Radionuclide imaging tests with [123I] Metaiodobenzylguanidine (MIBG), [18F] -fluorodeoxyglucose, [18F]-fluorodopa, or 68Ga-DOTA(0)-Tyr(3)-octreotate are useful for the diagnosis, staging and follow-up of pheochromocytomas (PHEOs) and paragangliomas (PGLs) (PPGLs). In addition to their ability to detect and localize the disease, they allow a better molecular characterization of the tumours, which is useful for planning targeted therapy with iodine-131 (131I) -labelled MIBG or with peptide receptor radionuclide therapy (PRRT) with [177Lu]-labelled DOTATATE or other related agents in patients with metastatic disease. In this review we detail the main characteristics of the radiopharmaceuticals used in the functional study of PPGLs and the role of nuclear medicine tests for initial evaluation, staging, selection of patients for targeted molecular therapy, and radiation therapy planning. It also offers a series of practical recommendations regarding the functional imaging according to the different clinical and genetic scenarios in which PPGLs occur, and on the indications and efficacy of therapy with [131I]-MIBG and 177Lu-DOTATATE.
Las pruebas de imagen con radionúclidos como [123I] Metayodobencilguanidina (MIBG), [18F]-fluorodesoxiglucosa, [18F]-fluorodopa o 68Ga-DOTA(0)-Tyr(3)-octreotate son de utilidad para el diagnóstico, la estadificación y el seguimiento de los feocromocitomas (PHEOs) y paragangliomas (PGLs) (PPGLs). Además de su capacidad de detección y localización de la enfermedad, permiten una mejor caracterización molecular, lo cual es de utilidad para la planificación de la terapia dirigida con MIBG marcada con yodo-131 (131I) o con la terapia con radionúclidos receptores de péptidos (PRRT) con DOTATATE marcado con [177Lu] u otros agentes relacionados en pacientes con enfermedad metastásica. En esta revisión detallamos las principales características de los radiofármacos empleados en el estudio funcional de los PPGLs y el papel de las pruebas de imagen con radionúclidos para la evaluación inicial, estadificación, selección de pacientes para terapia molecular dirigida y planificación de radioterapia. También se ofrece una serie de recomendaciones prácticas en cuanto al estudio funcional según los diferentes escenarios clínicos y genéticos en los que se presenten los PPGLs, y sobre las indicaciones y eficacia de terapia con [131I]-MIBG y 177Lu-DOTATATE.
Pheochromocytomas (PHEOs) and paragangliomas (PGLs) (collectively, PPGLs) are uncommon neuroendocrine tumours deriving from the neural crest cells of the sympathetic and parasympathetic nervous system.1,2 PHEOs arise from the adrenal medulla, and PGLs arise from paraganglia outside the adrenal medulla. PGLs may be located anywhere in the body and are classified as sympathetic or parasympathetic depending on their origin. PHEOs account for 80%–85% of chromaffin cell tumours, and PGLs account for 15%–20% of them.3 An estimated 0.2%–0.6% of patients with hypertension have PHEOs,4 and approximately 5% of patients have adrenal incidentalomas.5
There is a consensus that determination of fractionated metanephrine levels in plasma and/or urine is the diagnostic test of choice, and the initial test to make a diagnosis of endogenous hypersecretion of catecholamines in patients in whom it is clinically suspected.6,7 Once a biochemical diagnosis has been made, imaging studies must be performed to locate the catecholamine-producing tumour.8 To this end, 123I-metaiodobenzylguanidine (MIBG) scintigraphy, 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) and functional tests for somatostatin receptors (SSTRs) such as 68Ga-DOTA(0)-Tyr(3)-octreotate are useful for detecting lesions not detected by conventional imaging modalities1 (Fig. 1). In addition, functional tests should be considered prior to treatment decision-making in patients with malignant PPGLs and patients at increased risk of metastatic disease due to a large primary tumour, an extra-adrenal tumour, multifocality or recurrent disease.1,7 The type of nuclear medicine test must be suited to the tumour's characteristics, including location, size and catecholamine secretion profile, as well as the patient's particular genetic tests, since the sensitivity and specificity of the various tests vary widely depending on the context.2,9 The patient's genetic profile is of growing importance in guiding the selection of nuclear medicine tests for both diagnostic and therapeutic purposes.10,11 Each molecular imaging modality is based on unique cellular uptake mechanisms that depend on the tumour's molecular behaviour, which for its part is determined by the tumour's genetic profile.12 Functional tests also make it possible to plan targeted treatment with radionuclides with MIBG labelled with iodine-131 (131I-MIBG) or peptide receptor radionuclide therapy (PRRT) with DOTA-TATE labelled with 177Lu or other related agents; these treatments have shown promising results and limited toxicity in patients with metastatic disease.13–19
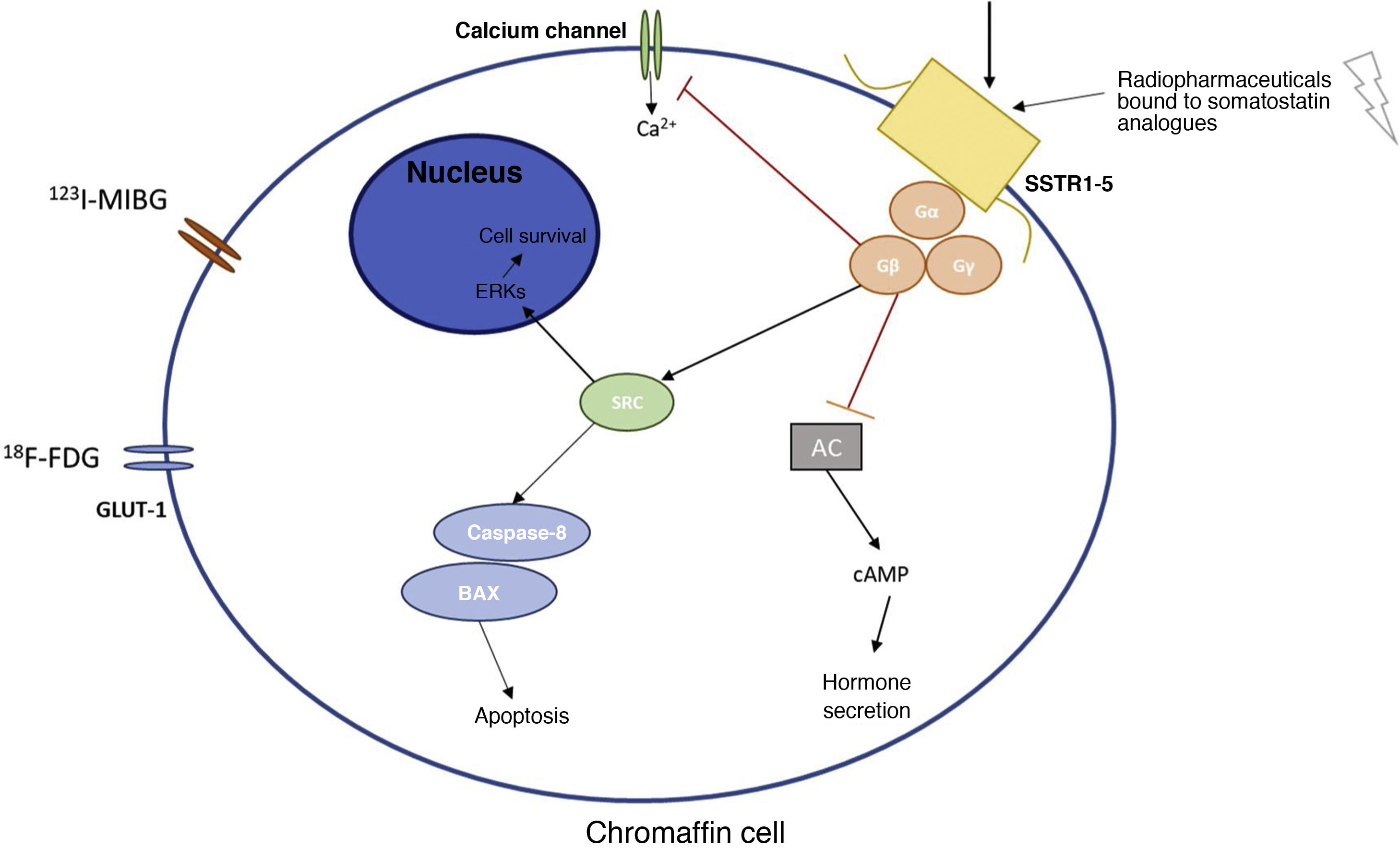
Main receptors and metabolic pathways of the chromaffin cell where the various radionuclides act.
Figure 1 explains the basic molecular mechanisms of the chromaffin cell and the main receptors used in diagnosis and treatment with radionuclides of PPGLs.
123I-MIBG: metaiodobenzylguanidine; 18F-FDG: fluorodeoxyglucose; AC: adenylate cyclase; cAMP: cyclic adenosine monophosphate; ERKs: extracellular signal-regulated kinases; GLUT-1: glucose transporter 1; SRC: proto-oncogene tyrosine-protein kinase; SSTR1-5: somatostatin receptor types 1–5.
