Probiotics may act as biological agents that modify the intestinal microbiota and certain cytokine profiles, which can lead to an improvement in certain gastrointestinal diseases.
ObjectivesTo conduct a review of the evidence of the role of probiotics in certain gastrointestinal diseases in adults.
Search methodsReview conducted using appropriate descriptors, filters and limits in the PubMed database (MEDLINE).
Selection criteriaThe MeSH terms used were Probiotics [in the title] AND Gastrointestinal Diseases, with the following limits or filters: Types of study: Systematic Reviews, Meta-Analysis, Guideline, Practice Guideline, Consensus Development Conference (and Consensus Development Conference NIH), Randomised Controlled Trial, Controlled Clinical Trial and Clinical Trial; age: adults (19 or older); language: English and Spanish; in humans, and with at least one abstract.
Data collection and analysisFull texts of all the Systematic Reviews and meta-analyses directly related to the review's objective were obtained, as well as the Randomised Controlled Trials of the studies that were considered relevant and of sufficient quality for this review.
Main resultsCertain probiotics, different for each process, have proven to be effective and beneficial in cases of acute infectious diarrhoea, antibiotic-associated diarrhoea, Clostridium difficile-associated diarrhoea, pouchitis and Helicobacter pylori infection eradication.
Authors’ conclusionsAlthough some probiotics have not demonstrated any benefit, there are certain gastrointestinal diseases in which the use of probiotics, true biological agents, can be recommended.
Los probióticos pueden actuar como agentes biológicos que modifican la microbiota intestinal y ciertos perfiles de citoquinas, lo que puede conllevar una mejoría en ciertos procesos gastrointestinales.
ObjetivosRealizar una revisión basada en la evidencia del papel de los probióticos en determinadas patologías gastrointestinales del adulto.
Métodos de búsquedaRevisión realizada utilizando los descriptores, filtros y límites adecuados en la base de datos PubMed (MEDLINE).
Criterios de selecciónSe han empleado los términos MeSH Probiotics [en el título] AND Gastrointestinal Diseases, con los siguientes límites o filtros: tipos de estudios: Systematic Reviews, Meta-Analysis, Guideline, Practice Guideline, Consensus Development Conference (y Consensus Development Conference NIH), Randomised Controlled Trial, Controlled Clinical Trial y Clinical Trial; edad: adultos (19 o más años); idioma: en inglés y español; en humanos, y que dispusieran, al menos, de un abstract.
Recogida y análisis de datosSe recuperaron los textos completos de todas las revisiones sistemáticas y metaanálisis directamente relacionados con el objetivo de la revisión, así como los ensayos clínicos aleatorizados de los estudios que se consideraron relevantes y de calidad para realizar esta revisión.
Resultados principalesDeterminados probióticos, diferentes para cada proceso, se han demostrado eficaces y beneficiosos en caso de diarrea aguda infecciosa, diarrea asociada a antibióticos, diarrea asociada a Clostridium difficile, pouchitis y en la erradicación de la infección por Helicobacter pylori.
Conclusiones de los autoresHay ciertas patologías gastrointestinales en las que se puede recomendar el uso de los probióticos, verdaderos agentes biológicos, y otras en las que no se ha demostrado beneficio.
The human gastrointestinal tract (GIT) houses the gastrointestinal microbiome, a complex and dynamic microbial ecosystem, which is estimated to feature more than 400 different species of bacteria,1 and which is responsible for important functions, including metabolic activities, trophic effects on the intestinal epithelium and interactions with the host immune system.2
The term “microbiome” (previously known as “intestinal flora”) refers to all microbes (bacteria, fungi, viruses, etc.) and their genetic components and environmental interactions in a particular environment.3 The term was added to the MeSH database in 2014.
The microbiota, the community of live micro-organisms residing in a particular ecological niche, such as the human large bowel (colon), acts as a barrier and prevents the colonisation of opportunistic and pathogenic micro-organisms.2 The intestinal microbiome is essential in the interaction between the intestinal epithelium and the mucosal immune system, and affects the development and homeostasis of normal mucosal immunity.3
Probiotics are defined as “live microorganisms which when administered in adequate amounts confer a health benefit on the host”.2–10 This is the definition proposed by the United Nations (UN) for the Food and Agriculture Organisation (FAO) and the World Health Organisation (WHO). The Royal Academy of the Spanish Language (RAE) has not yet added the term “probiotic” to its dictionary.
