To identify adverse events related to prone positioning in COVID-19 patients with severe disease and acute respiratory distress syndrome, to analyze the risk factors associated with the development of anterior pressure ulcers, to determine whether the recommendation of prone positioning is associated with improved clinical outcomes.
MethodsRetrospective study performed in 63 consecutive patients with COVID-19 pneumonia admitted to intensive care unit on invasive mechanical ventilation and treated with prone positioning between March and April 2020. Association between prone-related pressure ulcers and selected variables was explored by the means of logistic regression.
ResultsA total of 139 proning cycles were performed. The mean number of cycles were 2 [1–3] and the mean duration per cycle was of 22h [15–24]. The prevalence of adverse events this population was 84.9 %, being the physiologic ones (i.e., hypo/hypertension) the most prevalent. 29 out of 63 patients (46%) developed prone-related pressure ulcers. The risk factors for prone-related pressure ulcers were older age, hypertension, levels of pre-albumin <21mg/dl, the number of proning cycles and severe disease. We observed a significant increase in the PaO2/FiO2 at different time points during the prone positioning, and a significant decrease after it.
ConclusionsThere is a high incidence of adverse events due to PD, with the physiological type being the most frequent. The identification of the main risk factors for the development of prone-related pressure ulcers will help to prevent the occurrence of these lesions during the prone positioning. Prone positioning offered an improvement in the oxygenation in these patients.
Identificar eventos adversos secundarios al decúbito prono (DP) en pacientes con COVID-19 con síndrome de distrés respiratorio agudo (SDRA) moderado/severo, analizar los factores de riesgo para el desarrollo de úlceras por presión (UPP) en DP y describir la evolución oximétrica de estos pacientes durante el DP.
MétodoEstudio descriptivo retrospectivo realizado sobre 63 pacientes ingresados en la UCI de un hospital de segundo nivel, con neumonía por SARS-CoV-2, SDRA moderado/severo, ventilación mecánica invasiva, que precisaron maniobras de DP, durante marzo y abril de 2020. Se usó un muestreo no probabilístico consecutivo y se analizaron las variables seleccionadas a través de una regresión logística.
ResultadosSe realizaron un total de 139 sesiones de pronación. La mediana de sesiones fue de 2 [1–3] y la duración de 22horas [15–24] por sesión. La aparición de eventos adversos ocurrió en un 84,9% de los casos, siendo las fisiológicas (ej. hiper/hipotensión) las más frecuentes. Al comparar pacientes pronados que mantuvieron la integridad cutánea (34 de 63 pacientes, un 54%) versus los que desarrollaron UPP (29 de 63, un 46%), estos últimos presentaron los siguientes factores de riesgo: mayor edad, ser hipertensos, pre-albúmina <21mg/dl, mayor número de sesiones de prono y mayor gravedad al ingreso. Se observó un incremento significativo entre la PaO2/FiO2 previa al DP y en los diferentes cortes temporales durante el prono, además de una caída significativa tras despronar.
ConclusionesExiste una alta incidencia de eventos adversos debidos al DP, siendo los de tipo fisiológico los más frecuentes. La identificación de varios factores de riesgo para el desarrollo de UPP ayudará a prevenir la aparición de estas lesiones durante la pronación. La terapia de DP en pacientes COVID-19 con SDRA moderado/severo ha demostrado una mejora en los parámetros de oxigenación.
What is known/what it contributes?
The prone position is recommended in patients diagnosed with moderate/severe acute respiratory distress syndrome as a strategy to improve oxygenation and thus reduce mortality. This study shows physiological complications, especially haemodynamic complications, as the main adverse event secondary to prone positioning in patients admitted to the ICU for COVID-19 with moderate/severe acute respiratory distress syndrome. The following are presented as risk factors for pressure ulcers: older age, arterial hypertension, pre-albumin value <21mg/dL and greater number of prone positioning sessions.
Study implications
The standardisation of care for prone patients diagnosed with COVID-19 with moderate/severe acute respiratory distress syndrome in the ICU is proposed, thus reducing variability in care, which would lead to a reduction in adverse events.
In December 2019, a new type of coronavirus, SARS-CoV-2, which causes the disease known as COVID-19, was identified in China. On 30 January 2020, the World Health Organization (WHO) declared the COVID-19 epidemic a public health emergency, recognising it as a pandemic disease due to its high transmissibility and lethality on 11 March. The pandemic is estimated to have started in Spain on 17 March and ended its first wave on 22 May 2020, with a reported 234,824 cases confirmed by polymerase chain reaction (PCR) testing and 28,628 deaths as of that date (11.4% case fatality rate).1,2
The most common symptoms of COVID-19 are fever, cough and dyspnoea, but the infection can lead to more serious conditions, bilateral interstitial pneumonias, or acute respiratory distress syndrome (ARDS).3 It was estimated that during the first wave, 122,439 people in Spain required hospitalisation and 11,298 were admitted to Intensive Care Units (ICU).4 ARDS was the leading cause of mortality.5,6
For the specific management of acute respiratory failure in patients with COVID-19, the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC for its initials in Spanish) made three recommendations7: (I) early orotracheal intubation in patients with moderate-severe acute respiratory failure and/or signs of increased difficulty breathing; (II) sequence of orotracheal intubation with rapid induction without balloon resuscitative ventilation and planned difficult airway management protocol; and (III) prone positioning (PP) manoeuvre during the first 24h in patients with ARDS with PaO2/FiO2<150mmHg, assessing myorelaxation and repeating cycles until improvement.
