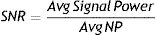“Noise power” (NP) is a measure that allows the assessment of the fast-firing synchronization of neural oscillations. We aimed to replicate higher gamma NP values in frontal and midline regions in patients with schizophrenia and re-evaluate its specificity to this disorder. We also aimed to assess the relationship of higher gamma NP values with drug treatment, chronicity, cognition and symptoms.
MethodsGamma NP values were obtained from electroencephalograms recorded during an oddball paradigm from 29 patients with schizophrenia, 27 with bipolar disorder and 36 healthy controls. We compared these values between the groups to evaluate the specificity to diagnosis. Altered gamma NP values were compared between the patients who had and had not received different treatments to assess the relationship with drug treatment. We also analyse the correlation between gamma NP values and chronicity, symptoms, and cognition.
ResultsCompared to controls, patients with schizophrenia presented increased gamma NP values in frontal and parietal midline regions, while bipolar patients showed increased gamma NP in the left frontal region. There was no significant relationship between drug treatment or chronicity with altered values. Increased gamma NP correlated with higher negative symptom scores in the schizophrenia group, but not with cognitive impairment in any of the groups of patients.
ConclusionsWe replicated an increase in gamma NP in patients with schizophrenia and found that this alteration was also present in a milder form in bipolar patients. These alterations seem to be independent of pharmacological treatment and illness duration.
Cognitive processes are based on accurate temporal relations of neural responses that are established by dynamic neural oscillations,1 especially in the gamma-frequency band (30−100 Hz).2 This cortical gamma oscillatory activity depends on a balance of neuronal excitation and inhibition (E/I balance), a fundamental mechanism for information processing in the brain.3–5 Although at the level of neurons the E/I balance can be defined in a simplified way as the maintenance of appropriate ratios of excitatory versus inhibitory synaptic inputs, at the level of neural circuits is highly multidimensional and complex.6,7 Disturbances in this balance could be due to increased excitatory (glutamatergic) signaling, or to a reduction in inhibition signaling (γ-aminobutyric acid -GABAergic)8 exercised by parvalbumin-positive interneurons that regulates pyramidal neurons firing, where NMDA receptors seems to play an important role.9 Consistent with this notion, a disruption in this E/I balance leading to aberrant oscillatory activity in the gamma range has been proposed in schizophrenia.10–12 Correspondingly, alterations in the gamma band have also been proposed to play a role in the pathophysiology and cognitive dysfunction in schizophrenia and bipolar disorder.13–15 Thus, the assessment of gamma cortical oscillatory activity related to E/I balance is considered a useful tool in the assessment of the electrophysiological basis of brain functioning and its alterations in schizophrenia and other mental disorders.16
Electroencephalography (EEG) is a widely used technique to measure oscillatory neuronal activity, providing high temporal resolution.17 Within the EEG frequency spectrum, gamma band oscillations are considered of special importance in neuroscience for a variety of reasons: (1) they are effective in supporting synchronization of neuronal firing,18 (2) seem to be involved in organizing the local neural circuits or assemblies that underlie higher brain functions,19 and (3) can be evoked or induced by sensory stimulation and cognitive tasks.20 In addition, numerous studies have reported abnormalities in the gamma band in schizophrenia, related to clinical symptoms and cognitive impairment.1,21–23
Research on the cognitive function of the human brain using EEG event-related potential (ERP) methods during task performance is generally focused on the analysis of stimulus-locked components. However, the activity registered after a target stimulus is composed not only of event-related activity, but also of activity linked to other concurrent cognitive processes inefficient for task resolution.24,25 Thus, other studies have been directed to investigate the neuronal activity generated during information processing that does not seem to be directly related in time to the stimulus, and has been generally considered simply as noise.26 This neuronal “noise”, different from the non-neuronal or artifactual noise, represents variations in brain activity that occur apparently at random, with no clear relationship to the assessed phenomenon.27 In fact, the assessment of this noise magnitude, which is subsequently denoted as ‘noise power’ (NP), has provided relevant information in the investigation of neural cortical activity in schizophrenia.28–31
Previously, our group has reported elevated NP values in the gamma band over frontal and midline regions associated with cognitive deficit and clinical symptoms in minimally treated patients with schizophrenia.28,30,32 These results suggested that increased background neural oscillatory activity –unrelated to task performance- in the gamma band may be related to worst cognitive and clinical status of patients with schizophrenia.
