As of August 5th, 2022, the World Health Organization (WHO) confirmed 26,017 cases of human monkeypox in the ongoing 2022 multi-country outbreak. Most persons were male and the median age was 37 years.1 A significant number of cases is reported among men who have sex with men (MSM). It is assumed that close contact during sexual intercourse may be the driving force behind this recent human-to-human transmission in high income countries.2
First monkeypox cases in humans were reported in the 1970s from the endemic regions of Western and Central Africa. Occasionally, these have been self-limited to zoonotic infections from rodents and primates to humans. Contrary to the current outbreak, a disproportionate number of cases have been described among children.3 Between 1980 and 1984, 111 out of 214 patients (51.9%) with primary infections due to the Central African clade, from the former Republic of Zaire, currently Democratic Republic of Congo, were under the age of 4, and 87 (40.7%) between the age of 5 and 14.4 Unvaccinated young males were probably at greater risk because of their exposure to mammals during hunting and playing activities.5 Secondary human-to-human transmission was predominately observed in children. More women were affected by secondary infections, suggesting child-to-mother transmission.3
As monkeypox infections increased in the 2000s, there seems to be a trend towards young adults in their twenties being affected.6 This tendency has been explained by several factors. It is important to note that the endemic regions of Africa have a much younger population than most of the world.7 However, the main reason for this change in age might be related to the presumably low vaccination coverage with the smallpox vaccine, which could provide some cross-protection against monkeypox.6 Since the smallpox virus has been declared eradicated in 1980, vaccination ceased to <30% of the global population.8 While most known outbreaks in the past were ascribed to the Central African clade, the 2022 multi-country outbreak is caused by the less severe Western African clade. This clade has only recently become an unexpected issue of concern to health authorities in West Africa, suggesting changes in monkeypox virus epidemic dynamics.6
