Sleep and circadian disturbances have been widely studied in patients with bipolar disorder. However, there is no clear evidence about the role of peripheral biomarkers of the circadian cycle in this population. This systematic review aims to identify potential endocrine blood biomarkers of circadian rhythms and study their relationship with sleep problems in these patients.
MethodsAn electronic search was performed of PubMed and PsycINFO databases. It included articles about the topic from 1991 through 2021. The search strategy was: ("peripheral biomarkers" OR "biological markers" OR biomarker OR cortisol OR melatonin OR orexin OR hypocretin) AND (blood OR serum OR plasma) AND (“sleep-wake” OR "circadian rhythm" OR sleep OR insomnia) AND "bipolar."
ResultsAfter excluding duplicates, 92 records were obtained. Only 5 studies met the inclusion criteria (n=499; bipolar disorder=125; unipolar depression=148; schizophrenia=80; controls=146). The endocrine parameters analyzed were: cortisol (3 studies), melatonin (1 study), and orexin-A (1 study). Overall, no significant associations were detected between these biomarkers and sleep disturbances, assessed with subjective (psychometric evaluation) and/or objective (polysomnography) measures.
ConclusionThis review highlights the lack of studies exploring the role of endocrine biomarkers related to circadian function in the pathophysiology of sleep disturbances in bipolar disorder.
Bipolar disorder (BD) is a severe affective disorder associated with functional disability. It has a prevalence of 1% and even up to 4% if bipolar spectrum subtypes are included.1 The underlying etiopathogenic mechanisms are not completely understood, but neuroendocrine system disturbances or inflammatory processes, as well as imbalances in oxidative stress parameters, have been proposed.2-4
It has also been hypothesized that the sleep-wake cycle is an involved mechanism, as rhythm disturbances are frequently identified in these patients.5,6 Although sleep disorders are evident during episodes of depression and mania, they are frequently present in euthymia,7 and they may affect the course of illness and functioning of patients with this affective disorder.8 In addition, rhythm disturbances have been identified earlier in the disease process, in people at risk for BD.9
Sleep-wake cycles are controlled by circadian rhythms with an influence from rhythmic hormones such as cortisol, melatonin, and orexin or hypocretin.10 Sleep function is partially regulated by melatonin (produced by the pineal gland), which is dark-dependently secreted and acts as a sleep facilitator, enabling sleep-wake rhythms to adapt to the circadian clock. Otherwise, cortisol shows peak levels in the early active phase, exhibiting an awakening response, and is also released in response to acute or chronic stress. The orexinergic/hypocretinergic neurons (lateral hypothalamus) also interact with other brain areas, coordinating these rhythms and stabilizing the wake state.11 The neuropeptide orexin plays a main role in sleep-wake status, but it is also involved in motor control, feeding behavior, and stress response.12
Some of these neuroendocrine parameters related to circadian function have been studied in patients with affective disorders, including patients with BD.13 For example, melatonin and cortisol secretion have been proposed as circadian parameters that might be considered potential trait markers of patients with BD.14 Specifically, melatonin is a neurohormone that has been associated with the pathophysiology of this disorder.13 Less explored is the orexin/hypocretin system, which has also been implicated in the pathophysiology of psychiatric disorders, including affective disorders and schizophrenia.15,16
Circadian dysregulation could be an important target in clinical practice and biomarker research. Moreover, some authors have suggested that the efficacy of mood stabilizers could be explained by their impact on circadian rhythm regulation, and on melatonin secretion in particular.14
Several studies have explored different assessment tools such as actigraphy, polysomnography, and blood melatonin monitoring to measure circadian disturbances. However, to our knowledge, there is no systematic review that compiles the main circadian endocrine blood biomarkers and specifically studies their relationship with sleep problems in patients with BD.
Thus, the present study aims to explore and synthesize existing evidence on endocrine blood biomarkers related to the sleep-wake cycle in patients with BD and to study their association with sleep disturbances in this population.
MethodsTo achieve the objectives of this review, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement17 recommendations were followed. The study has been approved by the local Research Ethics Committee.