In the present study we aimed to replicate these findings in a completely new sample of participants, comparing gamma NP values among schizophrenia patients, bipolar patients and healthy controls in order to re-evaluate the specificity of altered gamma NP values to clinical diagnosis and their correlation with symptoms and cognition using a denser EEG array (29 electrodes) than in our previous reports. On the other hand, since neural oscillations may be affected by a number of factors, we also aimed to assess the possible relationship between the altered gamma NP values with psychotropic drug therapy and illness duration.
Based on evidence of an E/I imbalance in schizophrenia33,34 related to an altered neural synchronization,35,36 and on findings from our previous research our main hypothesis was that gamma NP values during an oddball paradigm would be higher in schizophrenia but not in bipolar patients in comparison to healthy controls. Additionally, we expected to find that if these NP alterations reflect neurophysiological changes due to the disease, they were not explained by drug treatment nor illness duration. Our results could give credence to gamma NP alterations as a specific pathophysiological marker for schizophrenia.
Material and methodsSubjectsThe sample of participants in this study included 29 chronic schizophrenia patients (19 males), 27 euthymic type 1 bipolar patients (17 males), and 36 healthy subjects (23 males). All participants reported no hearing problems. Healthy controls were recruited through newspaper advertisements. Patients were diagnosed by one of the psychiatrists from the research group (VM), who was the treating clinician in most of the cases, through clinical interviews according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, 5th edition.37 The demographic and clinical characteristics of the sample are shown in Table 1.
Sociodemographic, clinical and cognitive data of patients and healthy controls.
| Schizophrenia Patients (N = 29) | Bipolar patients (N = 27) | Healthy controls (N = 36) | |
|---|---|---|---|
| Demographic and clinical: | |||
| Male: Female ratio | 19:10 | 17:10 | 23:13 |
| Age (years) | 41.00 (8.198) | 45.52 (9.916)# | 39.89 (8.528) |
| Illness duration (months) | 173.57 (105.529) | 193.91 (105.23) | N/A |
| Education (years) | 13.286 (4.088)# | 13.823 (3.395)# | 17.600 (3.406) |
| Paternal education (years) | 9.611 (3.913) | 10.600 (4.137) | 13.300 (4.739) |
| Pharmacology (N): | |||
| Antipsychotics | 29 | 16 | N/A |
| Lithium | 0 | 17 | N/A |
| Benzodiazepines | 17 | 12 | N/A |
| Anticonvulsants | 0 | 14 | N/A |
| Antidepressants | 8 | 11 | N/A |
| CPZ equivalents (mg/d) | 416.464 (203.522)** | 230.781 (123.773) | N/A |
| Symptoms (PANSS): | |||
| Positive scale | 12.04 (4.587)** | 7.33 (0.840) | N/A |
| Negative scale | 18.20 (8.246)** | 8.94 (2.508) | N/A |
| Total scale | 57.16 (19.482)** | 30.33 (3.236) | N/A |
| Cognition (BACS and WAIS-III): | |||
| Verbal memory | 33.61 (11.874)# | 35.63 (9.185)# | 49.21 (8.018) |
| Working memory | 15.36 (4.112)# | 17.47 (3.850)# | 22.26 (3.048) |
| Motor speed | 59.78 (19.551)# | 66.53 (13.397)# | 80.59 (11.904) |
| Verbal fluency | 17.04 (5.340)# | 20.79 (5.907)# | 28.70 (6.292) |
| Processing speed | 37.61 (12.819)# | 41.53 (12.317)# | 64.50 (10.805) |
| Problem solving | 16.46 (4.734) | 16.26 (2.978) | 17.82 (3.270) |
| Cognitive factor 1 | −0.823 (0.785)# | −0.432 (0.632)# | 0.870 (0.506) |
| Cognitive factor 2 | −0.030 (1.152) | −0.160 (0.823) | 0.112 (0.980) |
| Total IQ | 94.41 (11.937)# | 98.05 (9.925)# | 115.65 (8.800) |
| Neurophysiology: | |||
| Pz P3b amplitude (μV) | 1.222 (1.849) | 1.563 (1.714) | 1.780 (2.024) |
| Target trials included | 75.5 (8.12)# | 84.04 (19.57) | 93.69 (12.20) |
| Percentage of correct responses | 83.46 (18.83) # | 90.82 (11.11) # | 99.23 (0.99) |
| Percentage of omissions | 16.54 (18.83) # | 9.18 (11.11) # | 0.7711 (0.99) |
| Percentage of false alarm errors | 15.57 (18.57) # | 12.87 (16.80) # | 1.43 (1.97) |
| Average reaction time (ms) | 298.49 (46.87) # | 276.68 (58.75) # | 241.13 (41.84) |
Results are displayed as the mean (SD); N/A = not applicable; PANSS = Positive and Negative Syndrome Scale; BACS = Brief Assessment in Cognition in Schizophrenia Scale; WAIS-III Wechsler Adult Intelligence Scale
*p < 0.05 and **p < 0.01 between schizophrenia and bipolar groups.
