Chromogranin A (CgA) is the most abundant granin in gastroenteropancreatic neuroendocrine tumors (GEP-NETs). As a tumor marker is moderately sensitive and nonspecific. Despite the limitations of testing methods, which require careful interpretation, especially in the case of gastrinomas, patients treated with somatostatin analogs, and poorly differentiated tumors, it is the best tumor marker in GEP-NETs and may be of value in other tumors with neuroendocrine differentiation. CgA may be used as a marker in blood or tissue samples through immunohistochemical techniques. CgA levels correlate with tumor burden and extension and may be used for diagnosis and monitoring of GEP-NETs, especially midgut carcinoids and endocrine pancreatic tumors. It is also useful as a prognostic marker for the detection of recurrence and monitoring of response to different treatments.
La cromogranina A (CgA) es la granina más abundante en los tumores neuroendocrinos gastroenteropancreáticos (TNE-GEP). Como marcador tumoral es moderadamente sensible y poco específico. A pesar de las limitaciones de los métodos de medida que requieren una interpretación cuidadosa, especialmente en el caso de los gastrinomas, pacientes tratados con análogos de somatostatina y tumores pobremente diferenciados, es el mejor marcador tumoral en los TNE-GEP y puede ser útil en otros tumores con diferenciación neuroendocrina. La CgA puede ser usada como marcador en sangre o en muestra tisular mediante inmunohistoquímica. Las concentraciones se relacionan con la carga y la extensión tumoral y puede ser usada en el diagnóstico y seguimiento de los TNE-GEP, especialmente en los derivados del intestino delgado y neuroendocrinos del páncreas. Además es útil como marcador pronóstico en la detección de recidivas y en la monitorización de la respuesta a los distintos tratamientos.
The chromogranin/secretogranin family consists of a group of proteins derived from different genes but sharing a number of characteristics:
- (1)
Phylogenetic. Significant interspecies gene homology.
- (2)
Functional. Chromogranins are co-stored with molecules contained in dense secretion granules and are co-secreted by exocytosis by the regulated pathway. Unlike the constitutive secretion pathway, found in all cell types and characterized by the fact that recently synthesized proteins are transported by the cell using secretory vesicles for their immediate release without the need for a stimulus, the regulated pathway is characterized by its presence in more specialized cells (neuronal, endocrine, neuroendocrine, and polymorphonuclear neutrophils1) and by dense-core secretory granules, which is the name given to secretory vesicles in this pathway. These granules are characterized by:
- (a)
a maturation process consisting of the condensation and aggregation of protein material and the application of a specific coating in the presence of an acid and calcium-rich environment;
- (b)
a condensed charge of biogenic amines, hormone peptides, nucleotides, neurotransmitters, growth factors, and calcium (depending on cell type);
- (c)
strong staining with silver;
- (d)
electron-dense appearance in electron microscopy, and
- (e)
the ability to remain in the cell for periods of time, being released only in response to a stimulus which is specific to each cell type, both in normal and tumor cells.
- (a)
- (3)
Structural. They are water soluble, thermostable, acidic glycoproteins (due to the presence of abundant residues of acidic amino acids, glutamic acid and aspartic acid) with a sequence of 8–10 pairs of basic amino acids fairly preserved between species, which represent potential protease cleavage sites and bind to calcium with a low affinity but a high capacity.2–4 The initial protein is processed into smaller peptides in the intragranular milieu by proprotein convertase PC1/3, PC2, cathepsin,5,6 and to a lesser extent in the extracellular milieu by enzymes such as plasmin.7 Processing is different depending on cell type and is preserved despite tumoral transformation of the cell.8,9
The first member of this family to be isolated was chromogranin A, in 1965, from chromaffin cells of bovine adrenal medulla.10 Subsequently, chromogranin B was described in rat pheochromocytoma,11 and secretogranin II in bovine adenohypophysis.12
The main members of this family are chromogranin A (CgA, sometimes called parathyroid secretory protein), chromogranin B (CgB, sometimes called secretogranin I), and secretogranin II (sometimes called chromogranin C [CgC]).12–18 Other members of the family include secretogranin III (or 1B1075), IV (or HISL-19), V (or neuroendocrine protein 7B2), VI (or NESP55), and VII (or VGF).19
Tissue distributionThe granin family is widely expressed in different cells:
- -
Neuronal cells of the peripheral and central nervous system (cerebellum, brain cortex, hypothalamus, hippocampus, amygdala and, possibly, in astroglial cells).