Study selection criteriaThe inclusion criteria for the studies were: (1) quantitative or qualitative research that explored peripheral biomarkers related to the sleep-wake cycle in patients with any type of BD, (1a) endocrine parameters were melatonin, cortisol, orexin or hypocretin, (1b) biomarkers were measured in blood, serum, or plasma, (1c) the study included some parameter related to sleep or the sleep-wake cycle; (2) publications in the English or Spanish language; and (3) participation of patients of all ages. The exclusion criteria were: (1) review and meta-analysis articles and (2) articles that included patients with other diagnoses in addition to BD and that did not separate the results based on such diagnoses.
Search strategyThe PubMed and PsycINFO databases were searched from January 1991 through June 30, 2021. The search strategy used in each of these databases was the following: ("peripheral biomarkers" OR "biological markers" OR biomarker OR cortisol OR melatonin OR orexin OR hypocretin) AND (blood OR serum OR plasma) AND ("sleep-wake" OR "circadian rhythm" OR sleep OR insomnia) AND "bipolar."
Study selection processThis process was carried out in several phases. First (article identification phase), the results of the searches in the two databases were unified, and duplicate records were eliminated. Second (screening phase), the titles and abstracts of the records that potentially met the inclusion criteria were reviewed. If in doubt, we proceeded to read the full text of the article. Third (eligibility phase), full-text articles identified in the previous phase and any questionable articles were independently reviewed and read. Finally (inclusion phase), the articles included in the present systematic review were definitively selected.
Data extraction process for each studyThe following information was extracted from the selected articles: (1) title of the study, authors and year of publication, (2) size of the participant sample (BD and other participants separately), (3) characteristics of the participants (sociodemographic data, diagnosis, if they were outpatients or inpatients and phase of the disease at the time of assessment, if possible), (4) characteristics of the study (methodology, duration, and inclusion of a control group or not), (5) measurement of the biomarker (specific endocrine parameter, serum or plasma, measurement technique, and time of measurement), and (7) measurement of the sleep parameter (polysomnography, psychometric assessment, etc.).
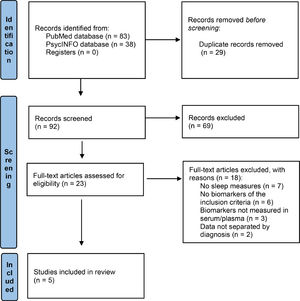
ResultsStudy selectionThe initial electronic database search from 1991 to 2021 yielded 92 articles. After an initial screening, only 23 met the inclusion criteria. Then, after a careful examination, 18 records were excluded for the following reasons: 7 did not include sleep measures, 6 did not measure the selected endocrine biomarkers (melatonin, cortisol, orexin or hypocretin), 3 included measures of these biomarkers in saliva, urine, feces, or cerebrospinal fluid (but not in serum or plasma), and 2 did not provide data separated by diagnosis (not only BD). We ultimately included 5 original studies that met all the inclusion criteria. The selection process is shown in Fig. 1.
Study characteristicsTable 1 presents a summary of characteristics and results.
Summary of study outcomes.
BD: bipolar disorder; MDD: major depression disorder; HC: healthy controls; HDRS: Hamilton Depression Rating Scale; CSF: cerebrospinal fluid; SZ: schizophrenia; PSQI: Pittsburg Sleep Quality Index; IL6R: interleukin-6 receptor; UD: unipolar depression; UDF: unipolar first episode; UDR: unipolar recurrent episodes; DEX/CRH: dexamethasone/corticotropin-releasing hormone
The eligible studies for this review included a total of 125 patients with BD in different phases of illness and a total of 374 participants all diagnoses combined (148 patients with unipolar depression, 80 patients with schizophrenia) and 146 healthy controls (HC). Only two studies included patients with an episode of mania (N = 35),18,19 and one single study included outpatients only in euthymia.20 The mean age of patients with BD varied from 34.4 (SD = 7.1 and 11.8, respectively)18,20 to 46.0 (SD = 12.9) years.21
All the studies were cross-sectional and included at least one control group. No interventional studies were identified.