Probiotics may be ingested in the form of any food supplement or as drugs.3,5,7 However, most commercial products are derived from food sources, especially cultured and fermented dairy products.4 They are available in multiple formulations that may contain just one or a combination of several probiotics, whose quantity varies widely from one product to another.3
The micro-organisms that are most commonly used as probiotics belong to the group of lactic acid bacteria (Lactobacillus) and Bifidobacterium. These are important constituents of the normal human GI microbiota.1,7 Other probiotics that are less commonly used but are also being researched due to their possible probiotic functions are strains of Streptococcus, Escherichia coli (E. coli) and Bacillus.2–4 Some non-pathogenic yeasts, such as Saccharomyces boulardii (S. boulardii) (from Litchi chinensis, a tropical fruit originating in southern China) that are not normally found in the GIT are also used.1,10
Probiotics improve the nutritional and microbiological balance of the GIT.1 They act as vectors that deliver their active components to several target sites in the GIT.6 Their destinations and effects vary by strain.7 Most effects occur only when live micro-organisms are ingested6; however, this may not always be necessary to achieve benefits.4
For a probiotic to be effective, it should survive the acidic environment of the stomach and transit through the bowel. This explains, in part, the requirement that probiotics should be ingested in high concentrations.3 Their survival during GI transit varies widely,3,4,6 since it depends on genera, species, strains, dose ingested, host-related factors (acid, biliary, and pancreatic secretions) and vector-related factors (foods, microencapsulation).6
Probiotics vary in their capacity to resist gastric acid and bile, their capacity to colonise the GIT and to have an influence on cytokines secreted by intestinal epithelial cells. As a result, the clinical benefits observed in some cannot necessarily be generalised to others.4 Their capacity for adhesion to the intestinal mucosa also varies by strain, thereby promoting competitive exclusion of pathogens and immunomodulation.6
The appropriate probiotic for each indication should be selected.3 Although probiotics are perceived and cause responses by immune and/or intestinal cells, their mechanisms of action are not entirely clear.4,8
The beneficial effects of probiotics on GI function are attributed to normalisation of permeability, restoration of the microbiota, improvement of barrier immune function, down-regulation of the pro-inflammatory immune response and rebalancing of pro-inflammatory and anti-inflammatory cytokines.3
The use of probiotics in different diseases is becoming increasingly widespread. A specific journal called Probiotics and Antimicrobial Proteins collects the main research studies being conducted on probiotics. This journal has an impact factor of 1.283.
The beneficial effects of probiotics on gastrointestinal diseases may be considerable (and demonstrated), possible or anecdotal. The gastrointestinal diseases on which probiotics have considerable beneficial effects include acute (viral) gastroenteritis, antibiotic-associated diarrhoea,11 travellers’ diarrhoea, pouchitis and irritable bowel syndrome (IBS). Those on which probiotics have possible beneficial effects include chronic idiopathic constipation (CIC; functional constipation, in the Rome IV classification), chronic gastritis due to Helicobacter pylori (H. pylori) and ulcerative colitis (UC). The benefits of probiotics have been anecdotal in other processes, such as acute pancreatitis, microscopic colitis (collagen), Crohn's disease (CD) and lactose intolerance.3–6
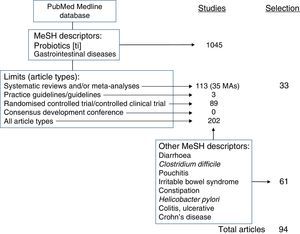
Review strategyThe literature search was performed in the PubMed database of the US National Library of Medicine (pubmed.gov). To review the scientific evidence, the Medical Subjects Headings (MeSH) terms Probiotics [in the title] AND Gastrointestinal Diseases were used, with the following limits or filters: study type: Systematic Reviews, Meta-Analysis, Guideline, Practice Guideline, Consensus Development Conference (and NIH Consensus Development Conference), Randomised Controlled Trial, Controlled Clinical Trial and Clinical Trial; age: adults (19 years of age or older); language: English (there are no studies on the general role of probiotics in GI disease in Spanish); in humans; and availability of at least an abstract.
All this was done according to the recommendations in a study by Shojania,12 but using the Systematic Reviews tool in addition to the Clinical Queries tool.
With this search strategy, 51 studies were retrieved. Of these, 28 were selected due to their relevance after ruling out childhood processes and others outside of the topics relevant to the review (colon cancer, HIV, surgery, concomitant use with other drugs).
All other studies were selected after searching for evidence with the strategy Probiotics[ti] AND each of the MeSH descriptors of the concrete entities reviewed (Diarrhoea; Clostridium difficile [C. difficile]; Pouchitis; Irritable Bowel Syndrome; Constipation; H. pylori; Colitis, Ulcerative; Crohn's Disease).
