Melanosis coli (MC) is a common condition characterized by a black or brown pigment deposited in the colorectal mucosa. It is a reversible condition that is influenced by many factors, such as living habits and bowel function. However, the epidemiology and etiology of MC are still unclear. Most studies show that there is a significant correlation between the use of anthraquinone laxatives and the occurrence of MC. At present, the mechanism of the apoptosis theory is widely recognized as regards the pathogenesis of MC. There is no specific clinical manifestation of MC, and its diagnosis is mainly based on a complimentary examination, such as endoscopic and histopathological tests. General treatment, such as changing living habits, is preferred, and medical or surgical treatment should not be considered in the absence of serious malignancy. The aim of this review is to systematically present and outline the concepts of the epidemiology, etiology, histopathology, pathogenesis, clinical manifestations, diagnosis and treatment of MC, in order to improve the understanding of this condition.
La melanosis coli (MC) es una entidad común caracterizada por el pigmento negro o marrón depositado en la mucosa colorrectal. Es una entidad reversible que está influenciada por muchos factores, como los hábitos de vida y la función intestinal. Sin embargo, la epidemiología y la etiología de la MC todavía no están claras. La mayoría de los estudios apoyan que existe una correlación significativa entre el uso de laxantes antraquinónicos y la aparición de MC. Actualmente, el mecanismo de la teoría de la apoptosis es ampliamente reconocido con respecto a la patogénesis de la MC. No existe una manifestación clínica específica de la MC, y el diagnóstico de la MC se basa principalmente en un examen auxiliar, como las pruebas endoscópicas e histopatológicas. Se prefiere el tratamiento general, como el cambio de hábitos de vida; por el contrario, no se debe considerar el tratamiento médico o quirúrgico en ausencia de una malignidad grave. El objetivo de esta revisión es entregar y resumir sistemáticamente los conceptos de la epidemiología, la etiología, la histopatología, la patogénesis, las manifestaciones clínicas, el diagnóstico y el tratamiento de la MC para mejorar la comprensión de esta entidad.
Melanosis coli (MC) is a noninflammatory bowel entity in which a brown or black pigment is deposited in the colorectal mucosa. MC is usually caused by the long-term use of anthraquinone laxatives, such as senna, rhubarb, aloe, rhamnus, and frangula.1,2 MC, which is common in people who suffer from long-term constipation, is a benign, reversible entity and can be gradually improved over one year after stopping the use of laxatives.2 It has been found that MC could be formed in a few months and could then return to normal within several months after discontinuing the use of laxatives.3 MC was discovered by Cruveilhier in 18294 and is frequently found in the cecum, colon and rectum but is rarely seen in the ileum and jejunum.5 However, Chaudhary et al.6 showed that MC could also involve the duodenum, ileum and jejunum. In 1857, Virchow formally put forward the concept of “melanosis coli”.7 MC is becoming increasingly common because there are many pathological conditions of the bowel that prompt the use of anthraquinone laxatives.8 To promote a better understanding of MC, we will summarize the recent progress made in MC research.