Only two studies used objective sleep measures, e.g., polysomnography/polygraphic recordings.19,20 Three studies were based only on subjective measures, specifically the Hamilton Depression Rating Scale (HDRS), using HDRS items that assess sleep disturbances (see Table 1 for further details). One study used both Pittsburg Sleep Quality Index (PSQI) and polysomnographic measures.20
BiomarkersThe selected studies were very heterogeneous with respect to the biomarkers analyzed. Only a single study included melatonin measures in serum 17(Bumb et al., 2016), and only one single study included a biomarker of the orexin/hypocretin system (Orexin-A) in plasma.22 The remaining three studies included cortisol levels in serum or plasma.
The study by Bumb et al.18 found a significant association between serum melatonin levels (determined by ELISA test) and diagnosis, this hormone level being lower in patients with major depressive disorder (MDD) versus patients with BD and HC (p = 0.003). The mean serum level in patients with BD was 11.4 pg/mL (SD = 5.2) versus 9.7 (SD = 5.6) in MDD. No difference was observed between BD and HC.
With regard to Orexin-A, Tsuchimine et al.22 observed different mean plasma levels of this neuropeptide in the three diagnostic groups and the HC group (F = 4.09; df = 3; p = 0.007) after adjusting for age, sex, and smoking status, with significantly lower levels in patients with BD (88.2 pg/mL [SD = 16.8]; p = 0.010).
Three studies that analyzed serum cortisol levels had different findings.
Ritter et al.20 reported that serum cortisol measured at 6:30 p.m. did not significantly differ between patients with BD and HC (p = 0.984) (see Table 1). It should be noted that all these patients were in euthymia. However, Linkowsky et al.19 studied cortisol levels in patients with an episode of mania in comparison with HC, reporting significantly higher cortisol levels during the nocturnal period (334 ± 69 vs 256 ± 50 nmol/L; p < 0.05) but not during the diurnal period.
In the study by Rybakowsky et al.,21 cortisol concentrations were measured in depressive patients with BD both at 16 hours after dexamethasone intake and after corticotropin-releasing hormone (CRH) infusion, thus, not comparable with previous studies. When compared with other groups (unipolar depression and HC), cortisol levels were significantly higher in bipolar patients with acute depression at both measures. Bipolar patients in remission had higher cortisol concentrations than unipolar patients within the 30 minutes after CRH administration.
Biomarkers and their association with sleep disturbancesThe study by Rybakowsky et al.21 found a significant correlation between insomnia (HDRS items) and both baseline cortisol and area under the concentration-time course curve (AUC) in bipolar subjects (p < 0.001). However, while only 8 patients with mania underwent the polygraphic sleep examination,19 their mean cortisol concentration during sleep was not correlated with the amount of wake or any other sleep stage.
Ritter et al.20 aimed to establish an association between markers of insomnia and inflammatory parameters (IL6 receptor in peripheral blood mononuclear cells) and reported that cortisol did not mediate the relationship between IL6R and PSQI, motoric arousals, change to wake or arousal index (all p>0.05).
It is worth mentioning that no association between melatonin levels and sleep disturbances assessed by HDRS were found in any of the diagnostic groups.18 Also, regarding Orexin-A levels, no correlations between this neuropeptide and any of the clinical variables were identified.22
DiscussionMain findingsIn this systematic review, we aimed to study specific endocrine blood biomarkers related to circadian function and investigate their association with sleep disturbances in people with BD. Despite the limited number of studies that met the restrictive inclusion criteria, it can be hypothesized that determining certain neuroendocrine biomarkers such as melatonin, cortisol, and the novel orexin/hypocretin system could have potential usefulness in patients with BD, since their concentrations may differ from those found in patients with other diagnosis or HC. However, despite their relation to circadian rhythms, it has not been possible to show any significant association with sleep disturbances in this population.
