Capnocytophaga canis is a capnophilic facultative anaerobic gram-negative bacillus. It belongs to the Flavobacteriaceae family and is part of the commensal oropharyngeal microbiota of dogs and cats mainly, together with Capnocytophaga canimorsus and Capnocytophaga cynodegmi.
We present the case of a 72-year-old male patient who presented symptoms of asthaenia and a loss of 10kg in recent months without fever or other accompanying symptoms. For three months, he had also presented persistent vomiting together with alternating episodes of watery diarrhoea and normal stools. His medical history included type 2 diabetes mellitus, as well as stage IV follicle centre lymphoma in 2007 with a complete response after six cycles of R-CHOP and two years of maintenance treatment. This was followed by progression several years later by R-CHOP and R-Bendamustine, which were interrupted due to the onset of atrial flutter.
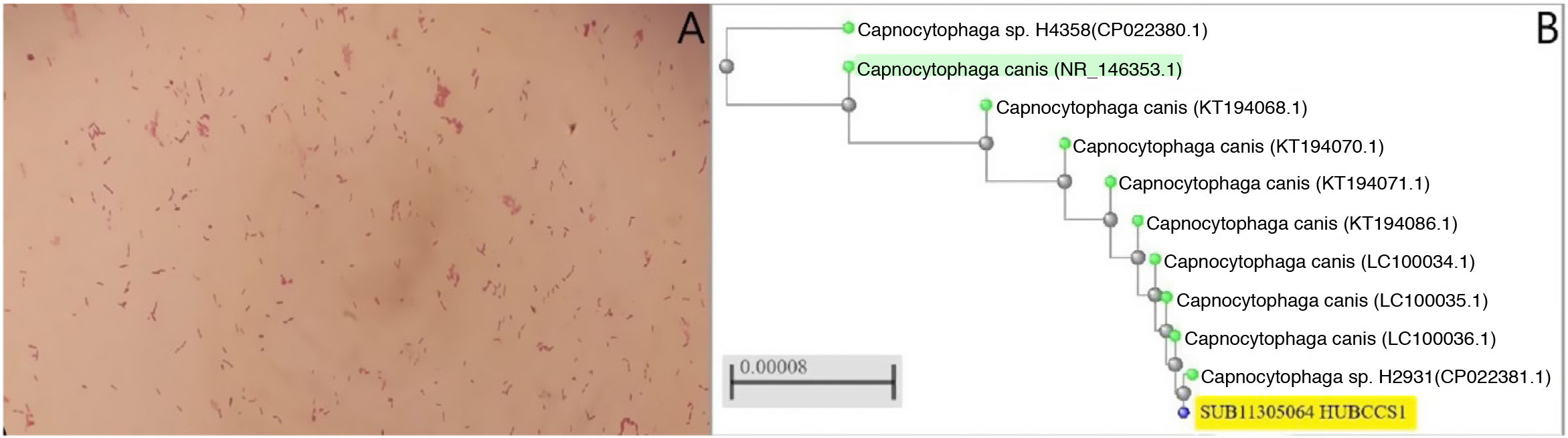
In the blood tests, mention should be made of CRP of 159.64mg/l [0–5] with 5.15×103μl leukocytes [4.5–11], with normal chest and abdominal X-rays. Twenty-four hours after admission, he presented a fever spike of 38.6°C and blood and stool culture samples were taken. The anaerobic bottles were positive at 52h and 107h, with gram-negative spindle-shaped bacilli (Fig. 1A). Re-inoculations were carried out on MacConkey, Brucella, chocolate and TSA+5% sheep blood agars (BD™) and incubated under microaerophilic (5% CO2) anaerobiosis conditions. After 10 days no growth was observed. After 48h, Campylobacter jejuni was detected in the BD MAX™ "Enteric Bacterial Panel" system, which was subsequently grown on Campy BAP (BD™)agar and presented susceptibility to macrolides. The patient was treated with oral azithromycin (500mg/24h for three days). In addition, a full-body computed tomography was performed in which only inguinal and external iliac chain swollen lymph nodes of up to 16mm were observed. in view of the symptoms and the result of the stool culture, this episode was considered gastroenteritis and transient bacteraemia due to C. jejuni, the patient was discharged and he completed a further three days of treatment with oral azithromycin.