- -
Endocrine cells (from the adrenal medulla, adenohypophysis, pancreas, gastrointestinal tract diffuse system, parathyroid glands, parafollicular or C cells of the thyroid gland, and bronchopulmonary cells).
- -
Neuroendocrine differentiation cells (prostate, spleen, thymus, heart, breast, placenta).
- -
In inflammatory sites in neutrophils.
The different members of the granin family have however different distributions. Thus, while CgA is predominately located in the adrenal medullar, gastrointestinal tract, and adenohypophysis, secretogranin II predominates in gonadotroph cells and pancreatic alpha cells.20–22
The presence of CgA in neuroendocrine cells has traditionally been described as ubiquitous, but its concentration varies depending on the type of tissue. Sympathoadrenal chromaffin cells are the main production and storage sites, followed in descending order by the adenohypophysis, pancreas, stomach, small bowel (jejunum and ileum), frontal cerebral cortex, parathyroid glands, and thyroid gland.22
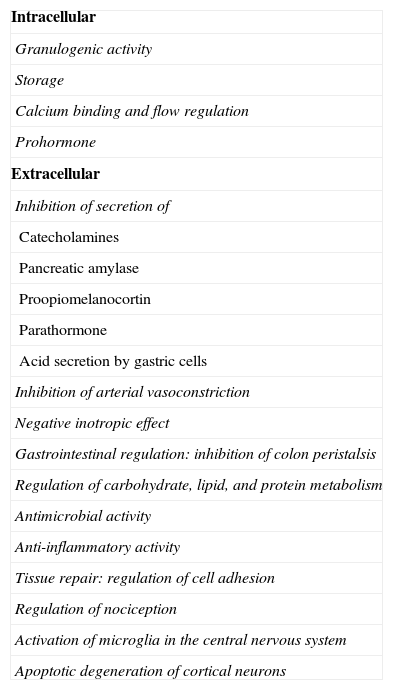
Roles of chromogranin and its derived peptides (Table 1)When speaking of CgA we usually refer to the 439-amino acid proprotein CgA with a molecular weight of 48kDa, derived from pre-CgA (a 457-amino acid preprotein) and whose gene is located at the 14q32.12 locus. As previously discussed, CgA acts as a prohormone, and after undergoing post-translational changes such as glycosylation, phosphorylation, or sulfation and/or tissue-specific proteolytic processing, gives rise to biologically active peptides controlling multiple physiological roles. Most roles consist of direct or indirect inhibitory regulation through autocrine, paracrine, or endocrine pathways (Table 1).
Actions of chromogranin and its derived peptides.
| Intracellular |
| Granulogenic activity |
| Storage |
| Calcium binding and flow regulation |
| Prohormone |
| Extracellular |
| Inhibition of secretion of |
| Catecholamines |
| Pancreatic amylase |
| Proopiomelanocortin |
| Parathormone |
| Acid secretion by gastric cells |
| Inhibition of arterial vasoconstriction |
| Negative inotropic effect |
| Gastrointestinal regulation: inhibition of colon peristalsis |
| Regulation of carbohydrate, lipid, and protein metabolism |
| Antimicrobial activity |
| Anti-inflammatory activity |
| Tissue repair: regulation of cell adhesion |
| Regulation of nociception |
| Activation of microglia in the central nervous system |
| Apoptotic degeneration of cortical neurons |
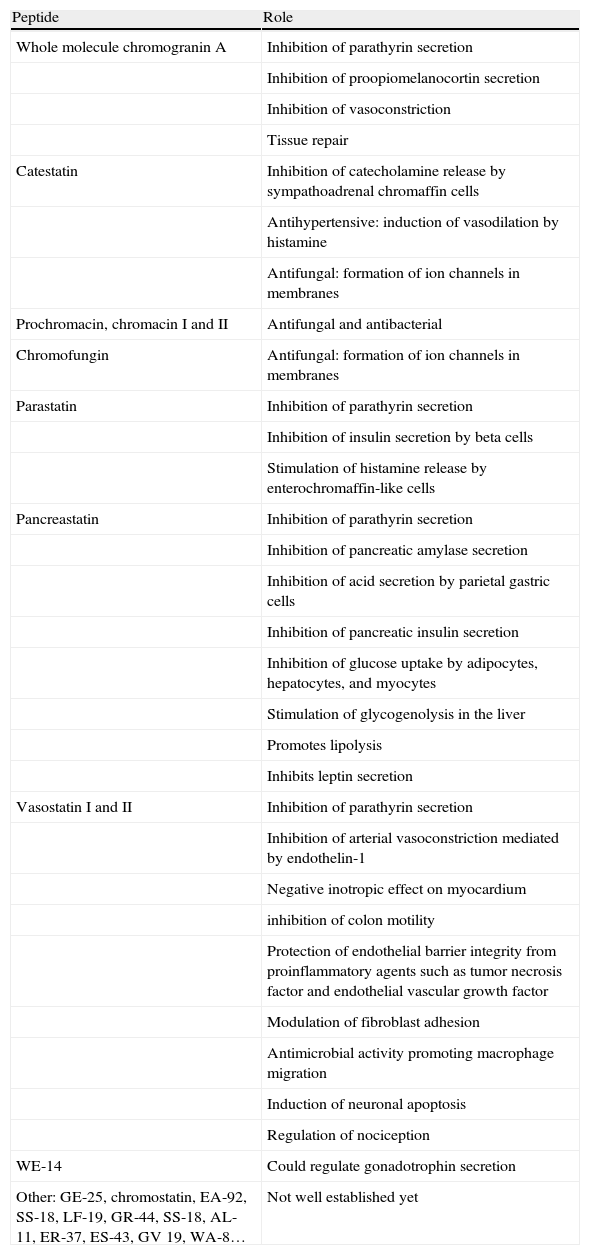