#p < 0.05 in comparison with healthy controls.
In patients, drugs and doses were stable during the 3 months prior to the EEG recordings. At the time of inclusion, chronic schizophrenia patients were all receiving atypical antipsychotics, 8 received antidepressants and 17 benzodiazepines. All the bipolar patients were euthymic: 16 were treated with antipsychotics, while 11 were not receiving this treatment and had not received it for at least the last six months, 17 were being treated with lithium, 14 with anticonvulsants, 11 with antidepressants and 12 with benzodiazepines (Table 1).
As in other previous studies of our group, the exclusion criteria were: (i) any neurological illness; (ii) history of cranial trauma with loss of consciousness longer than one minute; (iii) past or present substance abuse, except nicotine or caffeine; (iv) total intelligence quotient (IQ) under 70; (iv) for patients, any other psychiatric process; and (v) for controls, any current psychiatric or neurological diagnosis and/or treatment with drugs known to act on the central nervous system.
We obtained written informed consent from all participants after providing full printed and verbal information. The local ethical committee approved the study according to The Code of Ethics of the World Medical Association (Declaration of Helsinki).
Clinical, pharmacological and cognitive assessmentWe scored the clinical status of patients using the Positive and Negative Syndrome Scale (PANSS).38 The type of drug treatment (antipsychotics, lithium, benzodiazepines, anticonvulsants and/or antidepressants) was recorded after consultation with the patients’ psychiatrist or recent medical history. The dose of antipsychotic treatment was transformed into equivalents of chlorpromazine mg per day.
Cognition was assessed in all participants using the Spanish version of the Brief Assessment in Cognition in Schizophrenia Scale (BACS),39 including the following cognitive dimensions and tasks: verbal memory (word list learning), working memory (digit span), motor speed (token motor task), verbal fluency (word categories), attention and processing speed (symbol coding) and executive function/problem solving (Tower of London). IQ was estimated using the Spanish version of the Wechsler Adult Intelligence Scale (WAIS-III).40
EEG acquisition and processingEEG recordingEEG recordings were acquired using a Brain Vision® equipment (Brain Products GmbH; Munich, Germany) mounted in a 29-electrode cap (Electro-Cap International, Inc.; Eaton, Ohio, USA) and placed according to the modified 10/20 International System at Fp1, Fp2, F7, F3, Fz, F4, F8, FC5, FC1, FCZ, FC2, FC6, T7, C3, Cz, C4, T8, CP5, CP1, CP2, CP6, P7, P3, PZ, P4, P8, O1, OZ, and O2. Two additional electrooculography (EOG) electrodes were placed for monitoring blinks and both vertical and lateral eye movements.
EEG signals were referenced online to Cz electrode and recorded continuously at a sampling rate of 500 Hz. A high-pass hardware filter was applied (cut-off frequency = 0.50 Hz; time constant = 0.3 s; slope =12 dB/oct). Electrode impedance was kept under 5 kΩ during the recordings.
A 13 min long 3-stimuli auditory oddball paradigm was employed to elicit the P3b component of the P300 evoked potential. Participants were exposed to random series of 600 binaural tone bursts (duration 50 ms, rise and fall time 5 ms, intensity 90 dB and onset asynchrony of 1000 and 1500 ms) comprising target (500 Hz), distracter (1000 Hz) and standard (2000 Hz) tones with probabilities of 0.20, 0.20 and 0.60, respectively.41 Participants were comfortably seated in a quiet room and were instructed to keep their eyes closed and click a mouse button whenever they detected the target tone.
Signal pre-processing and processingEEG signals were off-line re-referenced to the average activity of all sensors and filtered using a 0.5–70 Hz band-pass filter. Subsequently the recordings were divided into 650 ms epochs starting 50 ms before the target stimulus onset. Only correctly identified target epochs were included in the analyses. Epochs were baseline corrected (50-ms pre-stimulus) and automatically rejected when exceeding a range of ± 70 μV in any of the 29 channels.