The prone decubitus position has been used for decades as part of the treatment of ARDS, providing physiological benefits such as improved oxygenation, optimisation of the ventilation-perfusion ratio with a reduction in shunt areas and more homogeneous ventral-dorsal redistribution of transpulmonary pressure by reducing dorsal compression.8 In addition, the response to PP could be considered more relevant if the decrease in partial pressure of arterial carbon dioxide (PaCO2) rather than the PaO2/FiO ratio is assessed.9
This technique is not free from complications: nerve compression, venous stasis, unscheduled extubation, limitation of diaphragm expansion, pressure ulcers (PUs), unscheduled catheter removal, retinal damage, transient reduction in arterial oxygen saturation (SatO2), vomiting, transient arrhythmias, etc.10,11
The aim of this study was to identify adverse events secondary to PP in patients admitted for COVID-19 with moderate/severe ARDS in the ICU, as well as to analyse the risk factors (RF) for the development of PUs caused by this position. Secondly, to describe the evolution of oxygenation and ventilation parameters during the PP in these patients.
MethodRetrospective descriptive study in an adult ICU of a second level general hospital. Approved by the Research Ethics Committee of the reference hospital (CEIC 2020/031).
Due to the high demand for critical care beds, it was necessary to expand from 12 structural beds to 15, as well as to provide space for resuscitation (10 beds) and operating theatre (12 beds).
Patients admitted to hospital in these 37 intensive care beds from 1st March to 30th April 2020 were included in this study.
Inclusion criteria: patients diagnosed with SARS-CoV-2 pneumonia, older than 18 years with moderate/severe ARDS according to the Berlin 2012 criteria,12 with invasive mechanical ventilation, analgesia and sedation, and in whom prone manoeuvres were performed, tolerating this position for at least one hour.
Variables: Demographic (age and sex) and physiological variables (body mass index - BMI), severity on admission (Simplified Acute Physiology Score [SAPS III]), comorbidities (cardiac, respiratory, endocrine, renal), time variables (stay in ICU, days of invasive mechanical ventilation), final outcome (discharge to ward, death in ICU, transfer to another hospital, death within 28 days after ICU discharge), PP manoeuvre (number of sessions required per patient and hours maintained on PP), adverse events during PP (injuries/PU, physiological adverse events, mechanical adverse events, other), adverse events during PP (injuries/PU, physiological adverse events, mechanical adverse events, other), adverse events during PD (injuries/UPP, physiological adverse events, other), adverse events during PD (injuries/UPP, physiological adverse events, other), mechanical adverse events, others), assessment of risk of developing PUs (current assessment scale of risk of developing PUs in ICU [EVARUCI] once a day) and associated risk factors (use of expanders and vasoactive drugs, absence of artificial nutritional support - enteral nutrition and/or parenteral nutrition -, albumin levels, pre-albumin and retinol-binding protein-RBP, collected on the day of PP), therapeutic effort (Nine Equivalents Manpower Score [NEMS], once daily), ICU experience of nurses, oxygenation and ventilation (at four time points: [I] prior to PD [II] after 1h on PD [III] within 12h after the start of PD and [IV] after detachment: PaO2, FiO2, PaCO2, PaO2/FiO2).
ToolsSAPS III: predicts the patient's vital prognosis by assessing through a series of items their previous situation, the cause and type of pathology causing admission, and their physiological state in the first hour of ICU stay. Score range between 0 and 229.13
EVARUCI: assesses the risk of developing PUs in the ICU through five factors: consciousness, haemodynamics, respiratory, mobility and others. Score range between 4 and 23.14,15
NEMS: measures the therapeutic effort of the nurse derived from the severity of the patient based on the assessment of nine activities related to ICU nursing work. Score range between 0 and 56.16–18
Data collectionThis was carried out by the research team through the clinical records. Patient selection was carried out through the ICU admission register with a diagnosis of SARS-CoV-2 pneumonia.
Data analysisThis is a descriptive analysis. Qualitative variables are described with absolute (n) and relative (%) frequencies, quantitative variables with mean and standard deviation (SD) when they followed a normal distribution, and with median and interquartile range [Q1-Q3] otherwise. The normality of the variables was assessed using the Kolmogorov-Smirnov test. The χ2 test or Fisher's exact test was used to study the association between qualitative variables, for quantitative and qualitative variables the Student's t-test or ANOVA if the data followed a normal distribution, and otherwise the Mann–Whitney U-test or Kruskal–Wallis.
The statistical programme SPSS version 25.0 (IBM Corp., Armonk, NY, USA) was used, assuming statistical significance if p-value <.05.
ResultsOf the 78 patients with SARS-CoV-2 pneumonia admitted to the ICU during the recruitment period, 63 met the inclusion criteria.