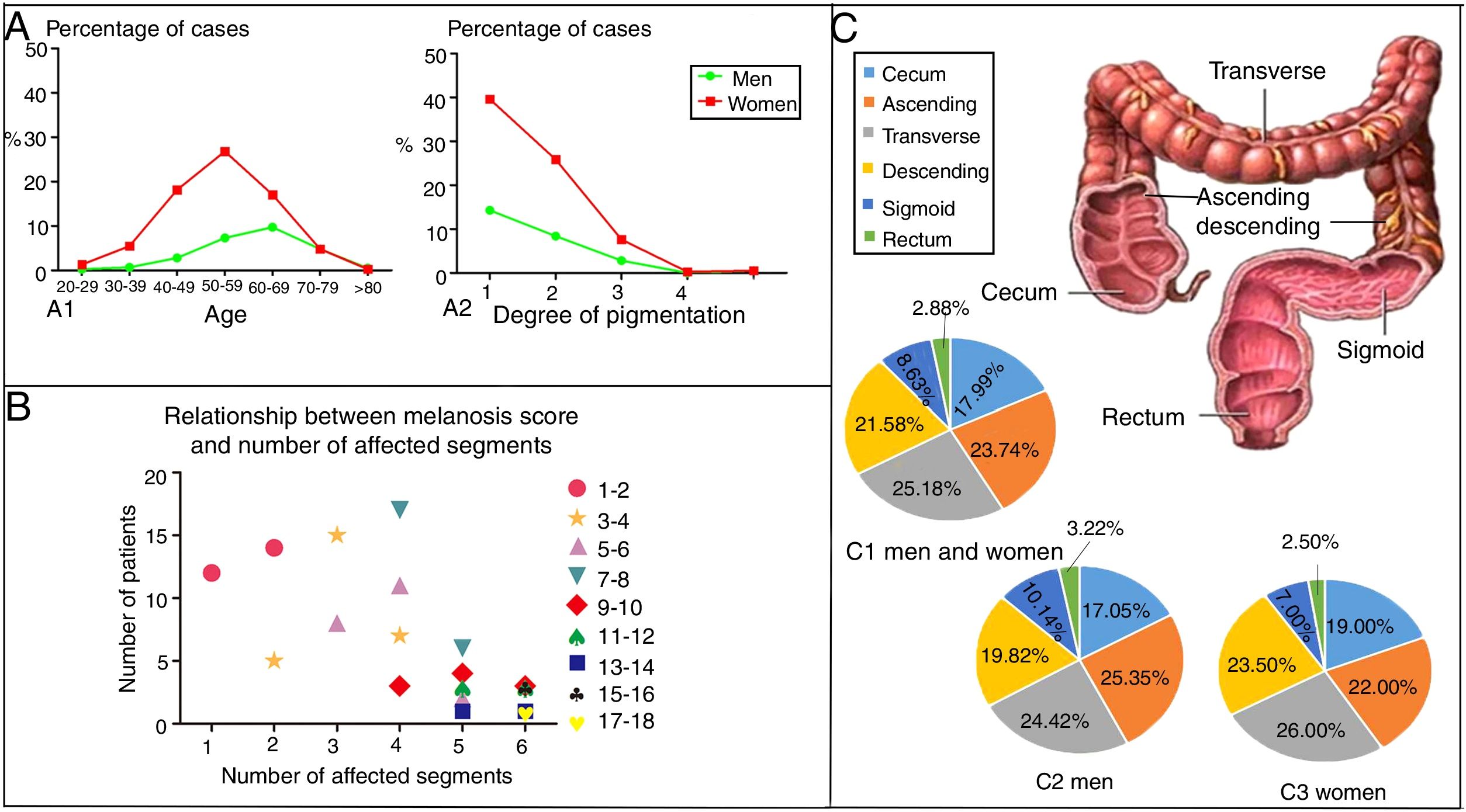
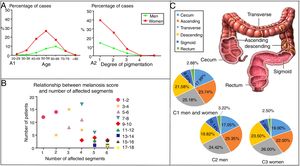
EpidemiologyWittoesch et al.9 showed that the incidence rates of MC range from approximately 0.82% to 1.13%. Among these cases, the majority of patients are women. Ninety-five percent of all MC patients are taking anthraquinone laxatives. Furthermore, MC occurs more often in the proximal colon (mouth side) and is relatively rare in the distal colon (anal side). In brief, the incidence rate of MC differs by sex and age; furthermore, the affected segments also differ10 (Fig. 1). There is a wide age range in people with MC, and it has been reported that a 4-year-old child who used senna syrup long-term secondary to constipation had MC. There is a lack of race-based or regional studies on this entity.11
The epidemiology of MC. (A) The distribution by age, sex and degree of pigmentation of 750 patients suffering from MC. As shown in (A1), women aged 50–59 years old accounted for most of the cases, while the most cases in men were found in the group aged 60–69 years. As shown in (A2), as the degree of pigmentation increased, the percentage of cases decreased. (B) Relationship between the melanosis score and the number of affected segments. The colon was divided into six parts, including the cecum, ascending colon, transverse colon, descending colon, sigmoid colon and rectum. The intensity of melanosis was determined by calculating the sum of the melanosis grades of individual segments. The grade of melanosis in each segment ranged from 0 to 3 according to the degree of pigmentation. As the melanosis score increased, the number of affected segments increased, and the number of patients decreased. Therefore, compared with a few affected segments with severe pathological character, more affected segments with lower grade of melanosis are easier to be seen in the same score of MC. (C) A total of 417 segments were affected by MC in 119 patients. As shown in (C1), the patients included both men and women. Only men are shown in (C2), and only women are shown in (C3). The incidence of affected segments was relatively higher for the ascending colon and transverse colon and lower for the sigmoid colon and rectum in both men and women.