Next, to improve signal artefact correction, we applied an independent component analysis (ICA) including EEG and EOG data (Brain Vision® software). We subtracted all components clearly corresponding to eye movement. Finally, a visual inspection was performed to manually reject remaining epochs still presenting a clear artefact. Subject data were included in the analysis only if 40 or more useful epochs were still available for the target condition. The average number of valid target tone segments per participant was 87 (SD 17.65) (Table 1).
P3b component calculationThe P3b component was calculated from the average of those valid segments for the target condition and defined as the mean amplitude in the 300–400 ms interval. The individual averaged data were then grand-averaged to calculate the P3b component of each study group (Fig. S1).
Gamma noise power (NP) calculationAs described in previous articles25,32 the epoch-segmented data were band filtered (35−45 Hz for the gamma band) and a spectrum analysis was applied using a fast Fourier transform (FFT) to estimate the total power in the gamma band expressed in μV2.
We calculated NP following the previous recommendations,31,42 based on signal-to-noise ratio (SNR) and the average total power in the 300−400 ms post-target stimulus segment. Both measurements were estimated using the Brain Vision® software (Brain Products GmbH; Munich, Germany.25,32
Since in the EEG recording neither the signal nor the noise powers are exactly known, average NP must be estimated with statistical methods. The signal power corresponds to the mean-squared amplitude of a series of the averaged trials,26 so that it was estimated by calculating the mean power of the averaged evoked single trials.24 NP could then be computed by subtracting this signal power from the mean total power of the single sweep:
SNR represents the quotient of the average signal power divided by the average noise power:
And from Eq. (1):Then, NP can be estimated byThereby, using formula (4) in every individual participant, we calculated the averaged NP for each electrode in the gamma band from the already extracted average total power and SNR values.
Statistical analysisAge and sex distribution were compared between groups using an analysis of variance (ANOVA, Bonferroni post-hoc) and a chi-square (X2) test, respectively. Years of education, BACS scores, P300 amplitude, and performance variables during the oddball task were contrasted between groups also using an ANOVA (Bonferroni post-hoc). PANSS scores, treatment doses and illness duration were compared between the schizophrenia and bipolar groups using t-tests for independent samples. These statistical analyses were performed using IBM SPSS Amos 24 for Windows.
Kolmogorov–Smirnov tests for gamma NP values resulted in a non-normal distribution for all 29-electrode measurements. Therefore, comparisons between groups on gamma NP were made using a non-parametric test (permutation test based on t statistics) using the FieldTrip toolbox for MATLAB.43 Here, a Monte-Carlo estimate of the significance probabilities and/or critical values were calculated based on randomizing our data 1000 times between the study conditions. Type I error across multiple comparisons was controlled using Bonferroni correction (p = 0.05/29 = 0.002).
To reduce the number of cognitive factors (the six BACS dimensions), we performed a principal component analysis (PCA) and saved each of the participants’ factorial scores for further analysis.
Spearman’s rho correlation coefficient was then used to assess the relationship between clinical (treatment dose, illness durations and PANSS scores) and cognitive (factor scores) variables, and gamma NP values in those channels with a significant p-value in the previous step. Additionally, we studied the possible relationship between the altered NP values with the following performance variables during the oddball task: average reaction time to target stimulus, and percentages of correct responses, omissions (false negatives) and false alarm errors (false positives).
To assess a possible relationship between the use of psychotropic medication with the gamma NP values, we performed a group comparison between patients receiving or not each type of drug (antipsychotics, lithium, benzodiazepines, anticonvulsants and antidepressants). In order to make this comparison with the larger number of subjects, we included all patients regardless of their diagnosis. We performed these comparisons using new non-parametric permutation analyses based on t-tests (FieldTrip toolbox).
Finally, multiple regression models were carried out to analyse the predictive capacity of the different clinical and cognitive factors (independent variables) on the gamma NP values (dependent variable). The regression models were made in those groups of patients and electrodes where significant alterations of gamma NP were found in the previous comparisons. The independent variables were: illness duration, chlorpromazine equivalents received, PANSS scores (positive and negative symptoms, as well as total score), estimated IQ, response parameters in the oddball task (percentage of correct responses, omissions and false alarm errors, as well as average reaction times), and the two general cognitive factors.
A database with the main data supporting the results is available (Mendeley Data, V2, doi: 10.17632/538wvc7rp8.2).