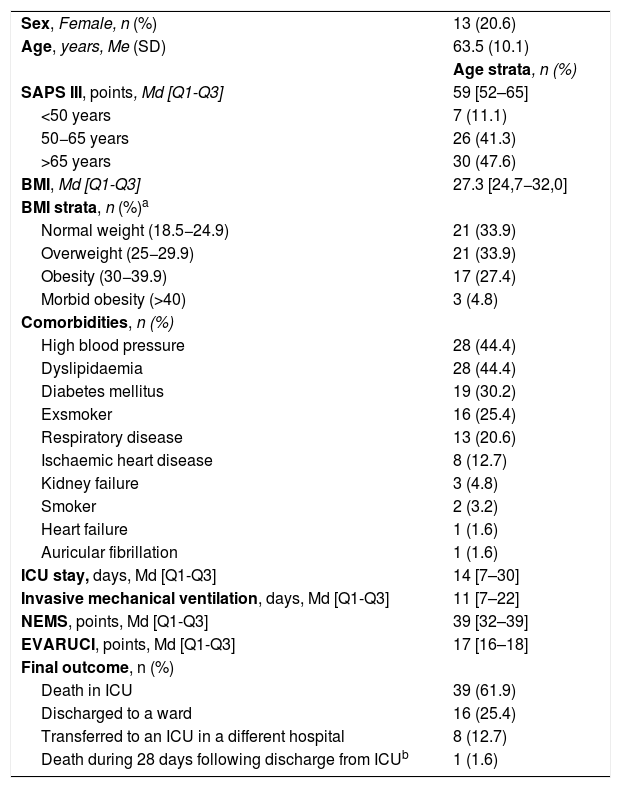
Descriptive data of the patients are shown in Table 1.
Study sample characteristics (n=63).
| Sex, Female, n (%) | 13 (20.6) |
| Age, years, Me (SD) | 63.5 (10.1) |
| Age strata, n (%) | |
| SAPS III, points, Md [Q1-Q3] | 59 [52–65] |
| <50 years | 7 (11.1) |
| 50−65 years | 26 (41.3) |
| >65 years | 30 (47.6) |
| BMI, Md [Q1-Q3] | 27.3 [24,7−32,0] |
| BMI strata, n (%)a | |
| Normal weight (18.5−24.9) | 21 (33.9) |
| Overweight (25−29.9) | 21 (33.9) |
| Obesity (30−39.9) | 17 (27.4) |
| Morbid obesity (>40) | 3 (4.8) |
| Comorbidities, n (%) | |
| High blood pressure | 28 (44.4) |
| Dyslipidaemia | 28 (44.4) |
| Diabetes mellitus | 19 (30.2) |
| Exsmoker | 16 (25.4) |
| Respiratory disease | 13 (20.6) |
| Ischaemic heart disease | 8 (12.7) |
| Kidney failure | 3 (4.8) |
| Smoker | 2 (3.2) |
| Heart failure | 1 (1.6) |
| Auricular fibrillation | 1 (1.6) |
| ICU stay, days, Md [Q1-Q3] | 14 [7–30] |
| Invasive mechanical ventilation, days, Md [Q1-Q3] | 11 [7–22] |
| NEMS, points, Md [Q1-Q3] | 39 [32–39] |
| EVARUCI, points, Md [Q1-Q3] | 17 [16–18] |
| Final outcome, n (%) | |
| Death in ICU | 39 (61.9) |
| Discharged to a ward | 16 (25.4) |
| Transferred to an ICU in a different hospital | 8 (12.7) |
| Death during 28 days following discharge from ICUb | 1 (1.6) |
BMI, Body Mass Index; EVARUCI, Current risk assessment scale for pressure ulcer development in intensive care; Md, Median; Me, Mean; NEMS, Nine Equivalents of Nursing Manpowe use Score; Q1, Quartile 1; Q3, Quartile 3; SAPS III, Simplified Acute Physiology Score; SD, Standard Deviation.
A total of 139 pronation manoeuvres were performed. The median [Q1-Q3] number of sessions patients underwent was 2 [1–3], with a median [Q1-Q3] of 22h [15–24] of PP therapy per session.
Therapeutic effort was greater than 32 points NEMS in 70.5% of shifts; 24.8% of the nurses had more than five years experience in critical care, 42.9% had worked in critical care for less than five years and 32.3% had never worked with critical care patients.