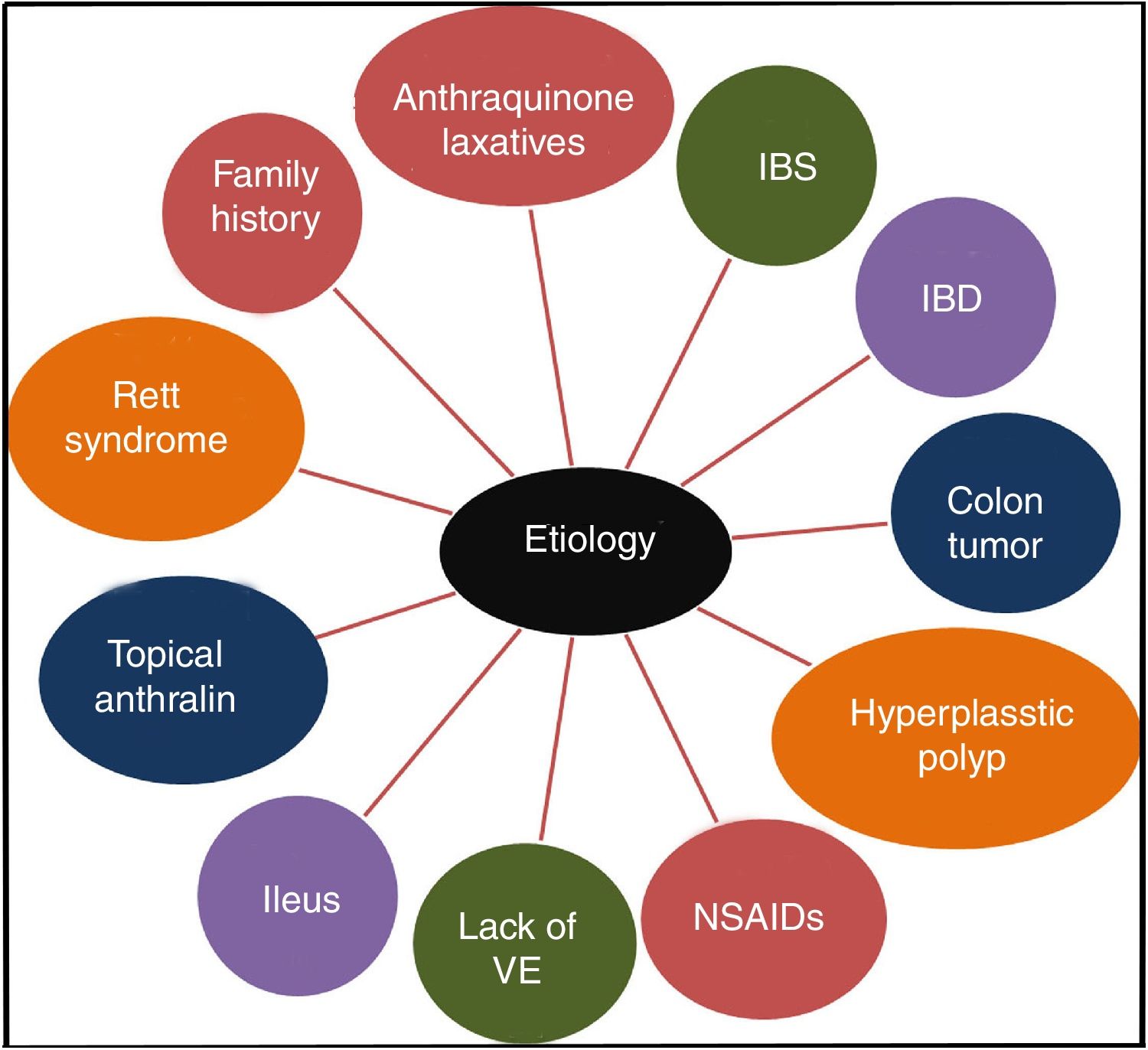
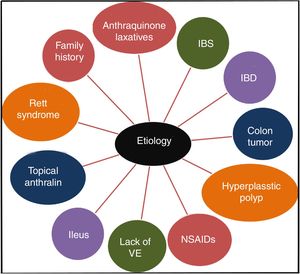
It has been confirmed that the long-term use of anthraquinone laxatives, which have been widely used as over-the-counter (OTC) drugs,12 can lead to MC,13 though the pathogenesis is not clear. This practice is the primary but not the only cause of the condition. Other causes, such as irritable bowel syndrome (IBS),14 inflammatory bowel disease (IBD), colonic neoplasms, and hyperplastic polyps, could also contribute to the onset of MC.13,15 Some studies show that MC may be connected to the use of non-steroidal anti-inflammatory drugs (NSAIDs),16 vitamin E (VE) deficiency, the intake of unsaturated fatty acids,17 environmental factors,18 family history,3 psoriasis19 and Rett syndrome.20 Feces stasis also plays an important role in the pathogenesis of MC. Samenius proposed that MC is more frequently observed in patients with an obstruction anywhere in the colon and rectum than in cases of chronic constipation where there is no mechanical cause for the constipation.8 A list of the etiologies of MC is shown in Fig. 2.
HistopathologyIt can be seen under a microscope that large numbers of macrophages with rich pigment are deposited on the mucosal lamina propria, and a small number of neutrophils and granulocytes can be visualized at the same time. Ewing et al.21 found many macrophages with rich pigment deposition on the mucosal lamina propria of the colon, the colonic submucosa and the submucosal lymphatic vessels through hematoxylin and eosin (HE) staining, which could also be discovered in the colonic mucosa and lymph nodes by periodic acid-Schiff staining (PAS), melanin staining, Fite staining and Congo red staining.22 Gomori iron staining is negative in the colonic mucosa and lymph nodes, as is the immunohistochemistry results for S100, keratin and HMB-45. CD86 is overexpressed in the cytoplasm and adjacent lymph nodes of macrophages with rich pigments. Regarding the infiltration of adjacent lymph nodes, Hall et al.23 concluded that yellow-brown spindle lymph nodes might contribute to the changes in MC, although the exact relationship between these two conditions is not clear. As shown by electron microscopy, large numbers of mononuclear cells with pigment granules are observed in the mucosa of MC, an observation that can be divided into 4 histological grades24 (Table 1). These changes cause the tissue or cell to malfunction and the nucleus to undergo degenerative changes, such as nuclear condensation and fragmentation.