ResultsThere were no significant differences between the groups in terms of sex distribution (X2 = 0.041, p = 0.98; Table 1). Bipolar patients were significantly older than healthy controls (mean difference = 5.63 p = 0.04). Illness duration was not significantly different between the groups of patients (Table 1).
Patients with schizophrenia had received significantly higher mean doses (in CPZ equivalents) of antipsychotics than bipolar patients (t = 3.307, p = 0.002; Table 1). The distribution of psychotropic drugs among patients with schizophrenia and bipolar disorder is also shown in Table 1.
Patients showed statistically significant lower scores compared to healthy controls in total IQ and in all BACS domains except Tower of London. The principal component analysis of the BACS scores yielded two independent factors (eigenvalue greater than 1) that explained 57.4% and 17.6% of the variance, respectively. The first cognitive factor included the verbal memory, working memory, motor speed, verbal fluency, attention and processing speed BACS scores, while the second cognitive factor corresponded to the executive function/problem solving domain (Tower of London). The first cognitive factor scores were significantly lower in both groups of patients compared to healthy controls (p < 0.001). As for the performance during the oddball task, patients had worse reaction times, gave fewer correct responses and made more errors by omission and false alarms (Table 1).
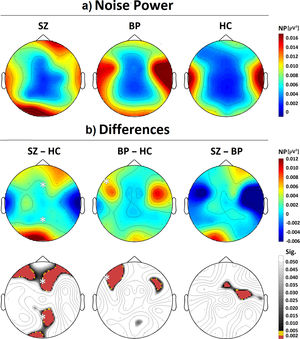
Between-group differences in gamma NPThe gamma NP values for each of the EEG electrodes are shown in Table 2 and topographically represented in Fig. 1. Our Bonferroni-corrected, non-parametric comparisons revealed significantly higher values of gamma NP in Fz (t = 3.460, p = 0.002), and Pz (t = 2.997, p = 0.002) channels in patients with schizophrenia compared to healthy controls. Bipolar patients, on the other hand, showed significantly higher values of gamma NP only in the F7 electrode compared to healthy controls (t = 2.702, p = 0.002). No significant differences, corrected for multiple comparisons, were found between patients with schizophrenia and bipolar disorder (Fig. 1).
Gamma noise power values (γ-NP) of patients and healthy controls (µV2).
| Schizophreniapatients(N = 29) | Bipolarpatients(N = 27) | Healthycontrols(N = 36) | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| γ-NP FP1 | 0.00967 # | (0.01026) | 0.00868 # | (0.00665) | 0.00534 | (0.00506) |
| γ-NP FP2 | 0.01509 # | (0.01557) | 0.00934 | (0.00811) | 0.00814 | (0.00922) |
| γ-NP F7 | 0.00780 # | (0.00967) | 0.00936# | (0.01153) | 0.00394 | (0.00304) |
| γ-NP F3 | 0.00600 # | (0.00689) | 0.00508 # | (0.00345) | 0.00315 | (0.00219) |
| γ-NP FZ | 0.00384 *# | (0.00200) | 0.00259 | (0.00161) | 0.00230 | (0.00160) |
| γ-NP F4 | 0.01044 # | (0.01720) | 0.00738 | (0.00669) | 0.00462 | (0.00433) |
| γ-NP F8 | 0.01003 | (0.00953) | 0.00989 | (0.00872) | 0.00634 | (0.00821) |
| γ-NP FC5 | 0.00794 | (0.01029) | 0.01438 # | (0.01528) | 0.00635 | (0.00773) |
| γ-NP FC1 | 0.00388 | (0.00290) | 0.00282 | (0.00159) | 0.00276 | (0.00367) |
| γ-NP FCZ | 0.00311 # | (0.00141) | 0.00243 | (0.00179) | 0.00215 | (0.00170) |
| γ-NP FC2 | 0.00583 *# | (0.00699) | 0.00271 | (0.00146) | 0.00263 | (0.00252) |
| γ-NP FC6 | 0.00880 * | (0.00945) | 0.01850 # | (0.02294) | 0.00860 | (0.01041) |
| γ-NP T7 | 0.01244 | (0.01790) | 0.01527 | (0.01167) | 0.01605 | (0.01760) |
| γ-NP C3 | 0.00715 | (0.01447) | 0.00636 | (0.00826) | 0.00374 | (0.00487) |
| γ-NP CZ | 0.00185 | (0.00099) | 0.00179 | (0.00088) | 0.00167 | (0.00088) |