While the role of CgA is not fully known, the role of its derived peptides is increasingly known,23–30 and it appears that many of the roles initially attributed to CgA, particularly extracellular functions, are actually mediated by these peptides (Table 2).
Roles of peptides derived from chromogranin A.
| Peptide | Role |
| Whole molecule chromogranin A | Inhibition of parathyrin secretion |
| Inhibition of proopiomelanocortin secretion | |
| Inhibition of vasoconstriction | |
| Tissue repair | |
| Catestatin | Inhibition of catecholamine release by sympathoadrenal chromaffin cells |
| Antihypertensive: induction of vasodilation by histamine | |
| Antifungal: formation of ion channels in membranes | |
| Prochromacin, chromacin I and II | Antifungal and antibacterial |
| Chromofungin | Antifungal: formation of ion channels in membranes |
| Parastatin | Inhibition of parathyrin secretion |
| Inhibition of insulin secretion by beta cells | |
| Stimulation of histamine release by enterochromaffin-like cells | |
| Pancreastatin | Inhibition of parathyrin secretion |
| Inhibition of pancreatic amylase secretion | |
| Inhibition of acid secretion by parietal gastric cells | |
| Inhibition of pancreatic insulin secretion | |
| Inhibition of glucose uptake by adipocytes, hepatocytes, and myocytes | |
| Stimulation of glycogenolysis in the liver | |
| Promotes lipolysis | |
| Inhibits leptin secretion | |
| Vasostatin I and II | Inhibition of parathyrin secretion |
| Inhibition of arterial vasoconstriction mediated by endothelin-1 | |
| Negative inotropic effect on myocardium | |
| inhibition of colon motility | |
| Protection of endothelial barrier integrity from proinflammatory agents such as tumor necrosis factor and endothelial vascular growth factor | |
| Modulation of fibroblast adhesion | |
| Antimicrobial activity promoting macrophage migration | |
| Induction of neuronal apoptosis | |
| Regulation of nociception | |
| WE-14 | Could regulate gonadotrophin secretion |
| Other: GE-25, chromostatin, EA-92, SS-18, LF-19, GR-44, SS-18, AL-11, ER-37, ES-43, GV 19, WA-8… | Not well established yet |
- (a)
Granulogenic activity: participation in the synthesis and maturation of secretory granules, especially in the aggregation of secretory products in the trans-Golgi network and in avoiding their rupture by controlling osmotic pressure.31,32 CgA is the only member of the granin family involved in this function, and is therefore called the on/off switch of granulogenesis.33
- (b)
Storage: the ubiquitous presence of CgA in neuroendocrine tissue and its co-secretion with molecules contained in the granule appear to suggest a role in their storage.