This review details the main characteristics of the radiopharmaceuticals used in the diagnosis of PPGLs and the role of functional tests for initial evaluation, including staging, selection of patients for targeted molecular therapy and planning of radiotherapy, in patients with PPGLs. It also offers a series of recommendations for clinical practice depending on different clinical and genetic scenarios in which PPGLs are seen.
Radiopharmaceuticals used for the diagnostic study of PPGLs123I metaiodobenzylguanidine scintigraphy/123I-MIBGMIBG is an iodinated guanidine analogue that shares a transport pathway across the cell membrane transporter system. In the cytoplasmic compartment, MIBG is stored in neurosecretory granules through vesicular monoamine transporters (VMATs). Furthermore, it is concentrated specifically in catecholamine-secreting tissues and tumours, enabling its specific detection.20 However, the detection capacity of 123I-MIBG scintigraphy may be seen to be altered by interaction with certain drugs such as opioids, tricyclic and other antidepressants, sympathomimetics, antipsychotics and some hypertensive agents21 (Table 1).
Main characteristics of radionuclides used in the functional diagnosis of a PPGL.
| Radiopharmaceutical | Characteristics | Biodistribution | Interactions | Image acquisition | Costs |
|---|---|---|---|---|---|
| 123I-MIBG | -Radioiodinated aralkyl guanidine.-Its structure contains the guanidine group of guanethidine bound to a benzyl group in which iodine has been introduced.-Functional similarity to adrenergic neurons and chromaffin cells of the adrenal medulla | -Rapid initial uptake in the liver (33% of the dose administered); much lower uptake in the lungs (3%), myocardium (0.8%), spleen (0.6%) and salivary glands (0.4%) | -Decreased uptake with administration of antihypertensive drugs (reserpine, labetalol or calcium-channel blockers), sympathomimetic agents (phenylephrine, ephedrine or phenylpropanolamine), cocaine, tricyclic antidepressants (amitriptyline or its derivatives or imipramine or its derivatives) or phenothiazine-Start thyroid blockade 24−48 h before administering iobenguane (123I) and continue for at least three days | -Planar and SPECT images are obtained 24 h after administration. | + |
| 68Ga-DOTA-peptides PET/CT | -These target somatostatin receptor (SSTR) subtype 2, which is the one that is the most commonly overexpressed in PPGLs | -Higher physiological uptake: spleen, followed by the kidneys.-Lower uptake: liver, pituitary gland, thyroid gland and adrenal glands.-High uptake may be seen in the uncinate process of the pancreas | -In patients with somatostatin analogues (SSAs), it is preferable to perform image acquisition on the day before or in the days before SSA administration-Corticosteroids can induce down-regulation of SSTR2s | -Image acquisition 40−90 min after injection.-The start time and duration of image acquisition will be adjusted depending on the equipment used and the characteristics of the patient and the tumour | + + + |
| 18F-FDOPA PET/CT | -An aromatic amino acid analogue that accumulates rapidly in target organs, especially in the striatum of the human brain and in lesions where 18F-FDOPA is converted to 18F-fluorodopamine (18F-FDA). | -Widely distributed activity in all body tissues.-Elimination through the kidneys; 50% is eliminated after 0.7 h and the other 50% is eliminated after 12 h. | -Carbidopa: fluorodopa bioavailability in the brain may be increased-Glucagon: fluorodopa uptake in the pancreas is seen to be affected by glucagon due to its interaction with the function of the pancreatic beta cells.-Haloperidol: increased accumulation of fluorodopa (18F) in the brain.-Reserpine: this can empty the contents of the intraneuronal vesicles and thus impede fluorodopa retention in the brain.-MAOIs: the use thereof can increase fluorodopa uptake in the brain. | -Imaging is performed 20−60 min after injection.-Optional imaging: early acquisition (10−20 min following radiopharmaceutical injection) focused on the abdomen (useful for locating abdominal lesions located near the hepatobiliary tract) | + + + |
| 18F-FDG PET/CT | -A glucose analogue that accumulates in all cells that use glucose as their primary energy source.-It accumulates in tumours with high levels of glucose exchange. After intravenous injection, the pharmacokinetic profile of fludeoxyglucose (18F) in the vascular compartment is biexponential | -Cellular fludeoxyglucose (18F) uptake occurs through a specific tissue transporter system, which is partially insulin-dependent and therefore can be influenced by intake, nutritional conditions and diabetes mellitus if present. | -All medications that modify serum glucose levels (e.g. corticosteroids, valproate, carbamazepine, phenytoin, phenobarbital, and catecholamines) can affect the sensitivity of the examination.-Administration of colony-stimulating factors causes increased fludeoxyglucose (18F) uptake in the bone marrow and spleen for several days. | -Image acquisition typically begins 45−60 min after FDG injection.-As long as sufficient activity remains for a suitable count rate, FDG PET can also be performed up to two to three hours after administration | + + |
PPGLs: paragangliomas and pheochromocytomas; SSTR2: somatostatin receptor type 2.