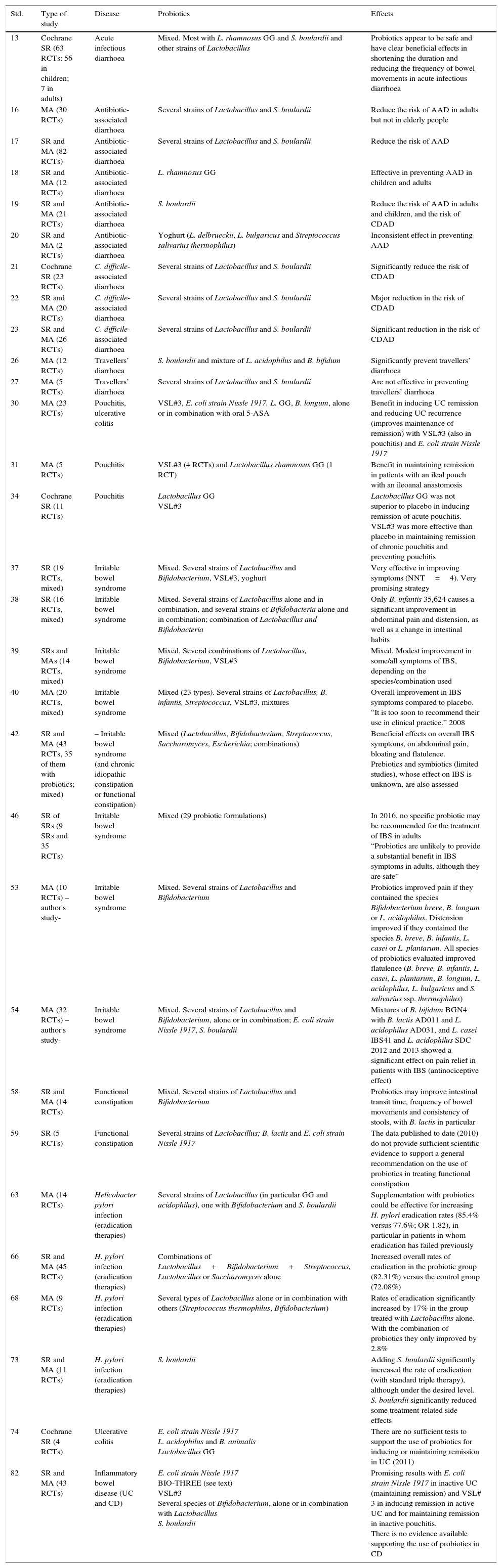
As the highest level of evidence and degree of recommendation correspond to systematic reviews (SRs) and meta-analyses (MAs), the most outstanding data and the data most applicable in clinical practice extracted from the evidence used to prepare this manuscript, a total of 33 studies, have been collected in Table 1.
SRs and MAs on the use of probiotics in different gastrointestinal processes.
| Std. | Type of study | Disease | Probiotics | Effects |
|---|---|---|---|---|
| 13 | Cochrane SR (63 RCTs: 56 in children; 7 in adults) | Acute infectious diarrhoea | Mixed. Most with L. rhamnosus GG and S. boulardii and other strains of Lactobacillus | Probiotics appear to be safe and have clear beneficial effects in shortening the duration and reducing the frequency of bowel movements in acute infectious diarrhoea |
| 16 | MA (30 RCTs) | Antibiotic-associated diarrhoea | Several strains of Lactobacillus and S. boulardii | Reduce the risk of AAD in adults but not in elderly people |
| 17 | SR and MA (82 RCTs) | Antibiotic-associated diarrhoea | Several strains of Lactobacillus and S. boulardii | Reduce the risk of AAD |
| 18 | SR and MA (12 RCTs) | Antibiotic-associated diarrhoea | L. rhamnosus GG | Effective in preventing AAD in children and adults |
| 19 | SR and MA (21 RCTs) | Antibiotic-associated diarrhoea | S. boulardii | Reduce the risk of AAD in adults and children, and the risk of CDAD |
| 20 | SR and MA (2 RCTs) | Antibiotic-associated diarrhoea | Yoghurt (L. delbrueckii, L. bulgaricus and Streptococcus salivarius thermophilus) | Inconsistent effect in preventing AAD |
| 21 | Cochrane SR (23 RCTs) | C. difficile-associated diarrhoea | Several strains of Lactobacillus and S. boulardii | Significantly reduce the risk of CDAD |
| 22 | SR and MA (20 RCTs) | C. difficile-associated diarrhoea | Several strains of Lactobacillus and S. boulardii | Major reduction in the risk of CDAD |
| 23 | SR and MA (26 RCTs) | C. difficile-associated diarrhoea | Several strains of Lactobacillus and S. boulardii | Significant reduction in the risk of CDAD |
| 26 | MA (12 RCTs) | Travellers’ diarrhoea | S. boulardii and mixture of L. acidophilus and B. bifidum | Significantly prevent travellers’ diarrhoea |
| 27 | MA (5 RCTs) | Travellers’ diarrhoea | Several strains of Lactobacillus and S. boulardii | Are not effective in preventing travellers’ diarrhoea |
| 30 | MA (23 RCTs) | Pouchitis, ulcerative colitis | VSL#3, E. coli strain Nissle 1917, L. GG, B. longum, alone or in combination with oral 5-ASA | Benefit in inducing UC remission and reducing UC recurrence (improves maintenance of remission) with VSL#3 (also in pouchitis) and E. coli strain Nissle 1917 |
| 31 | MA (5 RCTs) | Pouchitis | VSL#3 (4 RCTs) and Lactobacillus rhamnosus GG (1 RCT) | Benefit in maintaining remission in patients with an ileal pouch with an ileoanal anastomosis |
| 34 | Cochrane SR (11 RCTs) | Pouchitis | Lactobacillus GG VSL#3 | Lactobacillus GG was not superior to placebo in inducing remission of acute pouchitis. VSL#3 was more effective than placebo in maintaining remission of chronic pouchitis and preventing pouchitis |
| 37 | SR (19 RCTs, mixed) | Irritable bowel syndrome | Mixed. Several strains of Lactobacillus and Bifidobacterium, VSL#3, yoghurt | Very effective in improving symptoms (NNT=4). Very promising strategy |
| 38 | SR (16 RCTs, mixed) | Irritable bowel syndrome | Mixed. Several strains of Lactobacillus alone and in combination, and several strains of Bifidobacteria alone and in combination; combination of Lactobacillus and Bifidobacteria | Only B. infantis 35,624 causes a significant improvement in abdominal pain and distension, as well as a change in intestinal habits |
| 39 | SRs and MAs (14 RCTs, mixed) | Irritable bowel syndrome | Mixed. Several combinations of Lactobacillus, Bifidobacterium, VSL#3 | Mixed. Modest improvement in some/all symptoms of IBS, depending on the species/combination used |