- (c)
Calcium binding and flow regulation.
- (d)
Prohormone: activity as a peptide precursor through the intracellular and extracellular proteolytic processing of peptides.
- (a)
Inhibition of the secretion of: catecholamines by adrenal medulla (catestatin), pancreatic amylase mediated by cholecystokinin (pancreastatin), proopiomelanocortin (whole molecule CgA), parathyrin (whole molecule CgA, parastatin, pancreastatin, vasostatin), insulin by beta cells (parastatin, pancreastatin), and acid secretion by gastric parietal cells (pancreastatin).34
- (b)
Stimulation of histamine release by enterochromaffin-like cells (parastatin).
- (c)
Cardiovascular regulation through the inhibition of arterial and myocardial muscle contraction (whole molecule CgA, catestatin, vasostatin).
- (d)
Gastrointestinal regulation through the inhibition of colon peristalsis (vasostatin).35
- (e)
Regulation of carbohydrate, lipid, and protein metabolism (pancreastatin).
- (f)
Antimicrobial activity: first defense line of the host against invading microorganisms (catestatin, prochromacin, chromacin, chromofungin, vasostatin).36–38
- (g)
Anti-inflammatory activity (vasostatin).
- (h)
Regulation of the endothelial barrier and the modulation of adhesion of fibroblasts and endothelial cells in tissue repair and the remodeling involved in processes such as angiogenesis, inflammation, and neoplastic processes (whole molecule CgA, vasostatin).39,40
- (i)
Regulation of nociception (vasostatin).
- (j)
Activation of microglia in the central nervous system.41
- (k)
Apoptotic degeneration of cortical neurons (vasostatin).
Different test methods are available for measuring CgA. The most widely known include CgA-RIA (CIS bio international), DAKO CgA ELISA, and CgA by IRMA (a sandwich method with dual antibody against the fractions of 145–197 and 198–245 amino acids). Stridsberg et al., in a study comparing three commercial methods (RIA, IRMA, and ELISA), found differences in specificity, sensitivity, and positive and negative predictive value. RIA, using a monoclonal antibody, was found to be the most sensitive method, while IRMA, using two monoclonal antibodies, was the most specific method.42
A Spanish study compared the different methods of measuring CgA in 52 healthy subjects, 98 patients with benign conditions, 94 patients with tumor (not neuroendocrine) pathology, 20 patients with small cell lung cancer, and 79 patients with neuroendocrine tumors (NETs). Using the different cut-off normal values (6nmol/L, 60ng/mL and 90ng/mL for RIA, ELISA and IRMA respectively) abnormal values were found in a high proportion of patients with renal failure (76.7, 86.7 and 93.3% with ELISA, IRMA, and RIA respectively), other benign diseases (chronic gastritis, inflammatory bowel disease, liver disease, and heart failure), and other tumoral diseases (59.8% ELISA; 55.4% IRMA; and 37% RIA). The ROC curves comparing the area under the curve between healthy subjects and patients with NETs, or between subjects with no NET (excluding those with renal failure and small cell lung cancer) or with NET showed better results with ELISA (0.96 and 0.77) and IRMA (0.95 and 0.78) as compared to RIA (0.80 and 0.69).43
Several factors may account for the differences found in these studies:
- -
In each kit, the antibody is obtained from CgA molecules of different origin and size (CgA from Escherichia coli, CgA from urine of patients with carcinoid tumors)
- -
CgA levels are expressed in different units (nmol/L, ng/ML, U/L) and the definition of the concentration considered as the upper normal limit is also different.
- -
Differences between the antibodies used. The higher the number of epitopes in the CgA molecule that may be detected by an antibody, the greater its capacity to detect peptides derived from proteolytic processing of CgA and the more sensitive the method.
- -
Differences in patient study sample as regards type of NET may bias the results, because the processing of whole molecule CgA is different in each tissue.
