Patients with major depressive disorder (MDD) have high comorbidity with metabolic syndrome (MetS), although anxiety is prevalent comorbidity in MDD patients. However, there is no study on anxiety symptoms (AS) in MDD patients with MetS. Therefore, we aimed to identify the prevalence and risk factors of AS in patients with MetS who experienced a first-episode and drug naïve (FEDN) of MDD.
MethodsIn this cross-sectional study, 1718 FEDN of MDD outpatients with MetS were included. Sociodemographic data, clinical characteristics, suicidal attempts, and physical and biochemical parameters were collected. Hamilton Anxiety Rating Scale (HAMA), Hamilton Depression Rating Scale (HAMD), and Positive and Negative Syndrome Scale (PANSS) positive subscale were performed to detect the AS. Multiple linear regression analysis was used to analyze the correlation.
ResultsThe prevalence of AS in MDD patients with MetS was 85.96%, which was 1.79 times greater than that in patients with MDD alone (P<0.05). MDD patients with MetS had a greater rate of attempted suicide, a higher HAMD total score, and a higher diastolic blood pressure than MDD patients without AS (P<0.05). Their combination could distinguish AS in MDD patients. Moreover, HAMD score, thyroid-stimulating hormone (TSH) levels, PANSS positive score, and suicide attempts were related to HAMA scores in MDD patients with comorbid MetS (P<0.05).
ConclusionThere is a significant frequency of AS in MDD patients with MetS. Multiple clinical indicators and metabolic markers are associated with AS in patients with MDD and MetS.
Major depressive disorder (MDD) is a common psychiatric disorder in China, with a lifetime prevalence of 3.4% and a one-year incidence of 2.1%.1 It is considered the 2nd leading cause of entire life disability,2 and has been estimated as one of the leading causes of disease burden.3 MDD patients have high comorbidity with MetS, which is distinguished by the presence of abdominal obesity, high blood pressure (BP), high triglyceride (TG), decreased high-density lipoprotein cholesterol (HDL-C), and elevated fasting blood glucose (FBG) or diabetes mellitus (DM).4 Previous researches have demonstrated that there are comorbid MetS in approximately 20.0%−34.3% of MDD patients,5–8 which is 1.54 times higher than the general population.6 A population-based study among Russian, Somali, and Kurdish adults in Finland show that depressive symptoms are positively associated with MetS and individual components of MetS, including elevated TG and elevated waist circumference (WC), particularly hypertension.9
Recent researches have demonstrated that anxiety symptoms (AS) are highly prevalent in MDD patients, with prevalence ranging from 53.2% to 80.3%.1011 MDD patients with AS have more suicidal ideation and attempted suicide rates, as well as lower remission rates and higher functional impairment risk. In addition, there are more relapses in MDD patients with AS.1213 Several researches have explored the AS incidence in MetS patients, but there are inconsistent results. For example, Shinkov et al. have reported that patients with MetS have more anxiety scores.14 Moreover, a cross-sectional study of 321 Kurds shows an association between AS and MetS9 Another study shows similar results that MetS is strongly related to AS.15 Furthermore, associations between AS and different components of the MetS have been observed. A systematic review and meta-analysis recoveries a link between anxiety and a higher chance of developing hypertension.16 Skogberg et al. have claimed a positive association between AS and different components of the MetS, including elevated FBG and TG levels, and hypertension9 However, MetS are reported to be not correlated with a higher prevalence of anxiety.17 The inconsistency of previous studies has been attributed to sampling heterogeneity and pharmacological treatments. Therefore, the relationship between MetS and AS needs further investigation.
Given the high comorbidities with AS and MetS in MDD patients, especially a potential association between AS and MetS in the general population, researches on the studying incidence of AS and it's clinical correlates in MDD patients with MetS are very interesting. In this study, we hypothesized that there would be a high AS incidence in FEND MDD patients with MetS, and some socio-demographic features and clinical factors may be independently related to AS in MDD patients with MetS.