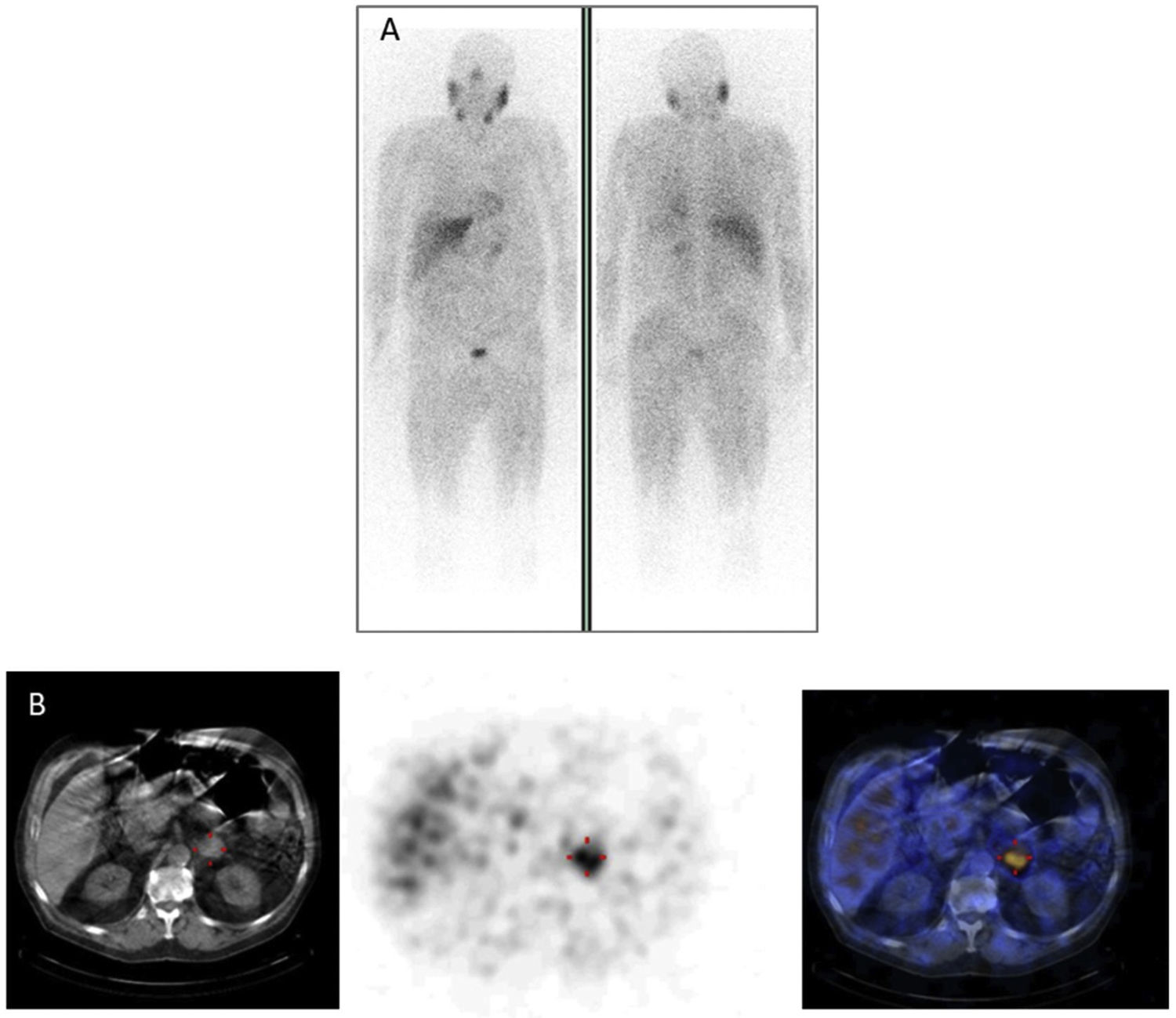
In patients with pheochromocytoma, 123I-MIBG scintigraphy sensitivity and specificity were estimated at 83%–100% and 95%–100%, respectively22 (Fig. 2). More extensive studies with larger numbers of extra-adrenal, multiple, recurrent and hereditary PGLs found a sensitivity of 52%–75%.23 At present, it is well known and has been repeatedly documented that 123I-MIBG scintigraphy should not be used in patients with PGLs associated with succinate dehydrogenase subunit B (SDHB) germline mutations, since the sensitivity of the test in this context is less than 50%.24 In cases involving head or neck PGLs, the sensitivity of 123I-MIBG scintigraphy is lower (18%–50%) than that of other functional imaging modalities, particularly 68Ga DOTA-somatostatin analogue (SSA)-using ones.25123I-MIBG scintigraphy is very useful for selecting potential candidates for treatment with 131I-MIBG in cases of malignant PPGLs.26,27
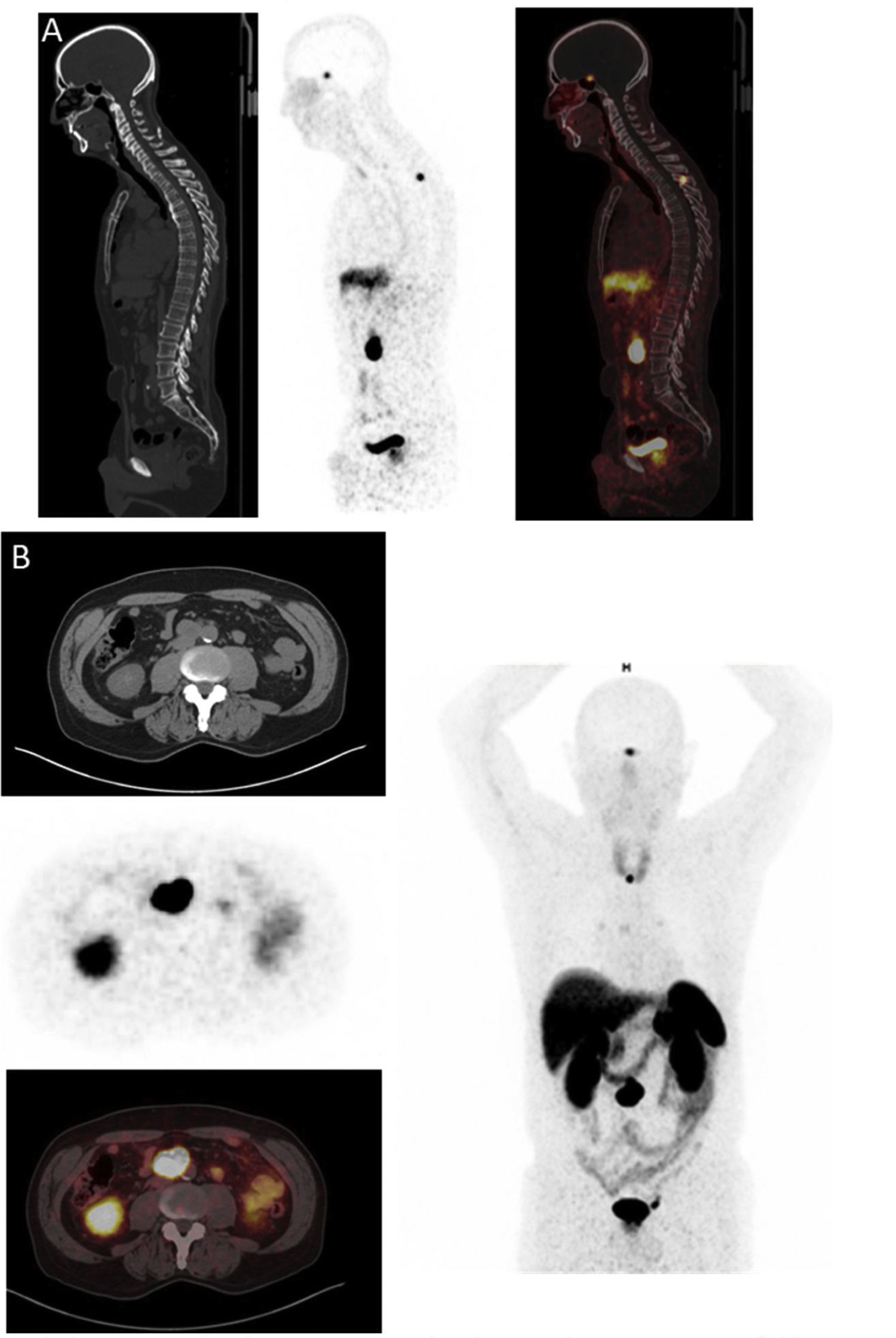
68Ga-DOTA-peptides PET/CT (Fig. 3)SSTR imaging using PET tracers has been performed with three different DOTA-coupled somatostatin agonists (SSTas): DOTA-Tyr3-octreotide (DOTATOC, edotreotide), DOTA-Tyr3-octreotate (DOTA-TATE, oxodotreotide) and DOTA-Nal3-octreotide (DOTANOC). 68Ga-SSTas target SSTR subtype 2 (SSTR2), which is the most commonly overexpressed receptor in PPGLs and is internalised in cells.28 SSTR subtype 1 (SSTR1) is also strongly expressed in some PPGLs, while the other subtypes are slightly expressed or not expressed at all. Low SSTR subtype 5 (SSTR5) expression in PPGLs represents an important difference from some neuroendocrine tumours affecting the gastrointestinal tract.29 A very important element to take into consideration is that treatment with cold SSAs can affect the accumulation of the radiopharmaceutical in different organs and tumour sites; hence, if possible, SSAs must be suspended before the test is performed (Table 1).
In a recent systematic review and meta-analysis, the combined detection rate of 68Ga-DOTA-SSA PET/CT was 93% (95% confidence interval [CI] 91%–95%); this was significantly higher (P < .001 for all) than for fluorodihydroxyphenylalanine (FDOPAlanine) labelled with 18F PET/CT (80%; 95% CI 69%–88%), 18F-FDG PET/CT (74%; 95% CI 46%–91%) and 123I/131I-MIBG scintigraphy (38%; 95% CI, 20%–59%).30 In addition, the usefulness of functional imaging with SSAs has recently been expanded, with the use of labelled SSAs such as PRRT using either lutetium-177 (177Lu) or yttrium-90 (90Y)31 (Fig. 3).