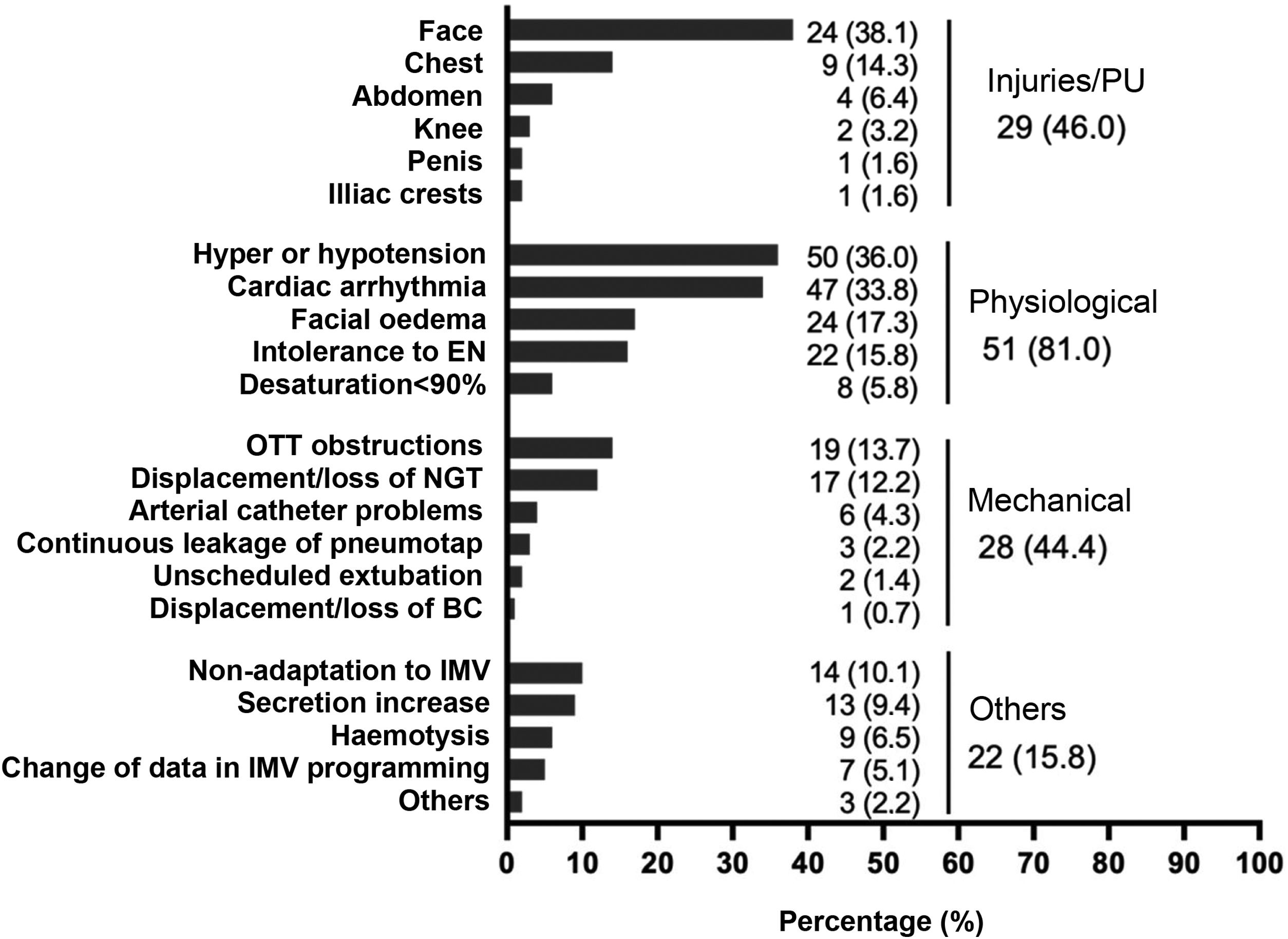
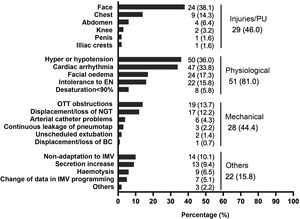
Adverse eventsIn 118 PD sessions (84.9%), patients experienced some type of adverse event (Fig. 1). No adverse events related to venous catheters were reported. It is worth noting the high percentage of PUs occurring on the face, observed in 82.7% of the patients who presented a PU (38.1% in the total number of patients).
Adverse events during prone positioning.
BC: bladder catheter; ; EN: enteral nutrition; IMV: invasive mechanical ventilation; NGT: nasogastric tube; OTT: orotracheal tube; PU: pressure ulcer.
Note: The right column reflects the number of patients or number of sessions where one or more injuries occurred. Injuries/PUs were assessed over the total number of patients (n = 63), assuming that, if a PU appears in more than one prone on the same patient, it is the same PU. Physiological, mechanical and other adverse events were assessed over the total number of prone positioning manoeuvres (n = 139).
In relation to the RFs for developing PUs, 33.3% were without parenteral nutrition and 42.2% without enteral nutrition. Median [Q1-Q3] albumin of 2.9g/dL [2.5–3.2], pre-albumin of 21.7mg/dL [9.7–30.2] and PBR of 6.1mg/dL [3.3–8.1] were identified.
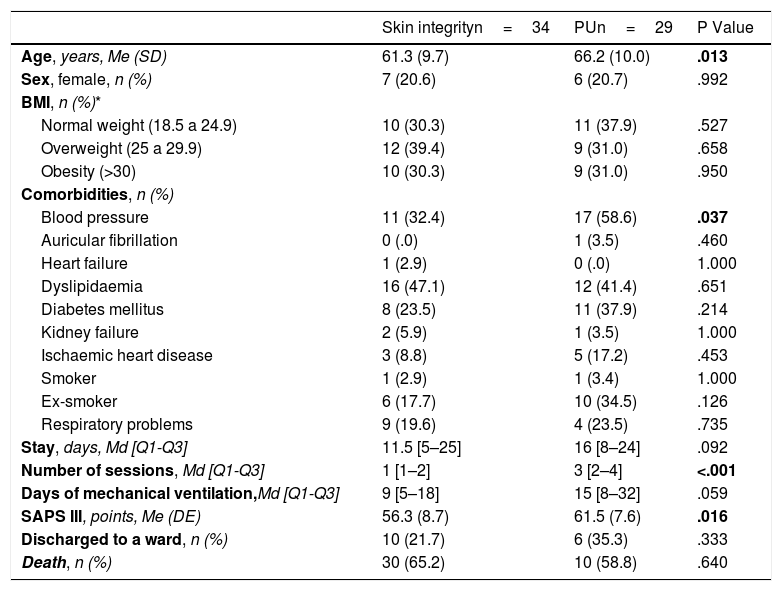
When comparing prone patients who maintained skin integrity (54%) vs. those who developed PUs (46%) (Table 2), it was observed that, statistically significantly, patients who developed PUs were older, hypertensive, were exposed to a greater number of prone sessions and presented greater severity on admission. A clinically relevant increase in the number of days of invasive mechanical ventilation was also observed.