Subjects and methodsSubjectsThis study was approved by the ethics committee of Shanxi Medical University's first hospital. All patients or their guardians provided their informed consent before participation. A total of 1796 outpatients from the Psychiatry department at The First Hospital of Shanxi Medical University between 2015 and 2017 were enrolled in this cross-sectional study. Inclusion criteria: (1) patients with a clinical diagnosis of MDD as per the Diagnostic and Statistical Manual of Mental Disorders (DSM-Ⅳ) 4th edition; (2) first-episode and no previous treatment with psychotropic medication; (3) age between 18 and 60 years; (4) Han Chinese; and (5) the total score ≥24 on the HAMD-17. Exclusion criteria: (1) patients with physical illness; (2) alcohol or drug abuse or dependence except for smoking; (3) organic brain diseases; (4) pregnant or lactating women; and (5) diseases that were consistent with other major Axis I disorders. However, there were 78 patients who were excluded due to the following reason. (1) pregnancy or lactation (n = 10); (2) substance use disorder (n = 9); (3) severe personality disorder (n = 15); (4) severe physical diseases (n = 9); (5) refused to participate in the study (n = 21); (6) not be interviewed due to acute clinical condition (n = 5) and (7) other unknown reasons (n = 9). Therefore, there were 1718 patients included in this study.
Demographics and clinical measurementsSociodemographic characteristics, such as age, sex, marital status, and education level, were collected via self-designed questionnaires. Two psychiatrists assessed depressive symptoms, AS, and psychotic symptoms by using the Hamilton Anxiety Rating Scale (HAMA), Hamilton Depression Rating Scale (HAMD), and Positive and Negative Syndrome Scale (PANSS) positive subscale, respectively. MDD patients with a total HAMA score of 18 or above were determined to have comorbid AS.11 These two psychiatrists underwent professional training before the study evaluation of PANSS, HAMD, and HAMA. The inter-rater correlation coefficients for the HAMD total score, HAMA total score, and PANSS positive subscale ranged from 0.82 to 0.85. In addition, suicide attempts in a lifetime were also assessed by face-to-face interviews. A WHO/EURO multi-center study18 provided the question "Have you ever attempted suicide in your lifetime?". When patients answer "yes" to this question, time variables, methods and the exact date of each suicide attempt need to be collected.
Measurement of physical and biochemical parametersWeight (kg) was divided by squared height (m2) to calculate body mass index (BMI). TC, HDL-C, LDL-C, FBG, TG, TSH, free triiodothyronine (FT3), free thyroxine (FT4), thyroid peroxidases antibody (TPOAb), antithyroglobulin (TgAb), and BP were tested. The criteria for the diagnosis of MetS were established by the Adult Treatment Panel of the National Cholesterol Education (NCEP ATP III),4 meeting any 3 of the 5 categorical cutoff criteria. (1) central obesity (WC >102 cm in males and >88 cm in females) or BMI ≥ 25; (2) TG ≥ 1.7 mmol/L or the treatment for this lipid abnormality; (3) HDL-C <1.03 mmol/L in men, and <1.29 mmol/L in women, or being treated with medication for this abnormality; (4) systolic BP ≥ 130 mmHg or diastolic BP ≥ 85 mmHg or diagnosed or being treated for hypertension; and (5) FBG ≥ 5.6 mmol/L or 2 h postprandial blood glucose ≥ 7.8 mmol/L or diagnosed or treated for diabetes mellitus.
Statistical analysisSPSS 26.0 (SPSS, Inc., Chicago, IL) was utilized for statistical analysis. Variables normality was checked by the Kolmogorov-Smirnov one-sample test. For the comparison of continuous variables, the independent sample t-test was utilized. The Mann-Whitney test and χ2 test were respectively used to analyze not-normally distributed and categorical data. To control for positive errors, Bonferroni correction was applied. Binary logistic regression (Backward: Wald) was applied. Univariate analysis was used to investigate the associations of several factors and AS in MDD patients with MetS. Additionally, the area under the receiver operating characteristic (AUC ROC) was employed to ascertain the discriminatory power of key indicators in separating individuals with and without AS. A statistical value of consistency considered acceptable19 ranged from 0.7 to 0.8 in general. Finally, to investigate the association of HAMA score with clinical and biochemical factors, a multivariate linear regression analysis was performed. GraphPad Prism 9.0 and Sigmaplot 14 were applied to plot the graphs. The P-value significance level was set at 0.05, two-tailed.
ResultsPrevalence of AS in MDD patients with MetS compared to MDD patients without MetSThe proportion of MDD patients with MetS was 34.40% (591/1718). MDD patients with MetS had higher PANSS positive symptom scores and HAMD scores compared to MDD patients without MetS (P<0.001). In addition, AS was more prevalent in MDD patients with MetS (85.96%) than in MDD patients without MetS (77.37%) (χ2=18.07, P<0.001, OR=1.79, 95%CI: 1.37–2.35).