The grades of MC and histological characteristics.
| Grades | Histological characteristics |
|---|---|
| 1 | Pigments confined to the mucous membranes, mainly occurring in monocytes and not present in epithelial cells. |
| 2 | High pigment density reaching the mucosal muscle layer. Some of the free pigmentation is present. |
| 3 | Pigment deposition in the mucosal and submucosal layers. |
| 4 | Marked presence of dense pigmentation in the mucosal muscle layer. This pigment can also be seen in local lymph nodes. |
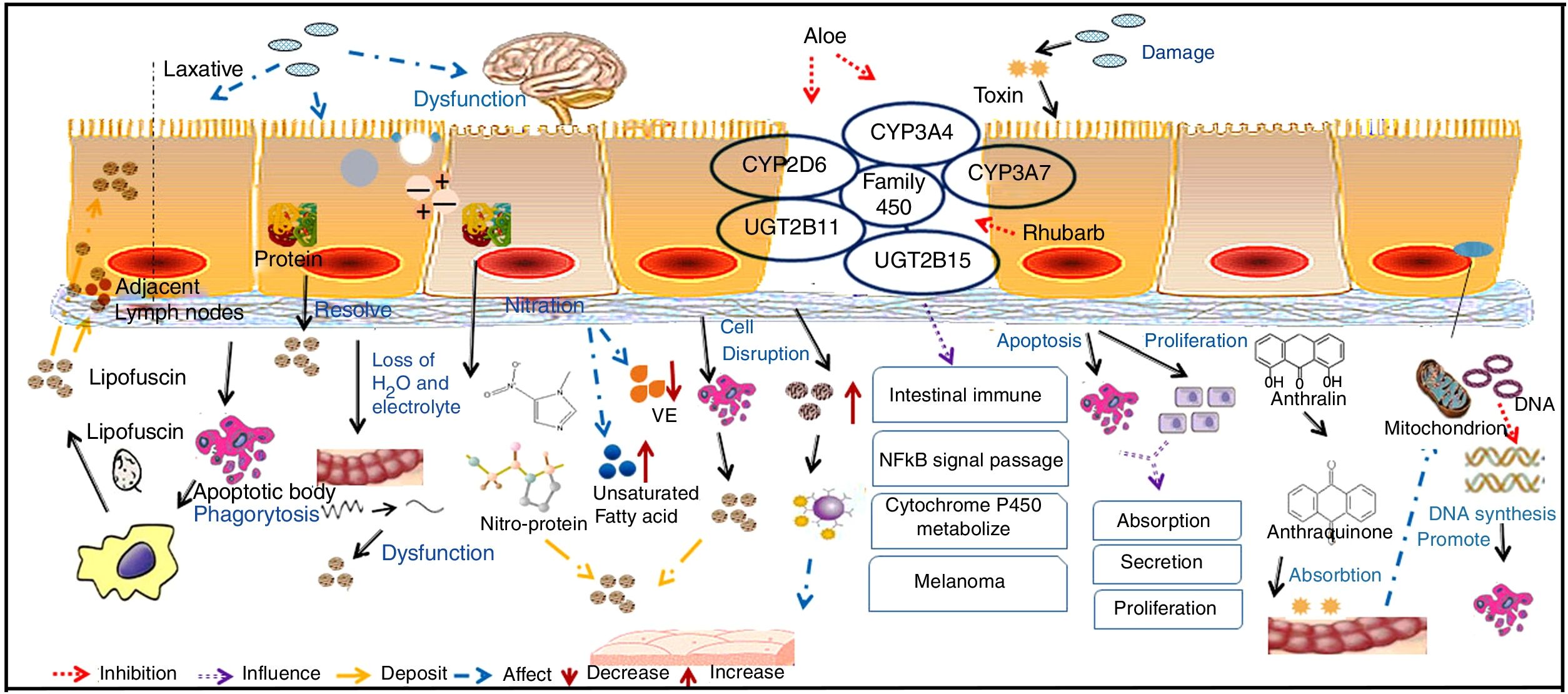
The pathogenesis of MC is not clear. However, some current theories are shown in Fig. 3.