99mTc-Hynic-TOC (Tectrotyd©) and 111In-OctreoScanScintigraphy with somatostatin analogues was first performed in 1987 in patients with SSTR-expressing tumours. At present, there are two radiopharmaceuticals; the most commonly used is 111In-pentetreotide (brand name: OctreoScan®). Pentetreotide labelled with indium (111In) binds specifically to SSTRs. The solution derived from reconstituting pentetreotide and labelling it with indium chloride (111In) is indicated for use as a supplementary technique in the diagnosis and management of gastroenteropancreatic neuroendocrine tumours (GEP-NETs), PPGLs and SSTR-carrying carcinoid tumours.32 Other radiopharmaceuticals have been developed based on the same somatostatin analogue as 111In-pentetreotide. In view of the favourable physical properties of 99mTc, 99mTc-N-α-(6-hydrazinonicotinoyl)-octreotide (99mTc-EDDA/HYNIC-Tyr3-octreotide) was developed; it has become the most commonly used isotope in nuclear medicine. EDDA/HYNIC-TOC labelled with technetium (99mTc) binds with high affinity to SSTR2 and SSTR5, as well as to subtype 3, with a lower affinity. Following radioactive labelling with sodium pertechnetate (99mTc) solution, the 99mTc-EDDA/HYNIC-TOC solution obtained is indicated in determining tumour location in patients with NETs with SSTR expression.
111In-pentetreotide may be useful for diagnosing PPGLs, but it is considered inferior to MIBG in cases of benign PHEOs.33 However, in patients with metastatic PPGLs, the sensitivity of 111In-pentetreotide appears to be higher.34
18F-FDOPA PET/CTPPGLs can absorb and decarboxylate amino acids such as dihydroxyphenylalanine (DOPA). This property depends on aromatic l-amino acid decarboxylase (AADC) activity. DOPA, the precursor to all endogenous catecholamines, undergoes uptake through l-amino acid transporters (LATs), primarily LAT1. 18F-FDOPA is converted to 18F-fluorodopamine (18F-FDA) by AADC and is stored in neurosecretory vesicles. PPGLs rapidly absorb 18F-FDOPA.35 To maximise uptake by the tumour, acquisition of static clinical PET imaging of PPGLs with 18F-FDOPA should start preferably 20 min after injection (Table 1).