CgA levels may be measured in plasma or serum. There is a positive linear association between both methods (r=0.9858; p<0.001).44 CgA studies in saliva to quantify stress level are available, but this method has not been used to study NETs.45
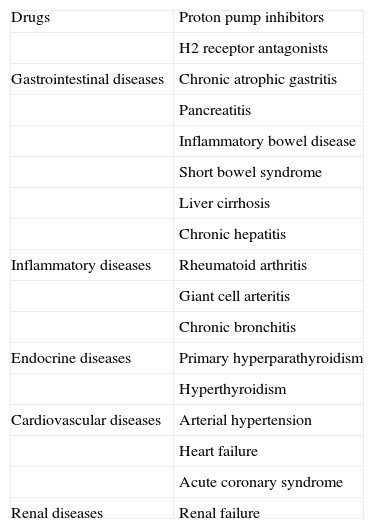
There are multiple causes of CgA elevation inducing false positive CgA measurements: drugs (proton pump inhibitors, H2 receptor antagonists), renal failure, high blood pressure, heart failure, acute coronary syndrome, hyperthyroidism and hyperparathyroidism, chronic atrophic gastritis (CAG), pancreatitis, inflammatory bowel disease, irritable colon, chronic hepatitis and liver cirrhosis, rheumatoid arthritis, chronic bronchitis, and exercise-induce stress (Table 3). High CgA levels have also been reported in giant cell arteritis, rheumatoid arthritis, and systemic lupus erythematosus.46 One of the most common causes of false positive results is the use of proton pump inhibitors. Hypergastrinemia caused by these drugs due to acid secretion inhibition represents a stimulus for gastric enterochromaffin cells. The elevation is greater in patients treated for longer than one year.47 We are of the opinion that, since the optimum discontinuation time of such drugs has not been shown, they should be withdrawn at least 14–10 days before CgA measurement. They may be replaced by ranitidine (which should be discontinued three days before) or other antacids.
False positive results in chromogranin A tests.
| Drugs | Proton pump inhibitors |
| H2 receptor antagonists | |
| Gastrointestinal diseases | Chronic atrophic gastritis |
| Pancreatitis | |
| Inflammatory bowel disease | |
| Short bowel syndrome | |
| Liver cirrhosis | |
| Chronic hepatitis | |
| Inflammatory diseases | Rheumatoid arthritis |
| Giant cell arteritis | |
| Chronic bronchitis | |
| Endocrine diseases | Primary hyperparathyroidism |
| Hyperthyroidism | |
| Cardiovascular diseases | Arterial hypertension |
| Heart failure | |
| Acute coronary syndrome | |
| Renal diseases | Renal failure |
Unlike CgA, CgB is not affected by the presence of renal failure or the use of proton pump inhibitors. This may make CgB a supplemental tumor marker, but it should be taken into account that it has a different tissue expression pattern and that limitations and differences regarding the procedure used and definition of the upper normal limit also exist in its measurement. In a study on 44 patients with carcinoid tumors, 17 with sporadic endocrine tumors of the pancreas (ETPs) and 11 with ETPs associated with multiple endocrine neoplasia type 1 (MEN 1), CgB sensitivity was 87%, as compared to 99% for CgA.18,48–50
Chromogranin A in neuroendocrine tumors in generalElevated CgA levels have been found in a variety of NETs, including pheochromocytoma, paraganglioma, neuroblastoma, Merkel cell skin carcinoma, gastrointestinal NETs, pancreatic islet cell tumors, medullary thyroid carcinoma, adenomas of the parathyroid glands and adenohypophysis, and also in a proportion of patients with bronchopulmonary NETs (including small cell lung cancer).