While the focus has been on MSM, little has been written about other populations at risk. With this flash survey we intended to gather valuable information in order to appropriately design future specific studies on vulnerable populations such as children and women and the current epidemiological situation in Europe. Especially considering that children are experiencing more complications and a higher mortality. In pregnant women vertical transmission has moreover been associated with stillbirth and congenital infection.9
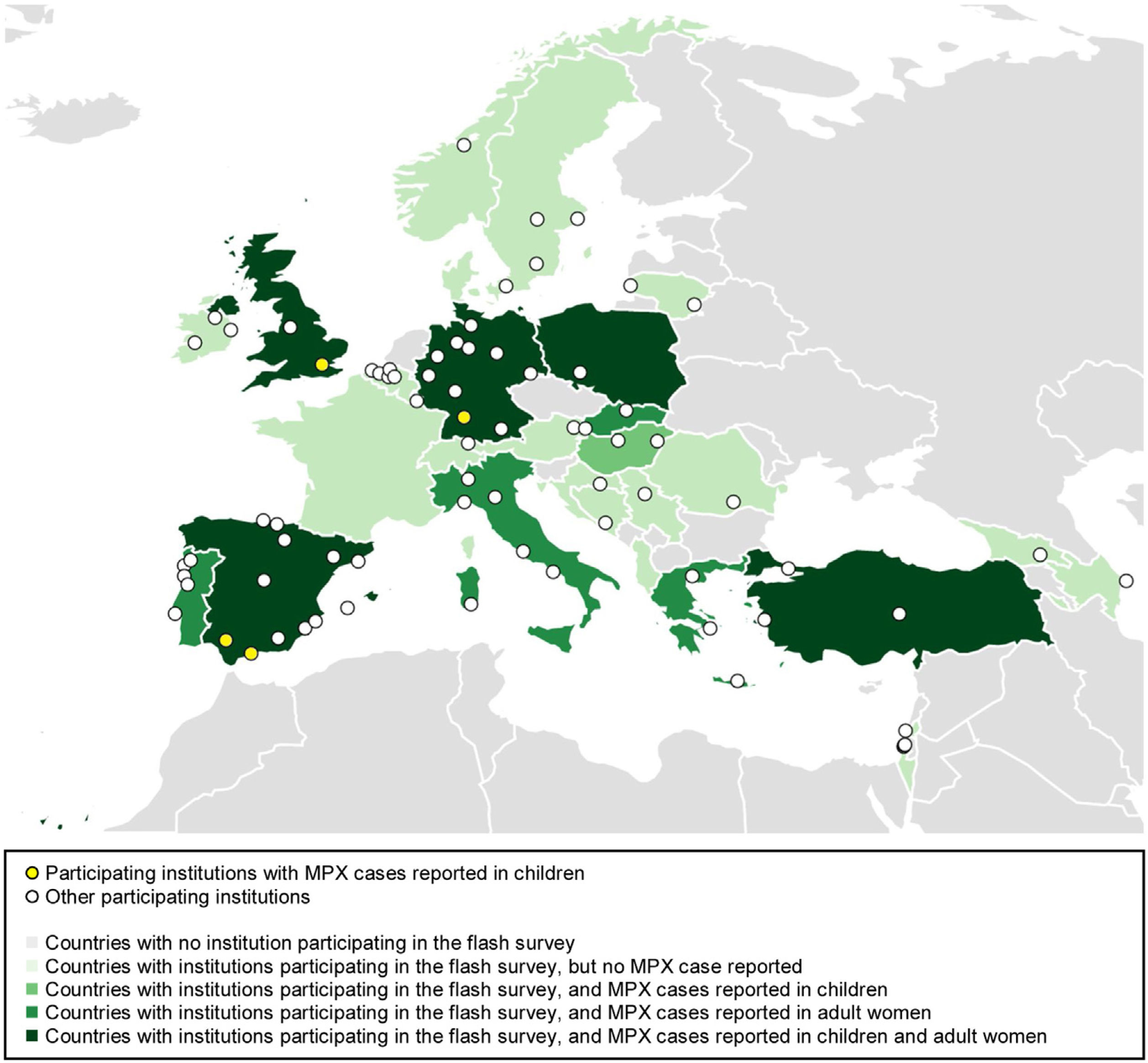
Between July 13th and August 3rd, 2022, 88 institutions from 27 European countries participated in our flash online survey regarding the prevalence of monkeypox in children and adult women in Europe (Fig. 1).
Distribution of participating institutions. Origin countries of participating institutions: Spain (n=18 [20.5%]), Germany (n=11 [12.5%]), Portugal (n=7 [8.0%]), Italy (n=6 [6.8%]), Belgium, Greece, and Turkey (n=5 [5.7%], each), Israel and Sweden (n=4 [4.5%], each), Ireland (n=3 [3.4%], Hungary, Lithuania, and United Kingdom (n=2 [2.3%], each), and Albania, Austria, Azerbaijan, Bosnia and Herzegovina, Croatia, Denmark, Georgia, Luxembourg, Norway, Poland, Romania, Serbia, Slovakia, and Switzerland (1 [1.1%], each). MPX, monkeypox.
In this online survey under the umbrella of the VACCELERATE Consortium (https://www.clinicalsurveys.net/uc/VACC_MPX_women_and_children/),10 participating institutions were asked to provide their numbers on persons evaluated for monkeypox and for confirmed cases locally, as well as the number of confirmed cases nationally. The largest numbers of evaluated children were reported from Belgium (n=47) and the United Kingdom (n=20). However, Spain (1.00) and Germany (0.33) were the countries with the highest ratios of confirmed cases. Spain (n=226) and Belgium (n=60) had the largest number of women evaluated for monkeypox. Among evaluated women the highest likelihood of infection was reported from Spain (0.08) and Portugal (0.06).