A recent meta-analysis that included 11 studies with 275 participating patients with PPGLs showed in an analysis based on lesions of 18F-FDOPA PET/CT that sensitivity and specificity were 79% combined.36 One advantage of 18F-FDOPA PET/CT over 123I-MIBG scintigraphy and other radiopharmaceuticals is its limited uptake by normal adrenal glands. This is very useful in detecting small PPGLs. 18F-FDOPA PET/CT is an excellent first-line imaging tool for head or neck PGLs, with a sensitivity greater than 90%.
With respect to metastatic disease, 18F-FDOPA PET/CT was found to work better for SDHB-negative PGLs than for SDHB-positive PGLs (sensitivity: 93% versus 20%, respectively). 18F-FDOPA PET/CT shows a very high sensitivity for detecting PPGLs associated with mutations in VHL, EPAS1 (HIF2A) or FH; these often are multiple and recurrent and occasionally exhibit quite a high potential for metastasis.37
A number of considerations should be taken into account with respect to interactions when PET is used with 18F-FDOPA. Interactions between PET with 18F-FDOPA and other drugs and other forms of interaction would include the following38:
- •
Fluorodopa bioavailability may be increased by prior treatment with aromatic amino acid decarboxylase (AAAD) inhibitors such as carbidopa (these block peripheral conversion of fluorodopa to fluorodopamine) or with catechol-O-methyltransferase (COMT) inhibitors such as entacapone or nitecapone (these decrease peripheral degradation of fluorodopa to 3-O-methyl-6-fluorodopa).
- •
Glucagon: fluorodopa (18F) uptake in the pancreas is seen to be affected by glucagon due to its interaction with the function of the pancreatic beta cells.
- •
Haloperidol: an increase in intracerebral dopamine caused by haloperidol may increase accumulation of fluorodopa (18F) in the brain.
- •
Reserpine: reserpine can empty the contents of the intraneuronal vesicles and thus impede fluorodopa (18F) retention in the brain.
- •
Monoamine oxidase inhibitors (MAOIs): concomitant use of MAOIs may increase fluorodopa (18F) uptake in the brain.
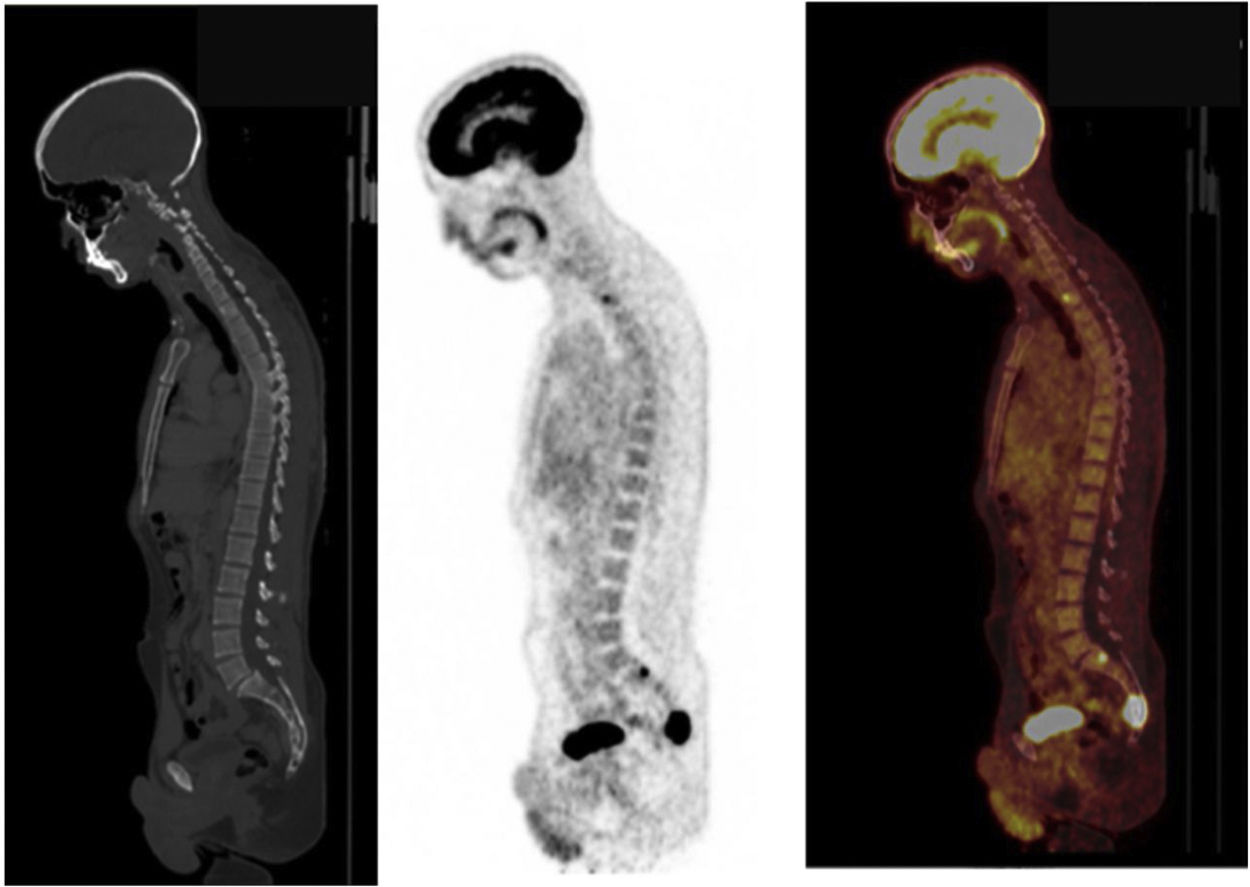
18F-FDG undergoes uptake by tumour cells through glucose membrane transporters and phosphorylation by hexokinase. 18F-FDG-6P does not follow other enzyme pathways and exhibits accumulation in proportion to the rate of cellular glycolysis. PGLs with underlying SDHx mutations exhibit more avid 18F-FDG uptake than other subtypes, primarily due to accumulation of succinate caused by blockage of the Krebs cycle39 (Fig. 4). Sensitivity is high (80%–100%), while specificity is low, as in any other malignancy40 (Table 1).