Prone positioned patients with skin integrity vs. prone positioned patients with PU.
| Skin integrityn=34 | PUn=29 | P Value | |
|---|---|---|---|
| Age, years, Me (SD) | 61.3 (9.7) | 66.2 (10.0) | .013 |
| Sex, female, n (%) | 7 (20.6) | 6 (20.7) | .992 |
| BMI, n (%)* | |||
| Normal weight (18.5 a 24.9) | 10 (30.3) | 11 (37.9) | .527 |
| Overweight (25 a 29.9) | 12 (39.4) | 9 (31.0) | .658 |
| Obesity (>30) | 10 (30.3) | 9 (31.0) | .950 |
| Comorbidities, n (%) | |||
| Blood pressure | 11 (32.4) | 17 (58.6) | .037 |
| Auricular fibrillation | 0 (.0) | 1 (3.5) | .460 |
| Heart failure | 1 (2.9) | 0 (.0) | 1.000 |
| Dyslipidaemia | 16 (47.1) | 12 (41.4) | .651 |
| Diabetes mellitus | 8 (23.5) | 11 (37.9) | .214 |
| Kidney failure | 2 (5.9) | 1 (3.5) | 1.000 |
| Ischaemic heart disease | 3 (8.8) | 5 (17.2) | .453 |
| Smoker | 1 (2.9) | 1 (3.4) | 1.000 |
| Ex-smoker | 6 (17.7) | 10 (34.5) | .126 |
| Respiratory problems | 9 (19.6) | 4 (23.5) | .735 |
| Stay, days, Md [Q1-Q3] | 11.5 [5–25] | 16 [8–24] | .092 |
| Number of sessions, Md [Q1-Q3] | 1 [1–2] | 3 [2–4] | <.001 |
| Days of mechanical ventilation,Md [Q1-Q3] | 9 [5–18] | 15 [8–32] | .059 |
| SAPS III, points, Me (DE) | 56.3 (8.7) | 61.5 (7.6) | .016 |
| Discharged to a ward, n (%) | 10 (21.7) | 6 (35.3) | .333 |
| Death, n (%) | 30 (65.2) | 10 (58.8) | .640 |
BMI, Body Mass Index; Md, Median; Me, Mean; PU, Pressure ulcer; Q1, Quartile 1; Q3, Quartile 3; SAPS III, Simplified Acute Physiology Score III; SD, Standard Deviation.
When oxygenation and ventilation parameters were assessed among patients who maintained skin integrity, 46.3% had a PaO2<80mmHg prior to PP vs. 35.3% of patients who developed Pus. At the time of PP, 4.7 vs. 13.7% who developed PUs. At 12h after PP 6.2 vs. 4.1% and after deproning 21.5 vs. 25%, respectively.
Those patients who maintained skin integrity had 89.3% PaO2/FiO2<150mmHgpre-PD vs. 88.3% of patients who developed PUs. At the time of PP, 43.7 vs. 45.3% who developed PU. At 12h after PP 36.1 vs. 32.1% and after deproning 49.3% vs. 45.8%, respectively. No significant differences were obtained in any parameter.
No significant differences were found when comparing the median [Q1-Q3] hours on PP in patients who kept their skin intact, where it was 22h [15–24] and in patients who developed PUs, where it was 23h [17–24].
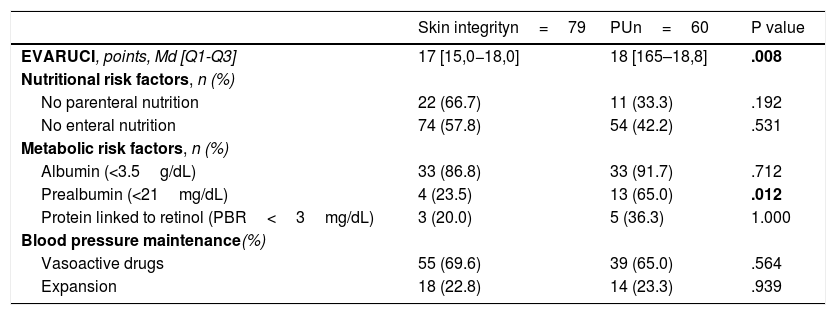
We analysed the possible RFs for PU development in the 139PP procedures performed (Table 3), and observed a significant difference in two RFs: those patients with higher EVARUCI and those PP sessions in which patients had a prealbumin value <21mg/dL. There is a loss of prealbumin data from 102PP sessions, due to the fact that this analytical determination was not routinely requested.