As a tumor marker, serum CgA is characterized by being moderately sensitive and non-specific. In addition, among general (and non-specific, such as neuron-specific enolase, carcinoembryonic antigen (CEA), or human chorionic gonadotrophin) tumor markers, CgA has the greatest sensitivity and specificity for detecting NETs.51–53
CgA is of great value in NETs where no marker is available or symptoms occur late in the course of the disease, in nonfunctioning NETs, which usually retain the ability to secrete CgA, and in NETs where limitations (such as fluctuations in serum catecholamine levels in pheochromocytoma) or disadvantages (such as 24-hour urine collection after dietary restrictions) exist in measurements of the marker, representing in these cases a more stable marker.54
In a sample of 211 patients with NETs, both gastroenteropancreatic (GEP-NETs) and other types, whose serum CgA levels were measured by polyclonal RIA (using human CgA from pheochromocytomas as tracer), with 175mcg/L for men and premenopausal women and 220mcg/L for postmenopausal women being considered as upper normal limits, the proportions of patients showing high CgA levels per type of NET were, in descending order: gastrinoma (100%, n=9), pheochromocytoma (89%, n=9), carcinoid tumors (80%, n=62), nonfunctioning ETPs (69%, n=13), medullary thyroid carcinoma (50%, n=26), small cell lung carcinoma (39%, n=23), neuroblastoma (33%, n=3), Merkel cell tumor (25%, n=4), nonfunctioning pituitary adenomas (20%, n=10), insulinoma (10%, n=21), and paraganglioma (8%, n=25). In this sample, CgA levels expressed as median (range) in mcg/L were found to be highest in carcinoid tumors, 688 (33–52340) mcg/L, followed in descending order by gastrinoma, 772 (289–1933) mcg/L; nonfunctioning EPTs, 306 (85–14750) mcg/L; pheochromocytoma, 275 (110–4674) mcg/L; medullary thyroid carcinoma, 184 (80–13900) mcg/L; small cell lung carcinoma, 149 (45–2948) mcg/L; neuroblastoma, 133 (117–238) mcg/L; Merkel cell tumor, 109 (84–1056) mcg/L; paraganglioma, 106 (50–11590) mcg/L; insulinoma, 105 (63–236) mcg/L; nonfunctioning pituitary adenomas, 131 (85–240) mcg/L; and GH-secreting pituitary adenomas, 71 (53–115) mcg/L.53,55
Differences between tumors may be explained by the fact that circulating CgA expression depends on a number of factors such as cell type of origin, degree of differentiation, density of secretory granules, tumor size and extension, and tissue secretion capacity. As regards the last factor, it should be noted that high CgA levels appear to be significantly and independently associated with the presence of other secretions.56
Chromogranin A in gastropancreatic neuroendocrine tumorsCgA is the most abundant granin in GPE-NETs and represents the best general marker in tissue (together with synaptophysin for immunohistochemical confirmation) and blood.
The variability in sensitivity (60%–100%) and specificity (70%–100%) ranges for circulating CgA reported in the literature for the different types of GEP-NETs depends on several factors:
- -
The clinical presentation of NETs, which depends in turn on the site of origin, histological tumor characteristics, primary tumor size, the route of dissemination, the presence or absence of metastases, the functionality or secretory activity of the tumor, the degree of neuroendocrine differentiation, and association with a hereditary syndrome.
- -
The procedure used for measurement and whether it is able to detect peptides resulting from the fragmentation process.
- -
The threshold considered as pathological, and how CgA variability is managed and studied in the control group of studies.
- -
Its ubiquity in normal tissue, which causes different physiological or non-neoplastic pathological events to be also able to increase it.
Together with 5-hydroxyindoleacetic acid (5-HIAA), CgA is the best marker for both the diagnosis and monitoring of patients with midgut carcinoid tumors. It may also be particularly helpful in those subtypes, such as rectal or bronchial tumors, where the elevation of 5-HIAA levels is less likely or in hindgut tumors, which are usually nonfunctioning and are increasingly diagnosed at an earlier stage in the setting of colorectal cancer screening, although CgA levels are low in these cases.