| 40 | MA (20 RCTs, mixed) | Irritable bowel syndrome | Mixed (23 types). Several strains of Lactobacillus, B. infantis, Streptococcus, VSL#3, mixtures | Overall improvement in IBS symptoms compared to placebo. “It is too soon to recommend their use in clinical practice.” 2008 |
| 42 | SR and MA (43 RCTs, 35 of them with probiotics; mixed) | – Irritable bowel syndrome (and chronic idiopathic constipation or functional constipation) | Mixed (Lactobacillus, Bifidobacterium, Streptococcus, Saccharomyces, Escherichia; combinations) | Beneficial effects on overall IBS symptoms, on abdominal pain, bloating and flatulence. Prebiotics and symbiotics (limited studies), whose effect on IBS is unknown, are also assessed |
| 46 | SR of SRs (9 SRs and 35 RCTs) | Irritable bowel syndrome | Mixed (29 probiotic formulations) | In 2016, no specific probiotic may be recommended for the treatment of IBS in adults “Probiotics are unlikely to provide a substantial benefit in IBS symptoms in adults, although they are safe” |
| 53 | MA (10 RCTs) – author's study- | Irritable bowel syndrome | Mixed. Several strains of Lactobacillus and Bifidobacterium | Probiotics improved pain if they contained the species Bifidobacterium breve, B. longum or L. acidophilus. Distension improved if they contained the species B. breve, B. infantis, L. casei or L. plantarum. All species of probiotics evaluated improved flatulence (B. breve, B. infantis, L. casei, L. plantarum, B. longum, L. acidophilus, L. bulgaricus and S. salivarius ssp. thermophilus) |
| 54 | MA (32 RCTs) – author's study- | Irritable bowel syndrome | Mixed. Several strains of Lactobacillus and Bifidobacterium, alone or in combination; E. coli strain Nissle 1917, S. boulardii | Mixtures of B. bifidum BGN4 with B. lactis AD011 and L. acidophilus AD031, and L. casei IBS41 and L. acidophilus SDC 2012 and 2013 showed a significant effect on pain relief in patients with IBS (antinociceptive effect) |
| 58 | SR and MA (14 RCTs) | Functional constipation | Mixed. Several strains of Lactobacillus and Bifidobacterium | Probiotics may improve intestinal transit time, frequency of bowel movements and consistency of stools, with B. lactis in particular |
| 59 | SR (5 RCTs) | Functional constipation | Several strains of Lactobacillus; B. lactis and E. coli strain Nissle 1917 | The data published to date (2010) do not provide sufficient scientific evidence to support a general recommendation on the use of probiotics in treating functional constipation |
| 63 | MA (14 RCTs) | Helicobacter pylori infection (eradication therapies) | Several strains of Lactobacillus (in particular GG and acidophilus), one with Bifidobacterium and S. boulardii | Supplementation with probiotics could be effective for increasing H. pylori eradication rates (85.4% versus 77.6%; OR 1.82), in particular in patients in whom eradication has failed previously |
| 66 | SR and MA (45 RCTs) | H. pylori infection (eradication therapies) | Combinations of Lactobacillus+Bifidobacterium+Streptococcus, Lactobacillus or Saccharomyces alone | Increased overall rates of eradication in the probiotic group (82.31%) versus the control group (72.08%) |
| 68 | MA (9 RCTs) | H. pylori infection (eradication therapies) | Several types of Lactobacillus alone or in combination with others (Streptococcus thermophilus, Bifidobacterium) | Rates of eradication significantly increased by 17% in the group treated with Lactobacillus alone. With the combination of probiotics they only improved by 2.8% |
| 73 | SR and MA (11 RCTs) | H. pylori infection (eradication therapies) | S. boulardii | Adding S. boulardii significantly increased the rate of eradication (with standard triple therapy), although under the desired level. S. boulardii significantly reduced some treatment-related side effects |
| 74 | Cochrane SR (4 RCTs) | Ulcerative colitis | E. coli strain Nissle 1917 L. acidophilus and B. animalis Lactobacillus GG | There are no sufficient tests to support the use of probiotics for inducing or maintaining remission in UC (2011) |