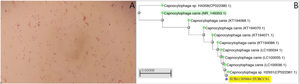
A) Gram-negative rod-shaped bacteria are observed after three days of incubation in the two anaerobic blood culture bottles. B) Phylogenetic tree obtained following comparison of the sequences of several strains of C. caniswith the sequence of the strain of the present case (accession number: SUB11305064 HUBCCS1) with the neighbour-joiningmethod. Our strain had a 99.57% identification match to neighbouring strains (CP022381.1, LC100034.1, LC100035.1 or LC100036.1, pertaining to the articles by Oldham and Duncan1 and Suzuki et al.2) and 99% with the reference strain (C. canis type strain LMG29146, highlighted in green in Fig. 1B) demonstrating the similarity between them.
A blood culture sample was extracted to sequence the 16S rRNA gene (Appendix B additional material), following the protocol proposed by Oldham and Duncan, and a 463bp sequence was obtained, which was analysed by BLASTR (version 2.13.0, available at: https://blast.ncbi.nlm.nih.gov/Blast.cgi)1. Capnocytophaga canis was identified with an identification coincidence of 99.57% with strains previously isolated from infections, and 99% with the reference strain (highlighted in green in Fig. 1B).2,3. Since the patient had already been discharged, no other complementary examinations were performed.
One year later he was re-admitted for septic shock and empyema due to Pasteurella multocida, which causes infection after bites, scratches or contact with cat saliva. After the patient had been questionned again, he reported having taken in a stray cat two years earlier, which frequently bit and scratched him. The finding of C. canis from the previous year was then taken into consideration. The sequence obtained previously after 16S sequencing of the rRNA was registered in GenBank (accession number: SUB11305064 HUBCCS1) of the NIH/NCBI. A phylogenetic tree was constructed using BLASTR (NCBI tree view) with the neighbour-joining method, using alignments by pairs of sequences and based on partial sequences of the 16S gene of between 853 and 1440bp or complete genome according to the strains (Fig. 1B).
C. canis grows with concentrations of 5%–10% CO2 or in anaerobiosis. It presents fastidious and slow growth (from 48h to six days) and does not grow on media such as McConkey agar. C. canis, C. canimorsus and C. cynodegmi are capable of causing infection in humans4–6.
One of the virulence factors recently described inC. canimorsus is the capsular polysaccharide (CPS), which seems to protect it against the bactericidal action of human serum and allows it to cause invasive disease7. Renzi et al. characterised several isolates from human clinical samples and healthy dogs, finding that some strains of C. canis and C. cynodegmi also presented CPS8.
Suzuki et al. characterised three cases of C. canis in 2016 from septic patients, and a case of septic shock caused by this microorganism was also described in 20202,9–11. In three of the cases the patients were heavy drinkers, and in addition one of them was asplenic, risk factors previously related to C. canimorsus7 infections. This is the second time that our institution has reported a case of C. canisinfection.
To conclude, C. canis has demonstrated its capability to cause infections in humans. An infection by this species must be ruled out, especially in immunosuppressed patients if there is contact with animals (bites, scratches or contact with their saliva) and antibiotic treatment should be initiated quickly to avoid fatal consequences.
FundingThis text has not received funding.
Author contributionsDomingo Fernández Vecilla: drafted the scientific text and reviewed the literature.
Estíbaliz Ugalde Zárraga: assisted with the molecular diagnosis (sequence in GenBank) and reviewed the case and the literature.
Mikel Joseba Urrutikoetxea Gutiérrez: reviewed the case, helped to modify it and reviewed the literature.
Felicitas Elena Calvo Muro: helped with the diagnosis, reviewed and suggested changes for the case.
Conflicts of interestThe authors declare that they have no conflicts of interest.