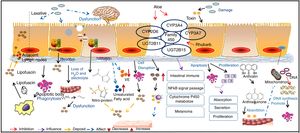
Mucosal epithelium cell apoptosis is involved in the pathogenesis of MCMost reports support the idea that pigment deposition is correlated with epithelial cell apoptosis caused by the use of laxatives. Moreover, it is now recognized that any cause of mucosal cell apoptosis can lead to this pigmentation, such as NSAIDs and IBD, which can also induce epithelial cell apoptosis.25 Some researchers have found that anthraquinone laxatives are decomposed by bacteria that consequently produce a type of rhubarb derivative in the caecum.26 The rhubarb derivative damages the intestinal epithelial cells and then promotes the effects of the laxative but then can lead to death of these cells, which are immediately engulfed by nearby macrophages. The macrophages generate tumor necrosis factor-a (TNF-a), which produces toxic effects on capillary vessels and then damages neurons, inducing cell swelling and decomposition. The main effects of this process are the breakdown of the cell nucleus and damage to the organelles.27 Apoptotic bodies then transform to lipofuscin under the action of heterogeneous lysosomes in the macrophages, which migrate into the mucosal lamina through the basement membrane. As a result, brown or black pigment will appear on the mucosal membranes.28 Some macrophages with abundant pigments may be present in the submucosal layer, submucosal lymphatic vessels and some other parts of the organ.21 In addition, GNG5, LPAR3, MAPK8, and PSMC6 might be candidate biomarkers associated with apoptosis in MC.29 Yamate et al.30 speculated that as the apoptosis of the mucosal epithelium or lamina propria layer is reduced, it could delay the progression of MC.
Intestinal dysfunction is involved in the pathogenesis of MCChanges in sympathetic function, electrolyte disorders and decreased water absorption lead to intestinal dysfunction. The unabsorbed residues deposit in the intestine, which forces the intestinal mucosa to fill with large quantities of black or brown pigment.31 The use of anthraquinone laxatives can result in the displacement of electrolytes and water loss in the intestinal tract and sequentially lead to the deposition of protein products after using such laxatives for a long period.
Oxidative damage and protein nitration are involved in the pathogenesis of MCResearch revealed that the positive immune markers of 3-nitrotyrosine (3-NT), 4-hydroxynonenol (4-HNE), and malondialdehyde (MDA) are significantly increased in a pig model of MC, among which 4-HNE and MDA have been identified as robust markers of oxidative stress, while 3-NT is a biomarker of endogenous peroxynitrite activity. This finding supported that oxidative damage and protein nitration are involved in the pathogenesis of MC. VE is an antioxidant substance that exists in the intestinal cell basement, and lipid peroxidation is produced by the interaction between free radicals and polyunsaturated fatty acids. As a result, VE deficiency and the intake of unsaturated fatty acids may contribute to the development of MC.17
Changes to mast cells in the colon are involved in the pathogenesis of MCMast cells secrete a variety of cytokines in the intestine and participate in the regulation of immunity. In addition, mast cells release allergic media and have a weak phagocytic function. When the number of mast cells increases, the disintegration and release of a large number of granules will cause allergic reactions in tissues. At the same time, this process will influence the function of intestinal immunity and will lead to the deposition of pigment on the mucosa.31 The long-term use of laxatives can cause epithelial cell damage, and the disruption of mucosal integrity triggers the release of immune competent cells, such as mast cell, in turn leading to the deposition of pigment on the mucosa.32
Direct toxic effects of drugs on epithelial cells are involved in the pathogenesis of MCIt has been concluded that anthraquinone laxatives would have direct toxic effects on colonic epithelial cells, which tend to produce lipofuscin, engulfed by nearby macrophages and then generate brown or black pigments.3 It has been suggested that anthraquinone laxatives produce toxic substances in the large intestine, where they damage epithelial cells.31 These substances alter the functions of normal cellular absorption, secretion and proliferation by inducing apoptosis, reducing the number of mucosal crypts and increasing cell proliferation.