Assessment of the risk of developing PUs and risk factors during each prone procedure.
| Skin integrityn=79 | PUn=60 | P value | |
|---|---|---|---|
| EVARUCI, points, Md [Q1-Q3] | 17 [15,0−18,0] | 18 [165–18,8] | .008 |
| Nutritional risk factors, n (%) | |||
| No parenteral nutrition | 22 (66.7) | 11 (33.3) | .192 |
| No enteral nutrition | 74 (57.8) | 54 (42.2) | .531 |
| Metabolic risk factors, n (%) | |||
| Albumin (<3.5g/dL) | 33 (86.8) | 33 (91.7) | .712 |
| Prealbumin (<21mg/dL) | 4 (23.5) | 13 (65.0) | .012 |
| Protein linked to retinol (PBR<3mg/dL) | 3 (20.0) | 5 (36.3) | 1.000 |
| Blood pressure maintenance(%) | |||
| Vasoactive drugs | 55 (69.6) | 39 (65.0) | .564 |
| Expansion | 18 (22.8) | 14 (23.3) | .939 |
Md, Median; Q1, Quartile 1; Q3, Quartile 3; PU, Pressure ulcer.
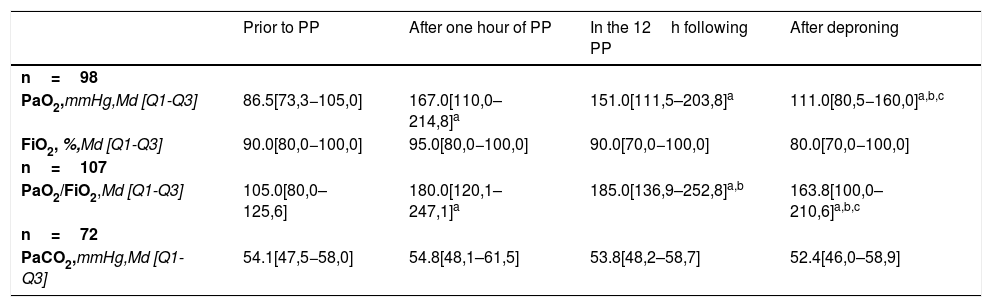
When assessing the evolution of oxygenation and ventilation variables during the pronation procedure in those patients with measurements at all times of the study (Table 4), we observed that the pre-PP PaO2 increased significantly during the first hour of PP (median difference: 78.5mmHg; p<0.001). Between 1 and 12h of PP, we observed a non-significant decrease (median difference: −4.5mmHg; p=0.494) and between 12h of PP and after deproning, we observed a significant drop (median difference: −29.5mmHg; p<0.001).
Oxygenation and ventilation variables.
| Prior to PP | After one hour of PP | In the 12h following PP | After deproning | |
|---|---|---|---|---|
| n=98 | ||||
| PaO2,mmHg,Md [Q1-Q3] | 86.5[73,3−105,0] | 167.0[110,0–214,8]a | 151.0[111,5–203,8]a | 111.0[80,5−160,0]a,b,c |
| FiO2, %,Md [Q1-Q3] | 90.0[80,0−100,0] | 95.0[80,0−100,0] | 90.0[70,0−100,0] | 80.0[70,0−100,0] |
| n=107 | ||||
| PaO2/FiO2,Md [Q1-Q3] | 105.0[80,0–125,6] | 180.0[120,1–247,1]a | 185.0[136,9–252,8]a,b | 163.8[100,0–210,6]a,b,c |
| n=72 | ||||
| PaCO2,mmHg,Md [Q1-Q3] | 54.1[47,5−58,0] | 54.8[48,1–61,5] | 53.8[48,2–58,7] | 52.4[46,0–58,9] |
PP, Prone position; Md, Median; Q1, Quartile 1; Q3, Quartile 3.
A significant increase was observed between pre-PP PaO2/FiO2 and the first hour of PP (median difference: 75.0; p<.001). Between 1 and 12h of PP a significant increase continues (median difference: 8.8; p=.028) and between 12h of PP and after deproning, we observed a significant drop (median difference: −32; p<.001).
With respect to PaCO2 prior to PP vs. at one hour of PP, we observed a non-significant increase (median difference: .4mmHg; p=.490), a non-significant decrease between one and 12h of PP (median difference: −.7mmHg; p=.159) and a non-significant increase between 12h of PP and values at deproning (median difference: .4mmHg; p=.809).