In patients with gastrinoma (Zollinger-Ellison syndrome, ZES), CgA levels are significantly higher than in patients with CAG. As local or distant disease progression occurs, concentrations gradually increase. However, the extent of increase reflects hyperplasia of gastric enterochromaffin-like cells caused by gastrin rather than gastrinoma size itself.57
In pancreatic NETs, CgA is the most helpful serum marker for diagnosis, staging, and monitoring, regardless of tumor functionality, because of its correlation with tumor size and progression, and its value for monitoring the course of disease and for assessing response to treatment. In functioning pancreatic NETs, CgA has a 64–199% sensitivity.58 Sensitivity in nonfunctioning ETPs ranges from 64% to 84%.59
In a study by Campana et al. where plasma CgA measurement using the DAKO CgA ELISA was analyzed in a sample of 280 patients (238 with NETs, 42 with CAG with and without hyperplasia of enterochromaffin-like cells, and 48 healthy subjects), the best cut-off value for separating patients with NETs from healthy subjects was found to be 18–19U/L (85.3% sensitivity and 95.8% specificity), and the best value for differentiating patients with NETs from subjects without NET (healthy subjects and patients with CAG) was 31–32U/L (75.3% sensitivity and 84.2% specificity). In the NET group, the origin was gastric in 5.9% (n=14), bronchial in 8.4% (n=20), intestinal in 35.7% (n=85), pancreatic in 39.5% (n=94), while 10.5% (n=25) of the 238 patients had ZES. The highest CgA levels, expressed as mean (standard deviation), were found in patients with ZES (1490.5 [3819.3] U/L) and the lowest in those with bronchial tumors (46.3 [94.6] U/L). Tumors arising in the stomach (78 [69.2] U/L), pancreas (322.2 [952.7] U/L], and bowel (380.1 [1224.9] U/L) had intermediate values with no statistically significant differences between them.60
Chromogranin A and tumor burden and extensionCgA levels in midgut carcinoid tumors and ETPs positively correlate to tumor size and extension.53,56,60–63 This is why circulating CgA levels are only slightly elevated in patients with insulinoma (as occurs with paraganglioma or pituitary adenoma), because these tumors are usually detected at an early stage of their course based on a characteristic clinical syndrome secondary to hormonal secretion or on compressive symptoms.64
In NET studies, CgA levels are higher in patients with metastases. They are also significantly higher in patients with diffuse metastases (distant lymph nodes, lung, bone, and spleen metastases) than in those with local metastases only. In other studies of patients with metastatic GEP-ENTs, CgA levels were also particularly related to the degree of liver extension.62,65 For the detection of metastases of carcinoid tumors, a combination of somatostatin receptor scintigraphy with CgA levels increases sensitivity from 80% to 93%.66
Chromogranin A as prognostic factor and survival predictorHigh CgA levels have been shown to be independent predictors of poor prognosis and decreased survival in GEP-NETs with metastases in general, and specifically for:
- -
Midgut carcinoid tumors.62,65,67,68 In a study of 310 patients with histopathologically documented carcinoid tumors (256 foregut, 39 midgut, and 6 hindgut tumors) where 350mcg/L were considered as the upper normal limit and the measurement method was not stated, plasma CgA levels higher than 5000mcg/L in midgut tumors acted as an independent poor prognostic predictor (survival of 33 months, as compared to 57 months for levels lower than 5000mcg/L).67
- -
Pancreatic NETs.69–72
It should be noted that this correlation with size and tumor extension and importance as a prognostic factor does not exist in three situations:
- (a)
Gastrinomas: these have high CgA levels, even in the absence of metastases, related to hyperplasia of enterochromaffin-like cells rather than tumor size.
- (b)
Poorly differentiated tumors: these have low or no CgA levels due to loss of the ability to synthesize and secrete CgA.73
- (c)
Tumors treated with somatostatin analogs: CgA levels are low because of the antisecretory effect, rather than the antitumor effect.74
In carcinoid tumors, the measurement of 5-HIAA in addition to CgA is recommended because it has a greater sensitivity for detecting tumor progression.52 In midgut tumors, CgA has shown an 83% correlation with tumor progression and a 100% correlation with hepatic progression. CgA has also been shown to be the first marker of recurrence, being more effective than 5-HIAA and imaging techniques.52,75,76 In a sample of 39 patients with metastatic midgut carcinoid tumors treated with somatostatin analogs, CgA had a greater correlation with physical function, quality of life, and prognostic value for survival as compared to 5-HIAA in 24-hour urine, for which none of these associations was found.77 In patients with carcinoid heart disease, the elevation of both CgA and NT-proBNP (N-terminal pro-brain peptide, a marker of left ventricular failure) is a diagnostic and survival marker.78
Assessment of treatment responseCgA monitoring may also be helpful in assessing the response to the different therapeutic options: surgery, medical treatment, radionuclide therapy, and liver transplantation.79,80 CgA could be considered as a biomarker of response in some studies with patients treated with everolimus for pancreatic NETs.80,81
Chromogranin A and multiple endocrine neoplasia type 1 syndromeThe monitoring of CgA levels is helpful in patients with MEN 1 because it allows for the early diagnosis of GEP-NETs and, thus, for early treatment. The identification of elevated CgA levels suggests the presence of an ETP (in general) and, to a lesser extent, a gastrinoma, because primary hyperparathyroidism and/or pituitary adenoma rarely increase such levels.