Similar to uptake of other PGL-specific radiopharmaceuticals, uptake of 18F-FDG during PET/CT is influenced by a genetic component. Although 18F-FDG PET/CT enjoys good acceptance for PHEOs associated with multiple endocrine neoplasia type 2 (MEN2), it only shows positivity in approximately 40% of patients. However, it shows strong diagnostic potential for metastatic PGLs, in particular those associated with mutations in SDHB (sensitivity by lesion: 83% for SDHB-positive tumours versus 62% for SDHB-negative tumours).41
Positron emission tomography combined with magnetic resonance imaging (PET/MRI) in PPGLsThe use of PET/CT with radiopharmaceuticals intended to locate and characterise PPGLs has proven useful in the management of these patients. Since they were introduced in 2010, PET/MRI imaging systems have proven comparable to PET/CT in oncology image acquisition and carry theoretical advantages, such as better soft-tissue contrast and delineation of local tumour extension with reduced exposure to ionising radiation as the CT component is replaced.42
On MRI, PGLs have the particular characteristics of being highly vascularised and having high signal intensity in T2-weighted imaging. MRI is more accurate in delineating the relationship of the head or neck PGL to the surrounding vascular and bone structures, and it is more sensitive than CT for detecting small PHEOs, while CT provides more information on bone destruction by head or neck PGLs.43 Therefore, if radiopharmaceuticals intended to study these patients with the potential advantages of MRI with hybrid equipment, i.e. 68Ga-DOTA-TATE PET/MRI or 18F-FDOPA PET/MRI, are used, this can result in improvements in diagnostic accuracy and delimitation of the primary neoplasm in patients with suspected PGLs, as well as improvements in detection of lymph-node and soft-tissue metastases. Multiparameter studies combined with the use of radiopharmaceuticals in hybrid equipment would enable improvements in the diagnosis of patients with hereditary syndromes and head or neck PGLs, particularly in the paediatric population, given the lower exposure to radiation compared to PET/CT.
64Cu-DOTA-TATE and 18F-flubrobenguane PETThe use of compounds labelled with positron emitters for image acquisition has achieved improvements in spatial resolution and the ability to measure tissue radioactivity. More-than-accredited experience with radiopharmaceuticals such as 68Ga-DOTA-peptides PET/CT and 18F-FDOPA PET/CT has enabled the development of similar radiopharmaceuticals. One of these radiopharmaceuticals, 64Cu-DOTA-TATE, has emerged as a novel PET tracer for SSTR image acquisition. Compared to 111In-DTPA-octreotide, it is superior in terms of both radiation dose and rates of lesion detection when tested directly in 112 patients.44 The lower energy range of 64Cu (17% beta (β)+; maximum positron energy 0.653 MeV) versus 68Ga (88% β+; maximum positron energy 1.899 MeV) theoretically leads to better special resolution, and 64Cu-DOTA-TATE's physical half-life of 12.7 h renders it appealing for routine use in clinical imaging in these patients.
The difference in the rate of lesion detection reported is related to the fact that, in the use of 64Cu, the range of positrons is substantially shorter and is linked to better detection of small lesions. However, the radiation load is higher for 64Cu-DOTA-TATE than for 68Ga-DOTATOC, primarily due to differences in the positron branching fraction. The positron branching fraction is 0.17 for 64Cu-DOTA-TATE and 0.89 for 68Ga-DOTATOC, meaning that a higher dose of 64Cu-DOTA-TATE must be injected to yield the same number of counts as for 68Ga-DOTATOC.45,46
The use of 18F-flubrobenguane was developed more recently and could shift the diagnostic paradigm in suspected PPGLs due to its similarity to MIBG plus the general advantages of PET such as image resolution and quality.33
Indications for molecular imaging studiesThe main indications for functional imaging are9:
Initial stagingCertain factors are significantly associated with metastatic disease; therefore, full-body functional imaging is preferable for all patients who meet any of the following criteria: SDHB, large tumours (>5 cm), extra-adrenal location or noradrenergic biochemical phenotype. In addition, a full preoperative functional study should also be considered in patients with high methoxytyramine levels.7,9,47
Head or neck PGLs are often multifocal and occasionally aggressive; therefore, in addition to functional tests, a detailed anatomical evaluation is needed that includes correlation with arterial and venous phases on CT or MRI and, for hybrid imaging techniques (single-photon emission computed tomography [SPECT]-CT or PET/CT), a detailed selective study of the area to be examined.9
Selection of patients for targeted molecular therapyAt present, success has been had with PRRT with DOTA-Tyr3-octreotate (DOTA-TATE, oxodotreotide) labelled with lutetium-177 (177Lu) or other SSAs labelled with yttrium-90 (90Y) in patients with inoperable/metastatic GEP-NETs and this has provided a great impetus towards their use for inoperable/metastatic PPGLs. There is extensive experience for the treatment of neuroendocrine tumours that accumulate MIBG inside.26,48
Nuclear imaging (PET and SPECT) provide very important information for planning targeted therapy with radionuclides with MIBG labelled with iodine-131 (131I) or PRRT with DOTA-TATE labelled with 177Lu or other related agents.49 In addition to confirming lesion uptake, it aids in personalised dosimetric evaluation of organs at risk and tumour targets.