| 82 | SR and MA (43 RCTs) | Inflammatory bowel disease (UC and CD) | E. coli strain Nissle 1917 BIO-THREE (see text) VSL#3 Several species of Bifidobacterium, alone or in combination with Lactobacillus S. boulardii | Promising results with E. coli strain Nissle 1917 in inactive UC (maintaining remission) and VSL# 3 in inducing remission in active UC and for maintaining remission in inactive pouchitis. There is no evidence available supporting the use of probiotics in CD |
| Std. | Type of study | Disease | Probiotics | Effects |
|---|---|---|---|---|
| 83 | MA (5 RCTs) | Ulcerative colitis (moderately active) | VSL#3 | VSL#3 associated with conventional treatment (5-ASA and/or immune modulators) is safe and more effective than conventional therapy alone in achieving response and remission in active UC of mild to moderate severity |
| 90 | Cochrane SR (1 RCT) | Crohn's Disease | Lactobacillus GG | There are no sufficient tests to draw conclusions on the efficacy of probiotics for inducing remission in CD (2008) |
| 91 | Cochrane SR (7 RCTs) | Crohn's Disease | Lactobacillus GG VSL#3 S. boulardii E. coli strain Nissle 1917 | There is no sufficient evidence to suggest that probiotics are beneficial for maintaining remission in CD (2006) |
Fig. 1 summarises the search strategy for that evidence and how the evidence used to draft the manuscript was selected.
The use of different probiotics in the groups of gastrointestinal diseases on which they have considerable effects and possible effects, in which they are recommended and are currently being used, are reviewed below, with variable results, as shall be seen.
Disease entities in which probiotics are being used with considerable beneficial effectsAcute infectious diarrhoeaMost studies have been conducted in infants and children. In a SR from the Cochrane database13 that analysed 63 studies including a total of 8014 participants, only 352 participants (4.4%) were adults 19 years of age or older. This MA found that probiotics reduce the overall risk of diarrhoea lasting four or more days by 59% and the average duration thereof by 25h. The two most commonly studied probiotics were Lactobacillus GG and S. boulardii.
Despite the great variability in the methodological quality of the trials, in general, probiotics, regardless of the strain(s) used, doses, causes (bacterial or viral), seriousness and country of study, demonstrated themselves to be safe and effective in reducing the duration and seriousness of diarrhoea, in addition to preventing the progression of the acute form (<14 days) to the persistent form. This goes against the general consensus that the effects of probiotics are strain-specific and that the results obtained with a probiotic cannot be extrapolated to other organisms, including related strains.14 Well-designed studies of specific regimens of probiotics in specific contexts are required. These studies must be as homogeneous as possible so that definitive conclusions may be drawn. Even so, the results are encouraging.15
Antibiotic-associated diarrhoeaA SR of the literature on the use of probiotics in treating antibiotic-associated diarrhoea (AAD) in adults (18–64 years of age) and elderly patients (≥65 years of age) analysed 30 randomised controlled trials that met the pre-established inclusion criteria. The MA of these trials suggested that administering probiotics as an adjuvant therapy to antibiotics is associated with a lower risk of AAD in adults, but not in elderly people.16 These results confirmed the results of prior MAs.17
Probiotics that have demonstrated their efficacy in preventing AAD include Lactobacillus rhamnosus (L. rhamnosus) GG18 and S. boulardii,19 but not yoghurt (Lactobacillus delbrueckii [L. delbrueckii] bulgaricus or Streptococcus salivarius [S. salivarius] ssp. thermophilus), as confirmed by the PROSPERO study.20 However, more studies are needed to pinpoint the optimal probiotic preparation dose, administration time and treatment duration.
