shared functional and structural brain pathway referred to as the BA-temporal poleOur findings indicate a significant reduction in rsFC between the BA and temporal pole across all three exercise groups compared to the control group. Additionally, we observed a significant correlation between rsFC and corresponding KOOS pain scores. These results underscore the potential of BA for pain relief across various physical exercise modalities. The basolateral complex, which processes emotional salience to sensory information, has been identified as crucial hub for nociceptive signal transmission (Llorca-Torralba et al., 2016) and modulation of pain behavior through descending pain control centers in the brain (Huang et al., 2019). Previous studies have demonstrated that physical exercise can alleviate anxiety and depression-like behaviors in rats by activating the 5-HT2C receptors located in the basolateral amygdala, indicating that this region may serve as a potential target for modulating emotional responses through physical exercise (Greenwood et al., 2012).
Additionally, we found that all three physical exercise modalities alleviate pain associated with KOA by modulating the rsFC of the BA-temporal pole pathway. Furthermore, we observed an increased FA values within this pathway, and these FA values were significantly positively correlated with pain relief. These results imply that different physical exercise interventions for KOA pain relief may share a common mechanism through rsFC of the BA-temporal pole pathway. The temporal pole is anatomically interconnected with the amygdala and plays a crucial role in socioemotional modulation. Damage to this region can lead to aberrant social and emotional processing (Olson et al., 2007). A study conducted by Dai revealed that patients with chronic migraine exhibited an increased functional connectivity density in the temporal pole, suggesting the involvement of this region in pain regulation (Dai et al., 2021). In the present investigation, participants underwent group training in each exercise modality. We postulate that the shared mechanism underlying all physical exercise modalities may be associated with the socio-emotional process, which is regulated by group training involving the rsFC of BA- temporal pole.
Physical training alleviates pain symptoms in KOA by modulating the functional connectivity of the amygdala-prefrontal brain pathwayOur findings indicate a significant decrease in rsFC between BA and mPFC in the cycling group compared to the control group, with rsFC showing a strong association with corresponding KOOS pain scores. The mPFC appears to play a crucial role in descending pain modulation pathways, and there is a positive correlation between greater severity of depressive symptoms and increased activation of the mPFC (Hawrylycz et al., 2012). Moreover, it has been proposed that physical exercise can modulate the activation of the mPFC and alleviate chronic pain (Shen et al., 2022), which is in line with our current study. These results suggest that cycling may ameliorate pain symptoms in patients with KOA by rsFC between the amygdala and mPFC. Compared to mind-body exercises such as Tai Chi and Baduanjin, cycling is a physical exercise that poses lower demands on motor control resources. We hypothesize that more cognitive resources can be directly allocated to the brain's pain modulation pathway, specifically involving the mPFC during cycling training.
Mind-body exercises mitigate pain symptoms in KOA by modulating cognitive processing brain regions, as compared to physical exerciseA significant decrease in rsFC between the CMA and DLPFC was observed in the Tai Chi group compared to the cycling group. This rsFC was also significantly associated with KOOS pain scores. The DLPFC, a critical region of the cognitive control network, plays an essential role in descending pain inhibitory mechanisms by modulating activity in various pain processing pathways including the descending pain suppression system and medial pain processing system (David & Massieh, 2017; Kong et al., 2019; Miller & Cohen, 2001; Todd et al., 2015). Previous studies have indicated that CMA is involved in nociception (Gonçalves et al., 2015; Zhou et al., 2019). Although no significant difference between groups was found based on our behavioral results, it is noteworthy that the Tai Chi group exhibited greater improvement in KOOS pain scores compared to the physical exercise group did. These findings suggest that mind-body exercises like Tai Chi may exert more positive effects on alleviating KOA-related pain through modulation of the CMA-DLPFC pathway.