Radiotherapy planningIncorporation of multimodal imaging into radiation therapy planning has increased accuracy in radiation administration. Molecular imaging can supplement morphological studies in difficult situations, particularly in evaluation of venous spread of large jugular PGLs and tumour recurrence in the surgical bed. It enables a more accurate determination of biological target volumes, a potential decrease in the likelihood of complications in surrounding normal tissue and the administration of a very high biologically effective dose to the tumour.49
Various radiopharmaceuticals can be used to manage PPGLs, with different radiochemical characteristics that render them suitable for every situation. The first thing to do is to distinguish radiopharmaceuticals and protocols for SPECT/CT versus PET/CT.50
Both 123I-MIBG scintigraphy and 111In-pentetreotide scintigraphy are well-established modalities for staging and restaging as well as monitoring of PPGLs. SPECT-CT is a more widely available modality and carries the advantage of sequential acquisition of morphological and functional data, thus increasing diagnostic capacity in terms of image interpretation and disease location while improving sensitivity. These techniques have some limitations, since they involve prolonged imaging, relatively long uptake times prior to imaging and some gastrointestinal tract artefacts, as well as thyroid blockade or temporary suspension of certain drugs that may interfere with suitable imaging interpretation. Low resolution in conventional SPECT imaging may also limit detection of small lesions.51
The use of PET/CT imaging has increased as it has some technical and clinical advantages over SPECT. At present, more accessible radiopharmaceuticals are available, which is why they play an important role in the evaluation of these tumours.52
Long-term follow-up with functional testsIn high-risk patients (young patients and patients with genetic disease, large tumours and/or PGLs), lifelong yearly follow-up should be offered.53 Currently, the best molecular imaging method for detecting metastatic PPGLs consists of using 68Ga-DOTA-SSA PET/CT, due to its high sensitivity.11,54,5518F-FDG and 18F-FDOPA are generally considered second-line and third-line options, respectively. The use of the combination plus a molecular test such as 68Ga-DOTA-TATE or 18F-FDG PET/CT can be considered in select cases, in patients with small lesions when there is a high likelihood of metastatic disease and in patients with SDHx.56 However, in asymptomatic SDHx carriers a study with molecular imaging tests is not recommended during childhood.57
Practical recommendations for the use of functional imaging in PPGLsAccurate identification of clinical context and genetic status of the patient enables personalised use of functional imaging modalities. Table 2 describes the recommendations of the European Association of Nuclear Medicine with respect to the use of imaging tests with radionuclides to study PPGLs.9
Proposed clinical algorithm for the use of functional imaging in cases of PPGLs according to the European Association of Nuclear Medicine.9
| 1st-line choice | 2nd-line choice | 3rd-line choice** | |
|---|---|---|---|
| Sporadic PHEO | 18F-FDOPA or 123I-MIBG | 68Ga-SSA | 18F-FDG |
| Head or neck PGLs | 68Ga-SSA | 18F-FDOPA | 111In-SSA/99mTc-SSA |
| Hereditary PHEO (except SDHx): NF1/RET/VHL/MAX | 18F-FDOPA | 123I-MIBG or 68Ga-SSA | 18F-FDG |
| Multifocal and/or metastatic extra-adrenal involvement and/or SDHx | 68Ga-SSA | 18F-FDG if SDHB or 18F-FDOPA if no SHDB | 18F-FDG and 123I-MIBG or 18F-FDG and 111In-SSA/99mTc-SSA |
PGL: paraganglioma; SDHx: succinate dehydrogenase.
These recommendations refer to those indicated by the European Association of Nuclear Medicine.
123I-MIBG scintigraphy or 18F-FDOPA PET/CT seem to be suitable for confirming the diagnosis of sporadic PHEOs, including non-functioning PHEOs, which rarely occur. Compared to 123I-MIBG scintigraphy, image acquisition by means of 18F-FDOPA positron emission tomography/computed tomography (PET/CT) entails fewer practical limitations and no drug interactions that might limit detection of pheochromocytomas.22
Head or neck PGLs18F-FDOPA and 68Ga-DOTA-SSA seem to be the most sensitive radiopharmaceuticals for PET image acquisition in sporadic cases. In patients with tumours associated with SDHx, 68Ga-DOTA-SSA PET/CT can detect very small lesions that may not be detected by 18F-FDOPA PET/CT.
If 68Ga-DOTA-SSA PET/CT is not available, then SSTR scintigraphy (Tektotryd/OctreoScan) can be used as an alternative, considering the limitations associated with the spatial resolution of SPECT. 18F-FDG PET shows high sensitivity in the context of these patients related to SDHx (especially SDHB) and can supplement 18F-FDOPA PET/CT for the detection of thoracic/abdominal PGLs.54
Retroperitoneal, extra-adrenal and non-metastatic PGLsFunctional imaging enables PGLs to be distinguished from neurogenic tumours, lymph node diseases and mesenchymal tumours. Therefore, the specificity of functional imaging represents a major contribution. Once a diagnosis of PGL has been made, the multiplicity of extra-adrenal locations must be considered. In this context, 18F-FDOPA and 68Ga-DOTA-SSA are more specific than 18F-FDG and are able to identify more lesions. Therefore, 68Ga-DOTA-SSA PET/CT is probably the preferred imaging modality at present, in particular for patients with SDHx mutations.58 FDG can provide genotype information closely linked to tumour behaviour (SDHB).22
Metastatic PPGLs18F-FDOPA shows very good outcomes in terms of detection of metastatic lesions in patients with sporadic PPGLs. However, its sensitivity decreases in the presence of SDHx mutations. 68Ga-DOTA-SSA has demonstrated better results compared to 18F-FDOPA, regardless of genetic history; 123I MIBG can lead to significant underestimation of metastatic disease and thus unsuitable treatment. Nevertheless, 123I-MIBG is a theragnostic radiopharmaceutical, and 123I-MIBG imaging can be used to determine whether a patient is a good candidate for 131I-MIBG therapy.
Role of metabolic therapy in PPGLsMetabolic therapy would be indicated in patients with metastatic PPGLs. This systemic treatment with radionuclides uses beta radiation-emitting isotopes coupled to MIBG or to SSAs.
Metabolic therapy with I131-MIBGThe diagnostic and therapeutic value of MIBG is based on its structural similarity to norepinephrine and a high affinity for and absorption in chromaffin cells. Radioactive iodine (I131) binds to the MIBG molecule to produce iobenguane I-131, which acts as a semi-selective agent for malignant PPGLs. This treatment can be considered for tumours that exhibit MIBG uptake on I123 scintigraphy, although it only works for approximately 60% of these cases.13–15 Similarly, a lower percentage of dopamine-secreting PPGLs exhibit iobenguane I-123 uptake.59–61
In patients with metastatic disease whose tumours show catecholamine secretion and MIBG uptake, the therapeutic value of 131I-iobenguane to relieve symptoms and achieve tumour regression or stabilisation has been demonstrated in large numbers of case series15,62–71 (Table 3). It should be noted that better objective responses are achieved in patients with limited disease and in patients with soft-tissue rather than bone metastases.64 On the other hand, it must be taken into account that external beam radiation therapy nullifies these tumours' ability to absorb MIBG, rendering treatment with 131I-iobenguane ineffective at any irradiated site.62
Efficacy of treatment with 131I-MIBG and 177Lu-DOTA-TATE and 90Y-[177Lu]Lu-DOTA-TATE PRRT in PPGLs reported in studies including more than 20 patients.