C. difficile-associated diarrhoea (CDAD)Antibiotics are widely prescribed drugs that may cause disruptions in the gastrointestinal microbiota, which may in turn reduce resistance to some pathogenic agents such as C. difficile. This may lead to C. difficile-associated diarrhoea (CDAD), a serious complication. As probiotics are live micro-organisms, it is believed that they may balance the gastrointestinal microbiota, thereby preventing this disorder.
In this regard, several studies have demonstrated that some probiotics are safe and effective in preventing CDAD. A SR from the Cochrane Collaboration (including 23 clinical trials, with a total of 4213 participants) suggested that probiotics significantly reduce the risk of CDAD by 64%. The incidence of CDAD was 2.0% in the probiotic group, compared to 5.5% in the control group with no treatment or with placebo.21
Other SRs and MAs, including 20 RCTs and 3818 patients, demonstrated that probiotics reduce the incidence of CDAD by 66%. In a population with an incidence of CDAD of 5%, probiotic prophylaxis would prevent 33 episodes per 1000 people.22
Another MA, which included 26 RCTs with 7957 patients, confirmed that the use of probiotics significantly reduces the risk of developing CDAD by 60.5%. Probiotics proved beneficial in both adults and children (reduction of 59.5% and 65.9%, respectively), especially among hospitalised patients. Lactobacillus, Saccharomyces and a mixture of probiotics were all beneficial in reducing the risk of developing CDAD.23Lactobacillus acidophilus (L. acidophilus) CL1285 and Lactobacillus casei (L. casei) LBC80R have also been shown to be effective.24
However, not all studies have demonstrated these results: an extensive RCT, which enrolled 2941 adults with exposure to antibiotics, confirmed that patients who received probiotics (a multi-strain preparation of L. acidophilus and Bifidobacterium bifidum [B. bifidum]) were not at reduced risk of CDAD.25
Regarding treatment, a MA published in 2012 evaluated the efficacy of probiotics in treating CDAD and suggested that probiotics are beneficial in treating this condition,17 although very few of the trials included had been designed specifically to evaluate this process.
Travellers’ diarrhoeaAcute diarrhoea is common in travellers, ranging from 5% to 50% depending on the destination.3,6 Most cases (80%) are due to bacterial infections. The most common infection is one of the seven types of diarrhoeagenic E. coli.2,3
One MA26 showed that some probiotics are safe and effective in preventing travellers’ diarrhoea and estimated that up to 85% of these cases may be prevented with probiotics. However, another subsequent meta-analysis27 did not confirm these results.
S. boulardii appeared to provide significant protection in these cases. L. rhamnosus GG and a mixture of probiotics consisting of L. acidophilus, Lactobacillus bulgaricus (L. bulgaricus), B. bifidum and Streptococcus thermophilus (S. thermophilus) also had a significant protective effect versus placebo.2,3,26
PouchitisProctocolectomy with an ileoanal anastomosis and ileal pouch is the treatment of choice for reconstructing intestinal continuity in severe refractory ulcerative colitis (UC) (and familial adenomatous polyposis) requiring surgery. The long-term complications observed following this procedure are most often acute and/or chronic inflammation of the ileal pouch, i.e. pouchitis. Up to 46% of patients with UC have at least one episode of pouchitis in the first 5 years following surgery,28 and 10–15% of patients with an ileoanal pouch develop serious pouchitis requiring long-term antibiotic use or removal of the pouch.29
Some observations and the high rate of response to several antibiotics have suggested that certain intestinal bacteria (increase in levels of Clostridium perfringens and absence of Streptococcus) play a significant role in the pathogenesis of pouchitis. Thus, it has been hypothesised that administering some probiotics may alter the microbiota and restore the intestinal mucosal barrier, thereby reducing the risk of inflammation of the mucosa of the pouch.4
In this regard, different studies have been conducted with certain probiotics that have confirmed their efficacy and safety in maintaining remission of pouch inflammation, achieved following antibiotic treatment, like 5-aminosalicylic acid,30,31 as well as in preventing acute pouchitis32 and recurrence of chronic pouchitis following induction treatment with antibiotics.33
The probiotic mixtures used in the different studies were VSL#3 (L. acidophilus, L. casei, Lactobacillus plantarum [L. plantarum], L. bulgaricus; Bifidobacterium longum [B. longum], Bifidobacterium breve [B. breve], Bifidobacterium infantis [B. infantis] and S. thermophilus)29,30; Trilac, which contains L. acidophilus, L. delbrueckii ssp. bulgaricus and Bifidobacterium bifidum, for 9 months; and Ecologic 825, a mixture of strains of B. bifidum, Bifidobacterium lactis (B. lactis), L. acidophilus, L. casei, Lactobacillus paracasei, Lactobacillus plantarum (L. plantarum), Lactobacillus salivarius and Lactococcus lactis,32 for 8 weeks.