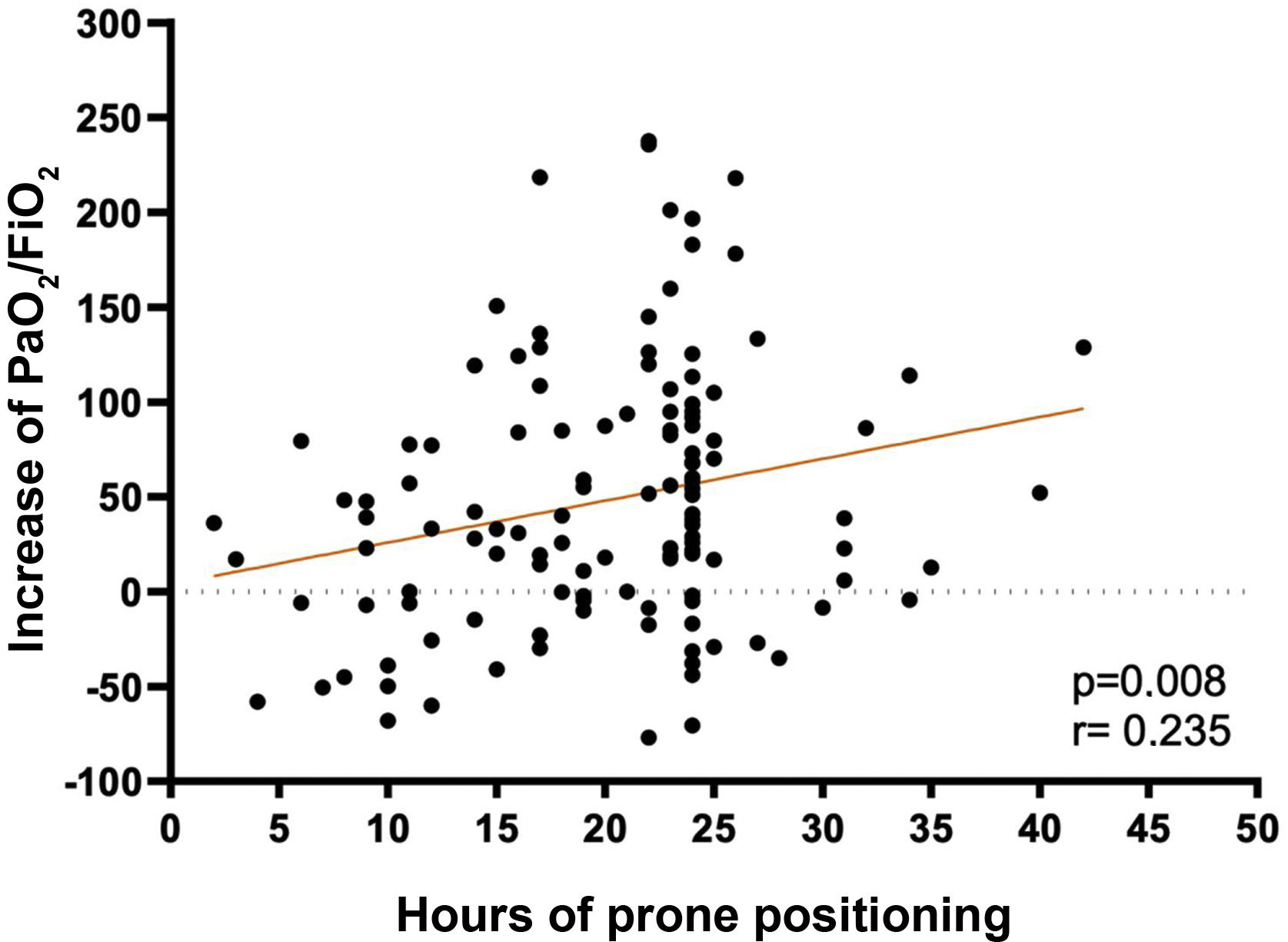
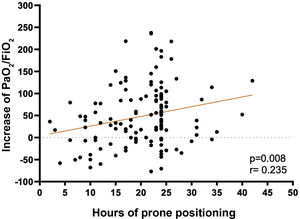
A low correlation (r=.235) was found between the hours of PP and the increase in PaO2/FiO2 at deproning, relative to the PaO2/FiO2 prior to prone (p=.008) (Fig. 2).
DiscussionThe results of this study report a high incidence of adverse events secondary to the PP position in patients admitted to the ICU for COVID-19 with moderate/severe ARDS. The RFs for the development of PUs among prone patients were: older age, hypertension, higher number of prone sessions and prealbumin values <21mg/dL. The prone position confirms improvement in respiratory parameters as time on PP progresses.
Authors such as, Araújo et al.19 report that 67% of patients report adverse events during PP in conditions similar to the patients analysed in this study.
These adverse events can be justified by the increased frequency of prone positioning, the difficulty of this technique, the unfamiliarity of the technique at the time, the instability of the patients and the therapeutic strain experienced nurses had to endure,20 one third of whom had had no previous contact with critical patient care.
Physiological adverse events are described as the most frequent, specifically those included in haemodynamic instability (hypertension/hypotension and cardiac arrhythmias), coinciding with the data of Taccone et al.,21 who report 72% (severe hypotension and arrhythmias). The same author describes a decrease in SatO2 in 63.7% of patients,21 which may be associated with fluid movements and changes in intrathoracic pressure,10,22 but there were few findings in this study.
In relation to facial oedema, the figures are lower than those reported by Araújo et al.,19 who reported 50%; Rodríguez-Huerta et al.,23 who reported 81.3%, and Concha et al.20 who reported 100%.
Another common adverse event relevant to nursing care is PUs. Girard et al.24 describe 56.9% of PUs in pronated patients, Sud et al.25 43.4%, Gattinoni et al.26 36%, and Araújo et al.19 report 50%, data very similar to those of this study. The latest study on the prevalence of PUs in Spain by the National Group for the Study and Advice on Pressure Ulcers and Chronic Wounds (GNEAUPP for its initials in Spanish)27 identifies a prevalence of PUs in the ICU of 14.9%, without differentiating whether the patient was prone positioned or not, being the hospital unit with the second highest prevalence.