Distinctive changes in brain activation patterns following the engagement in different physical exercise modalitiesCompared to the control group receiving a health education, we observed significant reductions in rsFC between the CMA and thalamus proper, as well as between the SFA and angular gyrus/precuneus in the Tai Chi group. Additionally, there was an increased rsFC between the BA and supramarginal gyrus. The angular gyrus and precuneus are crucial brain regions within the post-default mode network (DMN), which is typically deactivated during externally-focused attention-demanding tasks but activated during internally-driven and self-referential processes. Previous studies have shown alterations in DMN connectivity among adults with chronic pain (Farmer et al., 2012), where pain frequency and intensity were associated with changes in DMN connectivity at rest in adolescents experiencing varying levels of chronic pain (Jones et al., 2020). Our previous study also demonstrated that Tai Chi training modulates DMN activity in healthy older individuals, which aligns with our current findings (J. Liu, Tao et al., 2019). The angular/supramarginal gyrus, linked to salience detection, is part of the ventral attention network that may activate when attention is directed towards pain rather than away from it (Kucyi & Davis, 2015). A separate circuit contributing to secondary hyperalgesia and hypoalgesia has been identified through a series of studies involving ascending transmission pathways to the thalamus acting as "nociceptive discriminators" (You et al., 2022). Furthermore, an amygdala-thalamo circuit has been implicated in negative emotions such as fear memories (Silva et al., 2021). We speculate that altered rsFC between the amygdala and thalamus/DMN/supramarginal gyrus indicates that Tai Chi contributes to pain relief by modulating various brain regions involved in both nociception perception and cognitive processes.
In comparison to the control group, this study has demonstrated modulation of rsFCs in the BA-medial orbital gyrus and SFA-inferior occipital gyrus within the Baduanjin group. Unlike general resistance exercise, endurance exercise, and strength exercises, Baduanjin is characterized by symmetrical body postures and movements, controlled breathing, a meditative state of mind, mental focus, and an emphasis on coordinating movements with breathing while maintaining concentrated attention. The medial frontal cortex and adjacent orbitofrontal cortex have been extensively investigated for their role in reward-guided decision-making and adaptive behavior (Klein-Flügge et al., 2022), which can be highly beneficial for pain relief (Porreca & Navratilova, 2017). The occipital lobe serves as the brain's visual processing center associated with short-term visual memory and working memory functions (van Dam et al., 2015). Zheng et al. also found a significant increase in gray matter volume in the occipital lobe following Baduanjin exercise that partially aligns with our current findings (Zheng et al., 2021). Specifically located within the mammalian brain's anatomical region known as the visual cortex, inferior occipital gyrus plays a crucial role in visual processing. There is evidence suggesting that structural and functional abnormalities within the occipital lobe are implicated in pain pathology (Wang et al., 2023).
Compared to the control group, the cycling group demonstrated significant alterations in rsFCs within CMA-SMA, SFA-SMA, and SFA-postcentral gyrus. The SMA and postcentral gyrus are crucial hubs of the motor system. Prior research has indicated that patients with phantom limb pain exhibit extensive bilateral activation in the SMC and SMA during anteflexion of the amputation stump, confirming the role of the motor system in processing pain information (Dettmers et al., 2001). Previous studies have reported SMA activation after high-intensity Stationary cycling (Enders et al., 2016) as well as in complex regional pain syndrome (Bolwerk et al., 2013). Additionally, emotion processing also involves the SMA (Morawetz et al., 2017). Physical exercises such as cycling may produce greater modulation effects on networks and brain regions involved in motor function.
Physical exercise-related modulation of pain relief-related brain pathway (BA-mPFC) significantly correlated with the serum PD-1 level in patients with KOAWe observed a negative correlation between BA-mPFC rsFC and corresponding PD-1 levels in cycling groups, suggesting that anti-immunity may modulate pain relief after cycling exercise via BA-mPFC rsFC modulation. Previous studies have indicated the involvement of PD-1 in immune response regulation and its potential as an immunotherapy target (Ashrafizadeh et al., 2020; Lu et al., 2022; Policheni et al., 2022), while aerobic training can improve KOA symptoms by modulating immune markers (Gomes et al., 2016). Our findings suggest a significant association between decreased BA-mPFC rsFC and KOOS pain score as well as serum PD-1 level, indicating the potential modulatory role of anti-immunity in pain relief after cycling exercise through BA-mPFC rsFC modulation. However, no changes were observed in serum BDNF or Tim3 levels following 12 weeks of physical training, which is partially consistent with a previous study where no significant change was found in BDNF levels after one year continuous or interval aerobic training (Erickson et al., 2011). Further studies are needed to validate our findings.