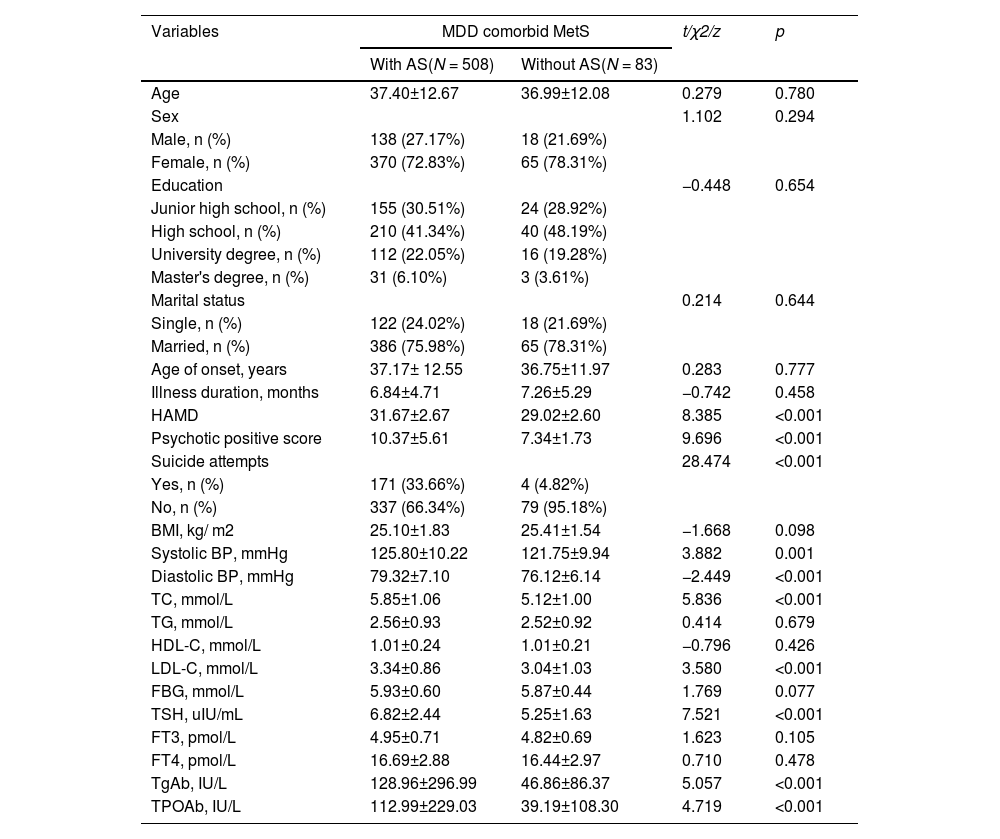
Comparison of clinical characteristics and biochemical indicators of AS and non-AS in MDD patients with MetSAs shown in Table 1, there were significant differences between the AS subgroup and the non-AS subgroup in terms of HAMD score, PANSS positive symptom score, suicide attempts, systolic and diastolic BP, TC, LDL-C, ATPO, ATG, and TSH (P ≤ 0.001). Among these variables, the AS subgroup had higher HAMD score, PANSS positive symptom score, suicide attempt rates, and higher levels of TC, LDL-C, TSH, TgAb, TPOAb, diastolic, and systolic BP compared with the non-AS subgroup (P<0.05).
Socio-demographic and clinical characteristics between MDD patients with comorbid metabolic syndrome with and without anxiety symptoms.
HAMD, Hamilton Rating Scale for Depression; HAMA, Hamilton Anxiety Rating Scale; BMI, body mass index; BP, blood pressure; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; FBG, fasting blood glucose; TSH, thyroid stimulating hormone; FT3, free triiodothyronine; FT4, free thyroxine; TgAb, anti-thyroglobulin; TPOAb, thyroid peroxidases antibody.