Sleep disorders, such as insomnia, and abnormal circadian rhythms are very common in patients with BD, even during the euthymic phase7. It has been suggested that sleep disturbances are a potential risk factor for both suicidal ideation and behavior in these patients, as has been observed in patients with other severe mental disorders such as schizophrenia. Patients with BD and previous suicide attempts more frequently experience comorbid insomnia, as well as a fragmented, poorer quality sleep.23,24
The circadian rhythm is a biological process that has an endogenous oscillation of about 24 hours. This rhythmicity is present in sleeping and feeding patterns, but also in brain wave activity, hormone production, and other biological activities.25 The suprachiasmatic nucleus located in the hypothalamus and the pineal gland is the main brain structure that controls circadian function, and it is known as the “master circadian pacemaker.” For example, the secretion of cortisol and melatonin exhibit a circadian pattern.
Cortisol secretion is significantly higher in patients with BD than controls at any phase (manic, depressive, or euthymic),26 but it is not a specific marker of patients with this disorder, as has also been described in patients with schizophrenia.27 Nevertheless, Ritter et al.20 did not find any significant differences in euthymic patients with BD versus HC.
Moreover, melatonin-receptor hypersensitivity to light has been proposed as a trait marker of mood disorders including BD,28 and some authors suggest that patients with BD could exhibit reduced nocturnal melatonin levels.29 Melatonin, also known as the dark hormone, regulates the secretion of other hormones, modulates the immune system, and participates in sleep modulation.30 However, the single study of this biomarker included in this systematic review identified higher serum levels of melatonin in patients with BD versus patients with MDD. This finding contrasts with findings in cerebrospinal fluid (decreased melatonin levels in patients with BD).18
Similarly, orexins (or hypocretins) are neuropeptides that play a key role in regulation of the sleep-wake cycle, but also of appetite, mood, and the reward circuit.31 Both orexin-A (hypocretin-1) and orexin-B (hypocretin-2) have been detected in the cerebrospinal fluid. While orexin-B undergoes accelerated degradation in the blood, orexin-A is also detected in blood. Dysfunction of the hypocretin/orexin system has been described in the pathophysiology of affective disorders.32 These neuropeptides have been studied in patients with affective disorders, but results are still inconsistent and contradictory. In a recent study, plasma orexin-A concentrations were higher in female and male patients with affective disorders compared with HC. These plasma concentrations were also significantly higher in the group of patients with BD versus the group with MDD.31 This contrasts with the finding of lower levels of orexin-A in patients with BD compared with healthy controls.22 Moreover, very few studies have analyzed the relationship of these neuropeptides with sleep disturbances in this population, and those that have been conducted have not identified any association.22
All of this, along with the results of this review, shows a picture of very inconclusive results on this issue, which is also of clinical interest, considering the impact of sleep disturbances on quality of life and functional outcomes of patients with BD.8
Limitations and strengthsThe results of this systematic review should be interpreted in the context of multiple limitations. First, the restrictive inclusion criteria led to analysis of a very limited number of studies with a very small sample size. Second, most of the articles included were of low quality, given their cross-sectional design or limited sample size. Furthermore, the combined results of the studies included are extremely heterogeneous, as only a single study analyzed the biomarkers melatonin and orexin-A. However, the three studies that explored cortisol levels barely considered confounding factors such as age or smoking status. In this sense, we would like to point out that it is essential to carry out both cross-sectional and longitudinal studies to analyze this question in order to obtain more consistent data. Finally, in the interest of exhaustivity, only those studies that measured endocrine biomarkers in serum or plasma have been included. Although there are published studies that analyzed these biomarkers in cerebrospinal fluid, feces, or urine, those were not been included in the present study.
On the other hand, some strengths must be mentioned. To our knowledge, there are no systematic reviews that specifically analyze endocrine blood biomarkers related to the sleep-wake cycle in patients with BD. Furthermore, this is a topic of clinical interest, as it represents a new targeting pathway in BD.
ConclusionsThis systematic review highlights the lack of studies exploring the role of peripheral endocrine biomarkers related to circadian function in the pathophysiology of sleep problems in patients with BD. Recognizing the importance of sleep and circadian rhythm disturbances as core symptoms of BD, future research should incorporate analyses of these biomarkers, with the objective of implementing their use in more personalized and precision medicine. Additionally, a precise knowledge of these neuroendocrine parameters could help identify novel therapeutic targets related to the circadian pathway for patients with this disorder.