| γ-NP C4 | 0.00356 * | (0.00323) | 0.00952 | (0.01600) | 0.00430 | (0.00610) |
| γ-NP T8 | 0.01224 * | (0.01862) | 0.02270 | (0.01837) | 0.01843 | (0.01808) |
| γ-NP CP5 | 0.00749 | (0.01041) | 0.00750 | (0.00778) | 0.00571 | (0.00816) |
| γ-NP CP1 | 0.00320 # | (0.00325) | 0.00199 | (0.00130) | 0.00185 | (0.00148) |
| γ-NP CP2 | 0.00324 | (0.00419) | 0.00204 | (0.00116) | 0.00179 | (0.00108) |
| γ-NP CP6 | 0.00731 | (0.01119) | 0.00765 | (0.00672) | 0.00698 | (0.01144) |
| γ-NP P7 | 0.01078 | (0.01663) | 0.01138 | (0.01366) | 0.00818 | (0.00855) |
| γ-NP P3 | 0.00363 | (0.00306) | 0.00421 | (0.00434) | 0.00322 | (0.00319) |
| γ-NP PZ | 0.00276# | (0.00178) | 0.00222 | (0.00150) | 0.00175 | (0.00086) |
| γ-NP P4 | 0.00415 | (0.00432) | 0.00368 | (0.00238) | 0.00290 | (0.00210) |
| γ-NP P8 | 0.00787 | (0.00884) | 0.00861 | (0.00675) | 0.00753 | (0.00884) |
| γ-NP O1 | 0.02057 # | (0.02518) | 0.01160 | (0.01323) | 0.00909 | (0.01025) |
| γ-NP OZ | 0.01721 # | (0.02282) | 0.01180 | (0.02045) | 0.00604 | (0.00813) |
| γ-NP O2 | 0.01213 | (0.01244) | 0.01160 | (0.01790) | 0.00729 | (0.01599) |
Results are displayed as mean (SD); comparisons between patients and healthy controls:
*p < 0.05 between schizophrenia and bipolar groups.
#p < 0.05 in comparison with healthy controls.
The comparisons that exceeded Bonferroni’s correction are shown in bold and italics (p < 0.002).
(a) Spatial scalp distribution of the grand-averaged gamma NP values for each group (µV2). (b) Distribution of differences between the groups and their statistical significance (p-values). The white asterisks represent the electrodes with significant differences after Bonferroni correction (SZ: schizophrenia patients, BP: bipolar patients and HC: healthy controls).
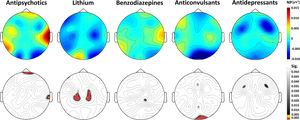
Non-parametric contrast analyses between the groups treated and untreated with the different types of drugs can be seen in Fig. 2. No significant differences were found in the electrodes where we had previously found altered gamma NP values.
Topographical maps of the differences in grand-averaged gamma NP values in patients receiving minus not receiving each type of drug treatment (above) and their statistical differences (below). No electrodes showed statistical differences after Bonferroni correction. Gamma NP values (µV2) above; p-values below.
We found no significant correlation between antipsychotic doses (expressed in CPZ equivalents) and gamma NP values in patients with schizophrenia in those electrodes where significant differences were found in the previous analysis. No significant correlations between illness duration and gamma NP values were found in patents with schizophrenia or bipolar disorder (Fig. S2)
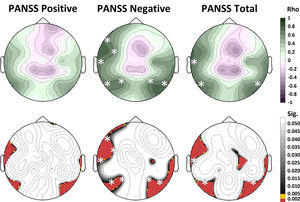
Relationship between gamma NP and clinical and cognitive factorsGamma NP values in patients with schizophrenia correlated significantly (after Bonferroni correction) with PANSS negative scores on F7 (rho = 0.642, p = 0.001), FC5 (rho = 0.612, p = 0.001), T7 (rho = 0.649, p < 0.001), P7 (rho = 0.627, p = 0.001), P3 (rho = 0.629, p = 0.001), P4 (rho = 0.581, p = 0.002) and P8 (rho = 0.712, p < 0.001) electrodes, and with PANSS total scores on T7 (rho = 0.668, p < 0.001), P7 (rho = 0.665, p < 0.001) and P8 (rho = 0.618, p = 0.001) electrodes (Fig. 3). No correlation was found between gamma NP values and PANSS scores in bipolar patients. No significant correlations between gamma NP values and BACS cognitive factor scores were found for any of the patient groups. Similarly, no significant correlations were found between altered gamma NP values and any of the performance variables in the oddball task.