| Author, year | Radionuclide | Number of patients | Efficacy (Response Evaluation Criteria in Solid Tumours [RECIST]) | Biochemical response |
|---|---|---|---|---|
| Severi S, 2021 (phase 2 CT)84 | 90Y-DOTATOC or 177Lu-DOTA-TATE | 46 | PR 8.7%, SD 71.7% | NR |
| Thorpe MP, 2020 (single-centre retrospective study)85 | 131I-MIBG | 125 | CR 1%, PR 33%, SD 53% | 59% |
| Zandee WT, 2019 (single-centre retrospective study)82 | 177Lu-DOTA-TATE | 30 | PR 23%, SD 67% | NR |
| Noto BR, 2018 (phase 1 clinical trial)15 | 131I-MIBG | 21 | CR 0%, PR 19%, SD 61.9% | 40% |
| Rutherford MA, 2014 (single-centre retrospective study)86 | 131I-MIBG | 22 | CR 5%, PR 14%, SD 59% | 20% |
| Van Hulsteijn, 2014 (meta-analysis)87 | 131I-MIBG | 243 | CR 11%, PR 27%, SD 52% | 51% |
| Fitzgerald PA, 2006 (phase 2 clinical trial)62 | 131I-MIBG | 30 | CR 13%, PR 50%, SD 1% | NR |
| Forrer F, 2008 (phase 1 clinical trial)19 | 90Y-DOTATOC or 177Lu-DOTA-TATE | 28 | PR 25%, SD 46% | NR |
| Loh KC, 1997 (multicentre retrospective study)64 | 131I-MIBG | 116 | CR 4%, PR 26%, SD 57% | 45% |
CR: complete response; CT: clinical trial; NR: not reported; PR: partial response; SD: stable disease.
Treatment with 131I-iobenguane can be repeated, normally at six-month intervals.65 Optimal dosimetry has not been established; the doses used have varied across prior studies.48,62,64–66,68–70 In general, patients present good tolerance to treatment; the main side effects are thrombocytopenia and transient mild leukopenia. Patients should be warned about the potential long-term risks of myelosuppression68,72,73 and possible increased risks of myelodysplasia and acute leukaemia,62,73 although it is not clear whether these risks are limited to those who receive high-dose therapy. On the other hand, hypothyroidism was observed in 3 out of 28 patients who received cumulative doses of 111–916 millicuries (mCi) in a series,69 and in 2 out of 10 patients in a second study with an average cumulative dose of 310 mCi.71 To prevent 131I-iobenguane uptake by the thyroid gland, thyroid blockade with potassium iodide should be started 24 h prior to administration of iobenguane I-131 and continued for at least five days. There is evidence that high-dose regimens may result in sustained complete response in a small number of patients, but carry an increased risk of serious toxicity.67,74
In conclusion, treatment with 131I-iobenguane should be considered in patients with suitable uptake on imaging tests with 123I-MIBG with an unresectable progressive PPGL, with symptoms of a disease not suited to locoregional management methods, or with a high tumour burden and a small number of bone metastases. However, for patients with rapidly progressing tumours or extensive disease with a bone predominance, chemotherapy is generally a preferable treatment option even if 123I-MIBG scintigraphy is positive.75
Peptide receptor radionuclide therapy (PRRT)PPGLs express SSTRs at levels similar to those of other neuroendocrine tumours, including gastroenteropancreatic neuroendocrine tumours.29,76–78 Therefore, patients whose metastatic PPGLs express SSTRs (as determined by uptake on scintigraphy with 111In-pentetreotide or, when available, PET with SSAs labelled with gallium-68 such as Ga-68-DOTA-TATE79–81), may benefit from treatment with radiolabelled SSAs such as 177Lu-DOTA-TATE.
The most commonly used radionuclides are DOTA-Tyr3-octreotate (DOTA-TATE, oxodotreotide) labelled with lutetium-177 (177Lu-DOTA-TATE) or other SSAs labelled with yttrium-90 (90Y-DOTATOC). The possible long-term side effects of treatment with radiolabelled SSAs may include a decline in kidney function, pancytopenia and myelodysplastic syndrome/acute leukaemia.72 The efficacy of [177Lu]Lu-DOTA-TATE and [90Y]Y-DOTA-TOC for malignant PPGLs has been reported in studies of isolated cases and small series16–19 (Table 3). A series of 28 patients with surgically unresectable progressive PPGLs received [90Y]Y-DOTA-TOC alone or sequentially with [177Lu]Lu-DOTA-TATE.19 Two cases of partial remission, five cases of minor response and 13 cases of stable disease were seen (disease control rate 71%). In a mean follow-up of 19 months, 10 of the 20 patients with objective response or stable disease continued to show no progression, and there were just two cases of mild haematological toxicity. In another series of 30 patients with inoperable or malignant PPGLs (17 with parasympathetic PGLs, 10 with sympathetic PGLs and three with PHEOs) were treated with up to four cycles of [177Lu]Lu-DOTA-TATE with an anticipated dose of 7.4 Gb per cycle.82 A partial response was seen in seven patients (23%) and stable disease was seen in 20 patients (67%), while three patients (10%) showed progressive disease. Mean progression-free survival was 91 months in patients with parasympathetic PGLs, 13 months in patients with sympathetic PGLs and 10 months in patients with metastatic PHEOs. Grade 3 or 4 subacute haematological toxicity occurred in six patients (20%). Two patients experienced a reversible subacute adverse event due to heart failure after a possible release of catecholamines.82 Finally, a recently reported series of 15 patients with metastatic or unresectable PPGLs who received PRRT with [177Lu]Lu-DOTA-TATE, with a median duration of follow-up of 27 months as of the start of PRRT, found progressive disease in three patients (20%), stable disease in eight patients (53%), a partial response in one patient (7%), a minor response in three patients (20%) and controlled disease in 12 patients (80%).83 Promising safety profiles with no long-term nephrotoxicity or haematological toxicity were seen. Therefore, treatment with [177Lu]Lu-DOTA-TATE is a safe, effective treatment modality for patients with metastatic/inoperable PPGLs. According to the recently published results of a phase 2 clinical trial, of the 56 patients included for treatment with [177Lu]Lu-DOTA-TATE or [90Y]DOTA-TATE, the tumour disease control rate was 80% (95% CI: 68.9%–91.9%).84
[177Lu]Lu-DOTA-TATE received approval from the United States Food and Drug Administration (FDA) in January 2018 to treat advanced gastroenteropancreatic neuroendocrine tumours that express SSTRs. However, this approval was not extended to PPGLs; such use remains investigational and should only be considered in somatostatin receptor-expressing tumours.
ConclusionFunctional imaging tests in nuclear medicine play an important role in the diagnosis, staging, treatment planning and management of PPGLs. The selection of the most suitable diagnostic modality is determined by the clinical and genetic context and the characteristics of the tumour (size and location). Treatment with I131-MIBG or [177Lu]Lu-DOTA-TATE may be useful in patients who have metastatic disease not suited to locoregional treatment and who show uptake on functional tests with MIBG in the former case or somatostatin receptor expression in the latter case.
FundingThis study did not receive funding of any type.
Conflicts of interestThe authors declare that they have no conflicts of interest.