The most frequent locations were the face and chest, as in the publication by Girard et al.24 (29% and 18%, respectively), with prone sessions of up to 16h. Studies on patients diagnosed with ARDS by COVID-19 undergoing PP manoeuvres, such as that of Concha et al.20 report 47% of PPUs in the face and 29% in the chest, with prone sessions of up to 48h; Rodríguez-Huerta et al.23 report 60% of PPUs located in the facial region, with sessions of more than 24h.
Some authors observe a clear relationship between the number of prone sessions performed on patients, the hours held in this position and the appearance of PUs on the face,20,23,28,29 while other authors find no relationship here.11,24,30 In this study, a significant relationship was only observed with the number of PP sessions received.
Patients admitted to the ICU are at high risk of developing PUs, with a mean incidence of 18.31% (range 3.3%–39.3%).31 ICU stay has been identified as the highest predictor of PU occurrence, where RF such as reduced mobility, friction and shear forces are 10 times more frequent compared to other units.32
A significant association between increasing age and the development of PUs was found in the publications of Lima Serrano et al.31 and Cox et al.33 In the study by Girard et al.24 age ≥ 60 years is considered a RF, as in this work. In the 2019 National Pressure Injury Advisory Panel34 clinical practice guideline, it is maintained that older age may have a possible impact on PU risk.
Authors such as Cox35 report that cardioivascular diseases are a PU RF in critically ill patients. In this study, the only detected cardiovascular RF was previously high blood pressure.
A relationship is established between pre-albumin value <21mg/dL prior to prone and the development of PUs, but with no significant difference for BMI and serum albumin levels, as in other studies on prone patients during the COVID-1936,37 pandemic. Previous articles have shown a correlation between low serum albumin levels and BMI and the development of PUs in ICU patients, but no mention is made of patient positioning on PP.24,31,38,39
Furthermore, it is observed that the higher the EVARUCI score, the more PUs, coinciding with the study by Roca-Biosca et al.40 A relationship has been found between the severity of the disease on the SAPS III scale and the appearance of PUs, an aspect that is supported by a review study using different scales (APACHE, SOFA, SAPS).38 No data have been found in the literature to support these results in patients with PP.
The results of this study maintain that the prone position improves oxygenation parameters. In the first studies on PP, Gattinoni et al.26 found an improvement without this leading to a reduction in mortality. The PROSEVA study by Guérin et al.10 argued that PP did reduce mortality, as well as improving oxygenation, and suggested maintaining PP for a longer time (16h).
More recent articles support this same trend, observing an increase in oxygenation in practically all patients, although a decrease in mortality is only seen in those diagnosed with severe respiratory failure, with a duration of therapy of at least 12h.11,19,41
There is no consensus on the optimal duration of the prone position. The study by Brazier et al.42 shows a relationship between longer duration and greater benefit. The use of PP for more than 12h per session in patients with moderate/severe ARDS has been included as an indication in clinical practice guidelines since 2017.43
The data from this study are consistent with an improvement in PaO2/FiO2 during PP hours, as is Douglas et al.,29 as well as a positive, albeit low, correlation between PP hours and increased PaO2/FiO2 on discharge.
Charron et al.9 propose monitoring ventilation by measuring PaCO2 and alveolar dead space to monitor the respiratory response to PP in patients with ARDS. The PaCO2 data collected in this study have not reported significant information.
All these findings should be evaluated with caution due to several methodological limitations that could compromise their external validity. As this was a single-centre retrospective cohort study and only restricted to the number of patients recruited in the indicated time period, it precludes a broad generalisability of the results. In addition, the burden of care due to the COVID-19 pandemic may have limited data recording and incident reporting. Failure to differentiate physiological adverse events "hyper/hypotension" due to the small number of cases, or to classify the degree of pressure injuries, could be presented as incomplete data.
Future lines of researchThe results of this study highlight the need for a universal protocol on the PP manoeuvre to help minimise adverse events resulting from PP and thus improve the quality of care.
As future lines of research, new studies are proposed to identify the RFs for the occurrence of adverse events and to support the design of detection tools, as well as to strengthen existing tools, such as the EVARUCI scale.
ConclusionsWe show a high incidence of adverse events due to PP in patients admitted for COVID-19 with moderate/severe ARDS in the ICU, with physiological adverse events being the most frequent.
In addition, several risk factors have been identified as contributing to the development of PUs in these patients (age, hypertension, number of PP sessions, pre-albumin values and severity on admission). Early detection and the development of protocols will help to prevent the development of these lesions.
It is corroborated that the use of PP therapy in COVID-19 patients with moderate/severe ARDS improves oxygenation parameters.
Contribution of the authorsThe authors of this paper participated in the research work as well as in the preparation of the article.
Each of them contributed substantially to the following aspects: conception and design of the study; acquisition, analysis or interpretation of the data; drafting of the article or critical review of the intellectual content; and final approval of the final version.
FundingThis study did not receive any type of funding.
Conflict of interestsThe authors have no conflict of interests to declare.
We would like to end this work by thanking the people who have helped us.
Firstly, we would like to thank Nieves Franco Garrobo (head of the Intensive Care Department) and Margarita Mas Lodo (intensivist) for their support and collaboration.
Thanks also to Blanca Sanjosé Montano for her help in the bibliographic review.