LimitationsSeveral limitations exist in the present study. Firstly, the sample size is relatively small. However, a previous study by Desmond suggested that for multiple comparisons, 24 subjects are required to achieve 80 % power at the single voxel level for typical activations in functional MRI neuroimaging studies (Desmond & Glover, 2002). Therefore, we believe our sample size is sufficient to investigate the effects and underlying mechanisms of mind-body and physical exercise in patients with KOA. Nevertheless, studies with larger sample sizes are needed to replicate our findings. Secondly, BDNF was sampled from blood rather than directly from the central nervous system which may be one reason why no significant group difference was found. Thirdly, we did not include pain duration as a covariate in fMRI data analysis due to no significant group difference among the four groups. Fourthly, medication was not included as a covariate since none of the subjects reported regular administration of pain medications. Furthermore, given the preliminary nature of this study, there was a lack of stringent controls for various confounding factors, such as the wide age range of participants. Future investigations should incorporate more rigorous variable control measures to bolster the robustness and scientific rigor of the findings. Finally, differences between weight-bearing (two mind-body exercises) and non-weight bearing activities (Stationary cycling and health education) may have affected modulation effect on pain relief, brain activation and molecular biological level among these three exercise modalities; thus, future studies should involve more rigorous experimental control over these influencing factors.
ConclusionIn conclusion, our results suggest that regardless of the physical exercise modality a 12 week-long exercise intervention can effectively alleviate pain symptoms in patients with KOA. The rsFC results reveal that all three physical exercise modalities selectively reduce the rsFC of BA-temporal pole compared to the education control group. The overlapping rsFC of BA-temporal pole (in all three exercise groups compared to the control group) and the rsFC of BA-mPFC (cycling group vs control group) are significantly associated with pain relief as measured by KOOS pain subscale. Additionally, we found a significant association between serum PD-1 levels and the rsFC of BA-mPFC. Finally, an increased FA value is found in the pathway connecting BA to temporal pole in mind-body exercises groups. Our results suggest that different physical exercise modalities can relieve KOA-related pain through both common and unique pathways.
Data availability statementThe data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Ethics statementThe study was approved by the Medical Ethics Committee of the Affiliated Rehabilitation Hospital of Fujian University of Traditional Chinese Medicine Patient consent statement Written informed consent was obtained from all participants.
CRediT authorship contribution statementJiao Liu: Data curation, Formal analysis, Writing – original draft. Weilin Liu: Writing – original draft. Jia Huang: Writing – original draft. Yajun Wang: Writing – original draft. Baoru Zhao: Writing – original draft. Peiling Zeng: Writing – original draft. Guiyan Cai: Writing – original draft. Ruilin Chen: Writing – original draft. Kun Hu: Data curation, Formal analysis. YouXue Tu: Data curation, Formal analysis. Meiqin Lin: Data curation, Formal analysis. Jian Kong: Conceptualization. Jing Tao: Conceptualization. Lidian Chen: Conceptualization.
This work was supported by Fujian Provincial Department of Science and Technology [grant No. 2017L2011]; NIH/NCCIH [grant R01AT006364, R01 AT008563, R21AT008707 and R61AT009310]; the National Rehabilitation Research Center of Traditional Chinese Medicine, Fujian Rehabilitation Industrial Institution and Fujian Rehabilitation Tech Co-Innovation Center [grant No. X2012001-Collaboration and No. X2021001-Collaboration].
We thank the professional instructors Dalu Qi, from the Fujian University of Traditional Chinese Medicine, and Chengwu Huang, from the Affiliated Apitherapy Hospital of Fujian Agriculture and Forestry University, for their training in the Tai Chi and Baduanjin exercises.