Additionally, all institutions were asked about their current treatment options and plans for treatment of monkeypox infections in children (classified as infants [0–1 year], toddlers [1–3 years], pre-schoolers [3–5 years], middle childhood [6–11 years], young teens [12–14 years], and teenagers [15–17 years]). Of 88 institutions participating in this survey, five (5.7%) reported confirmed cases. One institution from the United Kingdom reported consideration of off-labelled use of Imvanex® for post-exposure prophylaxis at any age and tecovirimat for treatment in infants. Two institutions from Spain, and one institution from Germany did not report having provided any specific treatment and one from Spain did not answer to this question.
With this flash survey, we would like to raise the need to raise awareness in populations such as children and adult women, as they can also develop monkeypox infection.
Authors’ contributionsJHG, OAC, and JSG contributed to study design and conceived the study idea.
JSG collected and validated the data, did the statistical plan and analysis.
JHG and JSG drafted the first version of the manuscript.
All authors contributed to data interpretation, manuscript writing and review of the manuscript.
Funding statementVACCELERATE has received funding from the European Union's Horizon 2020 research and innovation program (Grant agreement No. 101037867).
Conflict of interestsJHG reports no conflicts of interest.
OAC reports grants or contracts from Amplyx, Basilea, BMBF, Cidara, DZIF, EU-DG RTD (101037867), F2G, Gilead, Matinas, MedPace, MSD, Mundipharma, Octapharma, Pfizer, Scynexis; Consulting fees from Amplyx, Biocon, Biosys, Cidara, Da Volterra, Gilead, Matinas, MedPace, Menarini, Molecular Partners, MSG-ERC, Noxxon, Octapharma, PSI, Scynexis, Seres; Honoraria for lectures from Abbott, Al-Jazeera Pharmaceuticals, Astellas, Grupo Biotoscana/United Medical/Knight, Hikma, MedScape, MedUpdate, Merck/MSD, Mylan, Pfizer; Payment for expert testimony from Cidara; Participation on a Data Safety Monitoring Board or Advisory Board from Actelion, Allecra, Cidara, Entasis, IQVIA, Janssen, MedPace, Paratek, PSI, Shionogi; A patent at the German Patent and Trade Mark Office (DE 10 2021 113 007.7); Other interests from DGHO, DGI, ECMM, ISHAM, MSG-ERC, Wiley, outside of the submitted work.
JSG reports speaker honoraria from Gilead and Pfizer, outside of the submitted work.
The authors thank all participating institutions for their utmost contributions and support to the project and to all the individuals, associations, and individuals that have disseminated the link to the survey.






![Distribution of participating institutions. Origin countries of participating institutions: Spain (n=18 [20.5%]), Germany (n=11 [12.5%]), Portugal (n=7 [8.0%]), Italy (n=6 [6.8%]), Belgium, Greece, and Turkey (n=5 [5.7%], each), Israel and Sweden (n=4 [4.5%], each), Ireland (n=3 [3.4%], Hungary, Lithuania, and United Kingdom (n=2 [2.3%], each), and Albania, Austria, Azerbaijan, Bosnia and Herzegovina, Croatia, Denmark, Georgia, Luxembourg, Norway, Poland, Romania, Serbia, Slovakia, and Switzerland (1 [1.1%], each). MPX, monkeypox. Distribution of participating institutions. Origin countries of participating institutions: Spain (n=18 [20.5%]), Germany (n=11 [12.5%]), Portugal (n=7 [8.0%]), Italy (n=6 [6.8%]), Belgium, Greece, and Turkey (n=5 [5.7%], each), Israel and Sweden (n=4 [4.5%], each), Ireland (n=3 [3.4%], Hungary, Lithuania, and United Kingdom (n=2 [2.3%], each), and Albania, Austria, Azerbaijan, Bosnia and Herzegovina, Croatia, Denmark, Georgia, Luxembourg, Norway, Poland, Romania, Serbia, Slovakia, and Switzerland (1 [1.1%], each). MPX, monkeypox.](https://static.elsevier.es/multimedia/2529993X/0000004100000005/v2_202311091702/S2529993X23000242/v2_202311091702/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