Changes in gene expression are involved in the pathogenesis of MCLi et al.33 analyzed the expression profiles of the CYMP3A4, CYP3A7, UGT2B11 and UGT2B15 genes, which belong to the cytochrome P450 family. These genes usually participate in intestinal immunity, the nuclear factor κB (NF-κB) signaling pathway, cytochrome P450 metabolism and melanoma formation.34 It has been confirmed that aloe leaves can inhibit CYP3A and CYP2D6 expression in vivo and that rhubarb can inhibit the P450 family through antimutation actions.35 DNA microarray analysis indicated that in tissues with MC, CYP3A4 expression was significantly decreased compared with that in normal tissue. However, this specific mechanism requires further exploration. However, anthralin might be related to the inhibition of mitochondrial DNA synthesis and gene toxicity, as its chemical structure is not stable and it is easily oxidized to a free radical with biological activity.
Clinical manifestationsSymptoms and signsThere are no specific symptoms or signs of MC: the symptoms are usually those derived from constipation. Abdominal pain and abdominal distension are also often present.
Laboratory and auxiliary examinationsIn most cases, the serological examination is normal, and a small number of patients have hypokalemia, hypernatremia, or hypocalcemia.30
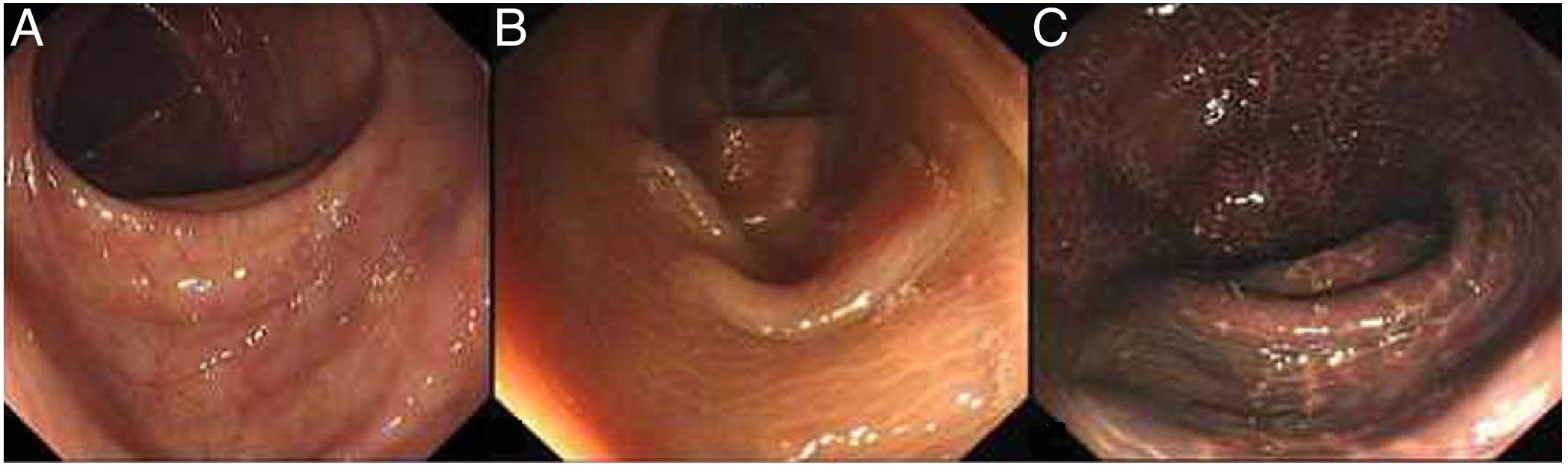
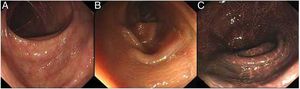
A colonoscopy examination is the most important tool in the diagnosis and differential diagnosis of MC. MC is visible with varying degrees of pigmentation on the colonic mucosa by colonoscopy (Fig. 4). It is usually manifested as a diffuse, irregular, dark brown mucosal discoloration on the right side of the cecum and colon, which is often compared to the skin of crocodiles or snakes.36 In severe cases, all of the colonic mucosa of the external rectum is black. The range and extent of the lesion can be determined by biopsy.