For pancreatic NET screening, although CgA has been shown to be the most sensitive NET marker, it cannot be the only diagnostic measure, and CgA measurement should be combined with regular imaging tests. In a study in 34 patients with MEN 1 where CgA was measured by RIA using an upper normal limit of 130mcg/L, high CgA levels indicated the presence of a pancreatic tumor with 100% specificity, but only 59% sensitivity.62,82
Chromogranin A in neuroendocrine tumors other than gastropancreatic tumors- -
Pheochromocytoma. CgA is helpful in the diagnosis and follow-up of pheochromocytoma for monitoring both treatment and tumor recurrence and progression. The isolated measurement of circulating CgA has a similar sensitivity and specificity to the measurement of catecholamines/metanephrines in plasma and urine, and it seems that a combination of both tests could increase yield. In addition, unlike as occurs with catecholamines, CgA measurement is not limited by fluctuations in serum levels. CgA levels decrease rapidly after surgery, while urinary catecholamine/metanephrine levels may take 2–3 weeks to decrease, and measurement is not altered by drugs for treatment. Serum CgA levels correlate with tumor size and extension, so that its value for screening patients with hereditary disease or small tumors is limited. The results regarding its role in discriminating between benign and malignant pheochromocytoma are controversial. In adrenal cortex tumors, CgA may be useful for differential diagnosis with poorly differentiated pheochromocytomas showing no catecholamine/metanephrine elevation.3,83–86
- -
Other neoplasms derived from chromaffin cells. As previously shown, sensitivity in paraganglioma and neuroblastoma is low (10% and 30% respectively) as compared to sensitivities higher than 85% in pheochromocytoma reported in several studies.53 However, in a study in 34 children diagnosed with neuroblastoma (in different stages), CgA, as measured by RIA with values higher than 42ng/mL being considered the upper normal limit, had a diagnostic value with 91% sensitivity and 100% specificity with correlation with tumor size and was reported to be a helpful predictor of survival.87
- -
Medullary thyroid carcinoma. The measurement of circulating CgA for the diagnosis of this tumor is not superior to calcitonin (CT) or CEA, except for dedifferentiated tumors that do not express CT or CEA.88
- -
Pituitary adenomas. Conflicting results have been reported for CgA in the diagnosis of gonadotropinoma, clinically nonfunctioning pituitary adenomas, and non-endocrine pituitary tumors.53,89,90 Its value for the etiological diagnosis of Cushing's syndrome has been pointed out, suggesting that high concentrations may indicate an ectopic origin.91 As in pheochromocytoma, it seems that the measurement of CgB and secretogranin II may have a diagnostic value in prolactinoma.3
- -
Parathyroid neoplasms. CgA does not appear to be of any value.
Elevated CgA levels in other tumors reflect their neuroendocrine differentiation in patients with pancreatic, colorectal, gastric, and prostate cancer. In breast carcinoma and hepatocarcinoma, the cause of such elevation is not clear.
In patients with adenocarcinoma of the pancreas, higher levels are found as compared to those with chronic pancreatitis and healthy subjects.92
CgA may be elevated in up to 34% of patients with colorectal cancer and is related to poorer prognostic after surgery.93
In prostate cancer, a correlation exists between immunohistochemistry and plasma levels, and CgA it has also been related to Gleason stage and unfavorable prognosis.94,95 Moreover, CgA measurement may help identify patients with advanced prostate cancer with no elevated prostate-specific antigen levels, those who could be eligible for adjuvant therapy, or those resistant to hormone suppression therapy.96 These are some of the factors leading to routine CgA measurement in clinical practice in the management of adenocarcinoma and mixed tumors of the prostate.
High CgA levels are found in more than 80% of patients with hepatocarcinoma, in which higher elevations are seen as compared to chronic hepatitis and liver cirrhosis.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Díaz Pérez JÁ, Currás Freixes M. Cromogranina A y tumores neuroendocrinos. Endocrinol Nutr. 2013;60:386–395.