Correlation between gamma NP values and symptom scores in patients with schizophrenia. A significant correlation was found between gamma NP values in the left frontal and temporo-parietal regions and PANSS negative and total scores. The white asterisks represent the electrodes with significant correlation after Bonferroni correction. Spearman’s rho above; p-values below.
Finally, two multiple regression models were performed in the group of patients with schizophrenia on the altered values of gamma NP in the Fz and Pz electrodes. Neither model was able to explain significantly the gamma NP from any of the independent variables (clinical or cognitive) proposed.
DiscussionThe study of measurements derived from cortical noise, such as noise power (NP) can be useful for the assessment of gamma power abnormalities32 which have previously been detected in schizophrenia and bipolar disorder.44,45 This may, in turn, give insight into the disruption in the balance between excitatory and inhibitory mechanisms in psychosis.10 Thus, in our approach, NP has been defined as the amount of neural activity registered by the scalp EEG that is not time-locked during a cognitive task. In this study, we investigated the specificity of altered NP values in the gamma band for schizophrenia, compared to patients with bipolar disorder and healthy controls, and the possible relationship with pharmacological treatment and illness chronicity.
According to our results, patients with schizophrenia showed significantly higher gamma NP values in the frontal and parietal midline regions compared to healthy controls. This finding is in line with the evidence of altered neural synchronization in schizophrenia,23 and can be taken as suggestive of an imbalance of excitatory and inhibitory neuronal activity that is involved in the pathophysiology of this disorder.46 It also was consistent with our previous reports of higher gamma NP values in the midline region in a completely different sample of patients,25,30 as well as with the higher gamma NP values in frontal regions in schizophrenia observed in other previous work.31 The regional distribution of these altered gamma NP values roughly corresponds to the default mode network (DMN).47,48 Failure to deactivate the DMN during the performance of a cognitive task has been described in schizophrenia,49 presumably related to a hampered GABAergic neurotransmission.50 Although the neurophysiological origins of gamma NP have not yet been clearly established, these findings suggest the existence of a common mechanism.
With respect to patients with bipolar disorder, our data showed a higher gamma NP in the left-frontal area when compared to healthy controls. This result may indicate the possible presence of an alteration of the E/I balance in bipolar disorder similar to that found in patients with schizophrenia, although milder and with an apparent different regional distribution. Although we were unable to find a relationship with patients' PANSS scores and BACS factors, some previous studies based on the analysis of EEG activity in patients with bipolar disorder have also reported alterations in left frontal regions, in these cases related to clinical and cognitive features of this disorder.51–54 In prior reports, our group found no significant differences between these two groups using completely different samples.28 This discrepancy may be related to methodological differences since the previous analyses were based on a lower resolution EEG recording (17- channels versus the current 29 channels) and a factor analysis of NP values was used,28 while here we performed a different approach of Bonferroni-corrected comparisons of the NP values in each of the electrodes. These current results support a milder alteration of gamma NP in bipolar disorder.
Our present study was aimed at assessing the neuronal activity in the gamma band. Nonetheless, another research work on NP in different EEG bands reported higher NP values across the entire frequency spectrum in patients with schizophrenia when compared to controls.31 However, in a previous study from our group we were unable to detect significant differences between patients with schizophrenia and the control group when NP values were specifically compared in the theta band.30 To our knowledge, no other works directly compare NP values in any EEG frequency band in patients with schizophrenia or bipolar disorder. Even though we have not been able to find significant differences in NP between patients with schizophrenia and bipolar patients, a study investigating the resting-state EEG power within specified frequency bands reported an increase in total power in the beta and gamma bands in patients with bipolar disorder relative to patients with schizophrenia,45 which points to a possible interrelationship between alterations in different frequency bands that we have not been able to detect in previous studies.
The significantly higher values of gamma NP in patients were not related to antipsychotic treatment, when comparing those bipolar patients receiving and those not receiving this type of drug treatment (all patients with schizophrenia were taking antipsychotics). In addition, gamma NP alterations were not related to treatment with the other psychotropic drugs studied (BZP, lithium, antidepressants or anticonvulsants). No relationship was found either between gamma NP values and illness duration. These results suggest that the alterations found in gamma NP in patients are independent of the drug treatment and the duration of illness. Although, as discussed below, due to the small size of the groups receiving or not each drug, this conclusion should be taken with caution.