In all cases, the Pouchitis Disease Activity Index significantly improved and the results were superior to placebo.
Finally, a SR from the Cochrane Collaboration34 concluded that VSL#3 was more effective than placebo in maintaining remission of chronic pouchitis and preventing the onset of pouchitis.
Irritable bowel syndromeThe pathophysiology of IBS remains unknown. However, several lines of epidemiological, physiological and clinical data have suggested that intestinal bacteria play a role in the pathogenesis of the disease.35,36
In addition, some physiological studies have demonstrated that manipulating the intestinal microbiota with probiotics may affect certain intestinal functions, such as motility and sensitivity, which appear to be relevant to the pathogenesis of IBS.37
Several systematic reviews and meta-analyses,37–43 as well as some clinical trials,44,45 although not others,46,47 appear to have confirmed that certain probiotics have overall beneficial effects in IBS on abdominal pain and distension as well as flatulence, compared to placebo, and may improve these patients’ quality of life.
However, in general, these are very mixed studies that have analysed and compared highly disparate combinations and doses of probiotics, and some have significant methodological deficiencies. Consequently, general recommendations cannot be established.
No specific strain or dose of any probiotic analysed appears to be consistently effective for improving IBS symptoms or quality of life.46 However, one study48,49 confirmed that symptoms significantly improved in the group of patients that received B. infantis (35,624) compared to placebo. In addition, the serum IL-10/IL-12 ratio normalised, suggesting that the probiotic may help to reduce a pro-inflammatory state associated with IBS.
L. plantarum is another probiotic that has been used with good results, superior to placebo, in the management of some symptoms in patients with IBS. Specifically, the DSM 9843 strain significantly reduced flatulence,50 and the LPO 151 and 299 V52 strains significantly reduced abdominal pain.
Our working group performed two meta-analyses to assess the effect of probiotics on IBS symptoms in general53 and abdominal pain in particular,54 and concluded that the beneficial effects of probiotics on each IBS symptom are probably strain-specific.
More data from high-quality RCTs dealing with specific profiles and symptoms are needed for probiotics to be recommended in the management of IBS.
Disease entities on which probiotics have possible beneficial effectsChronic idiopathic constipation (functional constipation)There are several potential mechanisms of action by which probiotics may be beneficial in functional constipation. First, they modify the gastrointestinal microbiota, which is abnormal in constipation.55 Second, probiotic metabolites may alter intestinal motility.56 Third, some probiotics increase production of lactic acid and short-chain fatty acids, thereby reducing luminal pH, which may improve colon peristalsis and shorten intestinal transit time.57
Based on this evidence, hypotheses as to the possible beneficial effect of certain probiotics on the management of CIC have been formulated. Several systematic reviews and meta-analysis have been conducted to assess this.42,58,59 These have concluded that, although probiotics have demonstrated some promising results in this regard, specifically an increase in the mean number of bowel movements per week, more high-quality RCTs are needed before they may be recommended in a standardised fashion in the management of CIC.
H. pylori infectionWhen H. pylori eradication is recommended,60 the rate of success is approximately 90% using a first-line treatment (in particular with concomitant quadruple therapy without bismuth, which has better results than classic triple therapy),61 and around 70% using a second-line treatment.62
In a 2007 MA, Tong et al.63 demonstrated that administering probiotics may both improve the rate of eradication and reduce the incidence of adverse events.
Subsequently, different studies and meta-analyses confirmed this,64–68 finding that the use of probiotics to supplement standard eradication therapy in patients infected with H. pylori may end up increasing the rate of eradication of the micro-organism by approximately 13% and decreasing the overall rate of adverse effects by approximately 41%, regardless of the patient's age, gender or dose of probiotics.66
Notable among the different types of probiotic used to improve the results of eradication therapies were Lactobacillus reuteri (L. reuteri),69 which demonstrated an ability to inhibit the colonisation of the human gastric mucosa by H. pylori,70 in addition to an ability to produce reuterin, a broad-spectrum antibiotic active against H. pylori.71 The DSM17648 strain of L. reuteri seemed especially effective for this.72
Adding S. boulardii also seemed to significantly increase the rate of eradication, although under the desired success level (80% versus 71% in the control group). This yeast also significantly reduced some treatment-related side effects.73
An economic evaluation is needed to establish the rate of cost-effectiveness of adding probiotics to H. pylori eradication treatment, since this undoubtedly increases treatment costs.