We focused on the risk factors of AS in MetS patients. The variables with significant differences in univariate analysis were included in logistic regression (Backward: Wald) to determine the risk factors of AS in MetS patients. The following factors were the risk factors for AS that were found in MDD patients with MetS, including HAMD score (B = 0.282, P < 0.001, OR = 1.325), suicide attempts (B = 1.563, P = 0.004, OR = 4.772), and diastolic blood pressure (B = 0.039, P = 0.043, OR = 1.040) (Table 2 and Fig. 1). In addition, the AUC ROC values for the different risk factors were 0.762 for HAMD, 0.644 for suicide attempts, and 0.635 for diastolic BP. Finally, when these parameters were combined, the combination of HAMD score, suicide attempts, and diastolic BP had a high AUC value of 0.81 to differentiate AS from non-AS (P < 0.0001, 95%CI = 0.7570–0.8556, Fig. 2).
Factors associated with anxiety symptoms in MDD patients with metabolic syndromes.
| Items | B | Wald statistic | P | OR | 95%CI |
|---|---|---|---|---|---|
| Suicide attempts | 1.563 | 8.383 | 0.004 | 4.772 | 1.657–13.747 |
| HAMD score | 0.282 | 24.558 | <0.001 | 1.325 | 1.186–1.482 |
| Diastolic Blood Pressure | 0.039 | 4.108 | 0.043 | 1.040 | 1.001–1.080 |
HAMD, Hamilton Rating Scale for Depression.
Multivariate linear regression analysis revealed that HAMD score (B = 0.380, t = 7.723, P<0.001), TSH (B = 0.186, t = 2.932, P = 0.004), PANSS positive subscale score (B = 0.284, t = 11.705, P<0.001) and number of suicide attempts (B = 0.743, t = 4.656, P<0.001) were all independently associated with HAMA score.
DiscussionThis is the first investigation to detect the incidence and related factors of AS in a large sample size of FEDN MDD patients with MetS. We found that AS prevalence was elevated by 1.79 times more in MDD patients with MetS (85.96%) than in patients without MetS (77.37%). Furthermore, HAMD score, suicide attempts, and diastolic BP were associated with AS in MDD patients with MetS, and their combination could distinguish AS and non-AS. Moreover, the HAMD score, TSH, PANSS positive subscale score, and the number of suicide attempts were related to the HAMA score in MDD patients with MetS.
In this study, we found that MetS may enhance the risk of AS in MDD patients. There is an association between AS and MetS in the participants of Kurdish origin.9 A meta-analysis of 18 cross-sectional studies revealed a statistically significant association between AS and MetS.20 More recently, a large population-based study21 has reported a prevalence of 30.2% in generalized anxiety disorder (GAD) among participants with MetS, which is higher than the prevalence of 20.9% among participants without MetS. However, the evidence about whether MetS increases the risk of AS is inconsistent. A previous study has shown an opposite result that MetS is not associated with AS.17 Furthermore, a cross-sectional study also obtains contradictory results that MetS is related to comorbid anxiety-depressive symptoms, not solely to anxiety or depressive symptoms, 22 suggesting that MetS does not increase the AS risk. Additionally, another cross-sectional study, part of the prospective Isfahan cohort study, also shows no significant association between AS and MetS.23
There are four reasons that may partially explain these differences. First, the heterogeneity of the subjects that are collected in other studies, which may influence AS, including the factors of the severity of depression and the various stages of economic load. Studies has reported that AS is positively associated with depression severity,24 and more AS is demonstrated in participants with large financial burdens.25 Second, antidepressants or benzodiazepines used by MDD patients may influence AS.26 A systematic review including 10 randomized controlled trials has reported that antidepressants usage is significantly associated with a reduction in AS.27 However, there is an opposite result that depression treatment leads to AS.28 Therefore, we selected patients with first-episode and untreated MDD to minimize the effects of antidepressants or benzodiazepines in this study. Third, MetS is defined differently according to different diagnostic criteria, such as NCEP-ATPIII,4 the Joint Committee for Developing Chinese Guidelines (JCDCG), and the International Diabetes Federation.29 Most importantly, the consistency of these criteria was not very good. In this study, the NCEP-ATPIII definition is used to measure MetS, which is a valid, reliable, and universal diagnostic criterion for MetS. Fourth, differences in the definition of AS should be considered. In STAR*D, a score ≥7 on the HAMD-17 anxiety/ somatization subscale is considered anxious depression in patients with MDD.30 However, HAMA-17 score ≥18 is identified as AS in patients with MDD.11 In our study, we used a HAMA-17 score of ≥18 to identify AS, which is more specific evaluation for AS.