Patients showed significantly worse performance than healthy controls on most of the cognitive functions studied. However, we did not find a significant relationship between the altered gamma NP values and the overall cognitive factor, which included all BACS scores except the Tower of London. Previous reports from our group found altered gamma NP values in the frontal-lateral region correlated with poorer performance in working memory and problem solving in patients with schizophrenia.30,32 Methodological differences could account for the discrepancy between previous and current results. In the previous studies the specific domains of cognition were assessed, while in the present work only one cognition factor was used. Moreover, it should be noted that the interpretation of the results must be done with care since the gamma NP values analysed were obtained during an oddball paradigm (an attention and working memory task) but not during the performance of the BACS cognitive battery, including multiple cognitive domains under which more complex neural processes are probably executed.55 However, we neither found a correlation between the altered gamma NP values and the variables of the oddball task performance. In a previous report we found an association between gamma NP and a cognitive composite score (using the MATRICS battery).28 Thus, we cannot discard a relevant relationship between cognition and gamma NP not detected in the present sample.
Patients with schizophrenia showed a direct relationship between the elevated gamma NP values in the left-frontal and parietotemporal regions and negative symptoms. These findings correspond approximately to the direct correlation between higher gamma NP and negative PANSS scores previously reported in patients with first episode schizophrenia.32 Although we have not been able to obtain a relationship between altered gamma NP and poorer cognitive performance in patients, the relationship with negative symptoms would suggest that increased inefficient high-frequency neural activity probably hinders the neural processes underlying normal cognitive and affective expression.
Our study has some limitations. First, our NP measure is, by definition, calculated during a cognitive task, but was not assessed in a resting state for comparison, since there is evidence in the literature of altered spectral power in the resting state gamma band in patients with first episodes and chronic schizophrenia.10,56 Second, although higher gamma NP values may indicate an increased level of cortical activation to the detriment of satisfactory selection of neuron populations, it is still arguable whether NP thus described originates exclusively from cortical activity (background activity generated by a variation of peak-latency and/or actual response differences) or it may be affected by the contamination of external artifacts.26 This may have limited relevance in our results since our EEG data were pre-processed applying an ICA. Third, the results related to pharmacological treatment should be taken with care, since when comparing medicated and unmedicated patients we were not able to obtain groups taking only one type of medication, and for some comparisons the sample size was small. In addition, future studies should study the relationship between drugs and NP alterations for homogeneous samples of patients according to their diagnosis. Fourth, the present study assessed only chronic patients, and it has not been possible to make comparisons of gamma NP with previous stages of illness (clinical high-risk subjects or first episode patients in the case of schizophrenia). Lastly, we cannot discard a state- rather than trait-dependence of gamma NP alterations in patients with bipolar disorder, which could be analysed in longitudinal assessments of depressive, euthymic and manic stages. It is plausible that patients have an inefficient basal hyperactivity and therefore future work should study NP at a resting state, which would allow better estimation of the specificity of this measure for schizophrenia versus bipolar disorder. Likewise, the inclusion of first episodes and even a longitudinal study of cases would make it possible to better delineate the evolution of gamma NP alterations in patients.
ConclusionsWe have confirmed previous findings showing that patients with schizophrenia have abnormally higher NP values in the gamma band in the midline, and that this alteration is present in a milder form in patients with bipolar disorder in frontal regions. These results are not explained by pharmacological treatment or illness duration. A plausible deficit in the mechanism of neural inhibition may underlay an inefficient cortical hyperactivity, and possibly, the increase of negative symptoms specifically in schizophrenia.
Author contributionsAll authors contributed to the study conception. VM designed the study. Material preparation and data collection were performed by BC, AL, MI, CC and AR. Data analysis was performed by BC, AD and VM. The first draft of the manuscript was written by BC, AD and VM. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
FundingThis work has been supported by the following grants: the “Instituto de Salud Carlos III” (PI18/00178), the “Gerencia Regional de Salud de Castilla y León” (GRS 1485/A/17 and GRS 1721/A/18), and the “Consejería de Educación de la Junta de Castilla y León” (VA057P17).
Ethical considerationsWe declare that the present study was conducted in accordance with the Declaration of Helsinki and we certify that formal approval to conduct the experiments described have been obtained from the review board of the University Hospital of Valladolid and could be provided upon request.
Conflicts of interestThe authors declare that they have no conflict of interest.
None.