The gradation of MC under endoscopy. The images were obtained from the Endoscopy Center of the Second Affiliated Hospital of Harbin Medical University. MC can be divided into three degrees according to the depth of pigmentation seen by colonoscopy. (A) Mild: the mucosa is edematous with diffuse brownish pigmentation and a snake-like appearance. (B) Moderate: the mucosa shows diffuse brownish pigmentation with yellow-brown granular pigment. (C) Severe: the entire colon is suffused with black-brownish pigmentation.
X-ray examination is not specific.37 Sometimes there will be a movement disorder of the colon that can be seen as the presence of stenosis at the junction of the sigmoid colon and rectum.
DiagnosisThe gold standard for diagnosing MC includes endoscopic manifestations and histological pathology. In addition, there is no significant correlation between adenomas and melanosis, but the presence of MC is associated with a significant increase in the detection rates of adenomas due to the lack of pigmentation in the adenomas themselves38 and vice versa; therefore, the presence of adenomas may also facilitate the diagnosis of early MC.39
Treatment and prognosisGeneral treatmentFirst, patients with MC should stop the use of anthraquinone laxatives. Additionally, developing good living habits, maintaining a positive attitude and consuming foods containing fiber and whole grains or fruits will be very helpful.40,41 At the same time, increasing the amount of exercise,42 particularly activities that exercise the abdominal muscles and the muscles of the anus or massage the abdomen, can appropriately aid in the process of defecation.
Medicinal treatmentThere is no proven treatment plan for MC except symptom minimization. However, a recently published case report showed that the condition of a 59-year-old woman who was previously confirmed to have MC by colonoscopy and biopsy was resolved after the patient began intravenous immunoglobulin administration despite continuing to take anthraquinone laxatives.43 However, the specific mechanism and long-term clinical effects are still unclear, and this approach may be a new method to treat MC in the future. For people with constipation, it is better to regulate the flora in the gastrointestinal tract and the shape of the feces by taking gastrointestinal motility drugs and promoting intestinal microbial preparation. Patients with polyps or tumors should be treated in a timely manner and should undergo colonoscopies regularly to prevent carcinogenesis.
Surgical treatmentSun et al.44 tested 48 patients with chronic constipation and demonstrated that patients with MC are more suitable for ileosigmoidal anastomosis (ISA) than cecorectal anastomosis (CRA).
ConclusionMC is a benign and reversible entity that is characterized by black or brown pigment deposited in the colorectal mucosa. MC is 95% related to the use of anthraquinone laxatives and thus is becoming increasingly common because there are many pathological conditions of the bowel that prompt the use of anthraquinone laxatives. Although the pathogenesis is not completely clear, the role of mucosal epithelial cell apoptosis, oxidative damage, retention of decomposed products, changes to the mast cells and other factors has aroused attention. There are no specific symptoms and signs of MC, as the condition mainly produces constipation, abdominal pain, and abdominal distension. The diagnosis of MC is mainly based on the observation of brown pigment deposits in the colorectal mucosa under the microscope or the presence of brown granules in the cytoplasm of submucosal macrophages. The treatment for MC is mainly to change the patient's way of life, and there are no targeted therapeutic drugs. Drug-induced MC is generally cured after discontinuing the taking of anthraquinone drugs for a year.
Authors’ contributionsYNN drafted the manuscript. RMT performed the acquisition and analysis of data. JSZ critically revised the manuscript. YNN and RMT plotted the figures. JSZ is the guarantor. All authors read and approved the final manuscript.
Financial disclosureThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors declare no conflict of interest.