Ulcerative colitisAlthough, initially, a 2011 SR from the Cochrane Collaboration74 concluded that there were no sufficient tests to support the use of probiotics to induce or maintain remission in UC, most studies, though small, have concluded that probiotics are equivalent, or at least not inferior, to standard therapy for maintaining UC remission.75
One trial76 reported that therapy with Lactobacillus GG may be more effective than standard therapy, with mesalamine, for prolonging recurrence-free time.
Most studies with probiotics in UC have been conducted with VSL#3 or E. coli strain Nissle 1917.77–79 Results with these probiotics have a degree of recommendation of A for maintaining UC remission and a degree of recommendation of B for inducing UC remission, according to the 4th° Triennial Yale/Harvard Workshop on Probiotic Recommendations.80
Thus, E. coli strain Nissle 1917 was as effective as a low dose of 5-ASA in preventing UC recurrence in adults.81
A MA that included RCTs comparing VSL#3 to controls (placebo or 5-ASA) demonstrated this probiotic to have a significant benefit versus controls for inducing UC remission.82 Another subsequent MA, which compared VSL#3 to placebo, demonstrated remission rates of 43.8% in patients treated with this probiotic versus 24.8% in patients with placebo.83 Previously, another RCT84 found that patients who received VSL#3 tended to experience a decrease of at least 50% on the Ulcerative Colitis Disease Activity Index (UCDAI) after eight weeks of treatment, compared to patients who received placebo (63 versus 41 per cent). Remission rates were also higher in the VSL#3 group (48 versus 32 per cent). However, histological results did not significantly improve with treatment with VS#3.
Finally, although benefits with probiotics have generally been anecdotal, studies have also been conducted with probiotics in Crohn's disease.
Crohn's diseaseThe vast majority of RCTs that have used probiotics in CD have been conducted in patients with inactive disease (in remission), to prevent clinical reactivation and/or endoscopic recurrence.
Studies conducted with different types of Lactobacillus85–87 and with E. coli strain Nissle 191788,89 have not demonstrated any of them to be superior to placebo in terms of preventing recurrence of the disease.
Two SRs from the Cochrane Collaboration concluded that the data available did not support the clinical efficacy of probiotic treatment in patients with CD either for inducing90 or for maintaining remission.91
In addition, one study92 demonstrated that S. boulardii, together with 2g daily of mesalamine, was superior to 3g daily of mesalamine alone in reducing clinical recurrences in patients with CD in remission. Another subsequent study93 did not confirm these results.
In general, with the possible exception of S. boulardii in certain populations (e.g. non-smokers), long-term treatment with probiotic micro-organisms does not seem to yield benefits in maintaining remission in patients with CD.94
Regarding induction of clinical remission, in general, there is no convincing evidence that probiotics show a significant capacity to increase the efficacy of conventional treatments in patients with CD.94
Table 1 presents the most outstanding data from all SRs and MAs used to prepare the article. From these, the following conclusions and recommendations may be drawn:
- (1)
Most of the studies reviewed were small, and many had significant methodological limitations. Consequently, it is difficult to draw unequivocal conclusions on the efficacy of the probiotics used. Large, well-designed, multi-centre, controlled clinical trials are needed to clarify the role of specific probiotics in different well-defined patient populations.
- (2)
There are considerable differences in terms of composition, doses and biological activity among various commercial probiotic preparations. Consequently, results with one preparation cannot be extrapolated to other preparations or all probiotic preparations.
- (3)
No strategy with a probiotic may currently be considered the standard primary treatment in any of the diseases reviewed above.
- (4)
Following assessment of the quality and consistency of the available evidence reviewed, in the author's opinion, certain probiotics, which vary by process, have been shown to be effective and beneficial in acute infectious diarrhoea, antibiotic-associated diarrhoea, C. difficile-associated diarrhoea and pouchitis and in H. pylori infection eradication, and thus may be recommended for use in these processes:
- -
L. rhamnosus GG and S. boulardii have clear beneficial effects in shortening the duration and reducing the frequency of bowel movements in acute infectious diarrhoea. Similarly, they appear to significantly decrease the risk of antibiotic-associated diarrhoea and C. difficile-associated diarrhoea. Supplementation with these two probiotics could be effective for increasing H. pylori eradication rates.
- -
VSL#3 in primary and secondary prevention of pouchitis, in addition to standard medical therapy.
- -
E. coli strain Nissle 1917 appears to be promising in maintaining UC remission and could be considered as an alternative in patients who are intolerant or resistant to 5-ASA preparations.
- -
The author declares that he has no conflicts of interest.
Please cite this article as: Sebastián Domingo JJ. Revisión del papel de los probióticos en la patología gastrointestinal del adulto. Gastroenterol Hepatol. 2017;40:417–429.