It has been shown that numerous risk factors influence AS in individuals with MDD. HAMD score, suicide attempts, and diastolic Bp were observed to be linked with AS in MDD patients with MetS in this study. A previous study has shown that AS is common in individuals with MDD throughout the lifespan, with almost up to 65% of MDD patients having comorbid AS.31 In addition, the term "anxious depression" has been used to describe a clinical condition characterized by depressive symptoms and AS, although not based on a DSM-5 or DSM-Ⅳ diagnosis.32 In addition, anxiety and depression often share many risk factors, such as female gender, and decreased household income.33 Interestingly, depression severity increases AS in patients with depression. For example, a recent multi-center, two-stage clinical trial has found that AS increases with depressive symptoms.24 Another study has revealed that comorbid AS is consistently related to more severe manifestations of depression.31 The correlation between HAMA and HAMD scores reported in this study further supports that depression severity is an independent risk factor for AS in FEDN MDD patients with MetS.
Moreover, several studies have assessed the association between suicide attempts and AS. Nevertheless, the results are inconsistent due to the heterogeneity of the samples. For example, Mathialagan et al. have found a statistically non-significant association between AS and suicidal behavior in a study of 122,020 American adolescents with MDD inpatient.34 Grunebaum et al. have found that suicidal MDD patients show less AS than those with non-suicide attempts.35 However, anxiety is also reported to be associated with suicidal ideation and overall suicide risk36 Pfeiffer et al. have found that individuals with MDD and GAD have 1.27 times the risk of dying by suicide than MDD patients without GAD.37 In this investigation, we discovered that an independent and positive association existed between AS and attempted suicide among FEDN MDD patients with MetS. We also discovered that there was a correlation between the HAMA score and the number of suicide attempts, indicating that suicide attempt is an independent risk factor for AS in patients with FEDN MDD.
Hypertension has been reported to be associated with AS. Patients with hypertension are at increased risk for AS because patients with hypertension have a 1.55-fold increase in the 12-month rate of anxiety disorders than patients without a hypertension diagnosis.38 Furthermore, the an increased risk of awareness of anxiety disorders in hypertensive patients.39 More importantly, anxiety is associated with diastolic BP40 In this study, we found that AS was associated with diastolic BP, suggesting that diastolic BP alone or hypertension is an independent risk factor for the progression of AS. A recent meta-analysis confirms a significant anxiety-hypertension association.41
This study also has some limitations. First, this is a case-control study that is unable to establish a causal link between relevant factors and AS in MDD patients with MetS. Therefore, a prospective cohort study is needed to confirm our results. Second, we do not collect data on lifestyle indicators, such as smoking status, dietary intake habits, and physical activity. Moreover, we could not obtain data on therapeutic interventions for hypertension, DM, increased TC and decreased HDL-C levels. Third, WC is not assessed, but BMI is used instead of WC. Fourth, in this study, all participants were Han Chinese population. Therefore, it is necessary to validate our findings in ethnically varied groups.
ConclusionIn conclusion, our findings indicate that AS prevalence in FEDN MDD patients with MetS is 85.96%. Compared with the non-AS subgroup, the AS subgroup has higher levels of TC, LDL-C, TSH, TgAb, TPOAb, and diastolic and systolic BP. They are more likely to attempt suicide and have more HAMD scores and PANSS positive symptom score. In addition, HAMD score, suicide attempts, and diastolic BP are independently associated with AS in FEDN MDD patients with MetS. In addition, HAMD score, TSH, psychotic positive score and number of suicide attempts are all independently related to HAMA score in MDD patients with MetS.
Ethical considerationsThis study was approved by the ethics committee of Shanxi Medical University's first hospital. All patients or their guardians provided their informed consent before participation.
Author contributionXianguang Zhang designed the study and collected the data. Jizhou Liu, Yonglan Yang and Yanjiang Zhang performed the analyses, and Jizhou Liu wrote the first draft of the manuscript. Xiangyang Zhang provided language help and writing assistance. All authors have approved the final manuscript.
Data availability statementThe data that support the findings of this study are available from the corresponding author upon reasonable request.
FundingThis work was supported by the Chinese National Programs for Brain Science and Brain-like Intelligence Technology (2021ZD0202102 to XYZ).
We thank all clinical psychiatrists, nurses, and patients who participated in the study. We also thank Key Laboratory of Mental Health, Institute of Psychology, CAS.