Increasing number of experimental and clinical studies suggest a strong relationship between hyperglycemia, oxidative stress, DNA damage and diabetic nephropathy (DN). Also, epidemiologic studies remark an enhanced risk of cancer with type 2 diabetes. This research aims to assess whether the X-ray cross complementing group 3 (XRCC3) gene T241M polymorphism (rs861539) and X-ray cross complementing group 1 (XRCC1) gene A399G polymorphism (rs25487) are related with predisposition to type 2 diabetes mellitus (T2DM) and to diabetic nephropathy in Turkish population.
Materials and methodsPolymerase chain reaction-based restriction fragment length polymorphism (PCR-RFLP) was performed to identify the distribution of genotypes and frequency of alleles of T241M polymorphism of the XRCC3 gene (XRCC3 T241M) and A399G polymorphism of the XRCC1 gene (XRCC1 A399G). The study population included 238 subjects residing in Istanbul, Turkey; 116 with T2DM, 50 with DN and 72 with normal glucose metabolism.
Results and conclusionPolymorphic Gln allele of XRCC1 gene was significantly related with T2DM and DN (OR 3.09, 95% CI 1.14–8.40 and OR 3.29 95% CI 1.23–8.80, respectively) however, there was no statistical association of XRCC3 T241M with T2DM or DN. The results of this study suggest that XRCC1 399Gln polymorphism is related with an increased susceptibility to T2DM and DN in the studied Turkish population.
Un número creciente de estudios experimentales y clínicos sugiere una sólida relación entre hiperglucemia, estrés oxidativo, daño en el ADN y nefropatía diabética (ND). Además, los estudios epidemiológicos advierten mayor riesgo de cáncer con diabetes de tipo 2. Esta investigación tiene como objetivo evaluar si el polimorfismo del gen T241M del grupo 3 (XRCC3) complementario cruzado de rayos X (rs861539) y el polimorfismo del gen A399G del grupo 1 (XRCC1) complementario cruzado de rayos X (rs25487) están relacionados con la predisposición a la diabetes mellitus de tipo 2 (DM2) y a la nefropatía diabética en la población turca.
Materiales y métodosSe realizó un polimorfismo de longitud de fragmento de restricción basado en la reacción en cadena de la polimerasa (PCR-RFLP) para identificar la distribución de genotipos y la frecuencia de los alelos del polimorfismo T241M del gen XRCC3 (XRCC3 T241M) y el polimorfismo A399G del gen XRCC1(XRCC1 A399G). La población de estudio incluyó a 238 individuos que residían en Estambul, Turquía; 116 de ellos con DM2, 50 con ND y 72 con metabolismo de la glucosa normal.
Resultados y conclusiónEl alelo Gln polimórfico del gen XRCC1 se relacionó de manera importante con DM2 y ND (OR: 3,09; IC95%: 1,14-8,40 y OR: 3,29; IC95%:1,23-8,80, respectivamente). Sin embargo, no hubo asociación estadística de XRCC3 T241M con DM2 o ND. Los resultados de este estudio sugieren que el polimorfismo XRCC1 399Gln está relacionado con un aumento de la susceptibilidad a la DM2 y a la ND en la población turca estudiada.
Diabetes mellitus (DM) is a metabolic disease which occurs by deficiency in insulin secretion and/or insulin action that leads to inefficiency in metabolism of carbohydrate, lipid and protein.1 Diabetes mellitus is recognized as a prevalent worldwide disorder with growing incidence. According to the World Health Organization (WHO) data, the number of deaths caused by diabetes was 1.5 million in 2012 and currently 422 million people have diabetes mellitus in the world and the number of patients with diabetes is expected to more than double in the next 20 years.2 According to data from the International Diabetes Federation, 12.8% of the adult population of Turkey suffer from diabetes and there were over 6.7 million cases in 2017.3
Type 2 diabetes mellitus (T2DM) usually causes both microvascular and macrovascular complications. Diabetic nephropathy (DN) is a late and one of the major chronic complication of T2DM and finally progresses to end-stage renal disease (ESRD) with multifactorial pathogenesis and the mechanism has not been fully understood.4,5 Increasing number of studies suggest that there is a link between hyperglycemia, oxidative stress, DNA damage and diabetic complications.6–9
In recent years; epidemiologic studies remark an association between many type of cancers (such as colorectal, liver, bladder, breast, pancreas and renal cancer) and T2DM.10–15 Increasing risk of cancer among diabetic patients may be linked with enhanced DNA damage and reduced DNA repair capacity. Also, DNA repair gene polymorphisms may modulate DNA repair capacity and affect predisposition to T2DM and its vascular complications.
The X-ray repair cross-complementing group 3 (XRCC3) is a RAD51 family gene and encodes a protein that is participated in the homologous recombination repair of DNA double-strand breaks (DSB) to maintain chromosomal stability located on human chromosomes 14, at 14q32.3.16,17 T241M (rs861539), the most commonly investigated polymorphism of XRCC3 gene consists of a C/T transition leading to an amino acid substitution from Thr to Met at codon 241 and this substitution may result in DNA repair capacity change.18 On the other hand, the other investigated gene X-ray repair cross-complementing group 1 (XRCC1) is located on chromosome 19, at 19q13.2 and encodes the XRCC1 protein which is a crucial enzyme that plays a series of coordinating role in stages of the base excision repair (BER) system due to its interactions with several proteins. Fifty polymorphic regions have been identified for XRCC1 gene and the most frequently studied genetic variant is Arg399Gln that consists of a G/A transition leading to an amino acid substitution from Arg to Gln at codon 399.19,20
XRCC3 T241M and XRCC1 A399G have been studied as risk factors for various cancer development in different populations.21–25 However, studies addressing the association of these polymorphisms with susceptibility to T2DM and the diabetic complications have been relatively rare. Therefore, aim of this study was to assess whether the T241M polymorphic variant of XRCC3 gene and A399G polymorphic variant of XRCC1 gene are associated with susceptibility to T2DM and DN in Turkish population.
Materials and methodsStudy populationThe study population consisted of total 238 subjects from Istanbul/Turkey (age ranged between 23 and 83 years), including 116 patients with T2DM (77 females and 39 males), 50 patients with DN (15 females and 35 males) and 72 healthy volunteer with normal glucose metabolism (51 females and 21 males). Newly diagnosed T2DM or DN patients were not included in the study for accuracy's sake. Therefore, patients had been under therapy at least 1 year during the sample collection as indicated in Table 1. Diagnosis of diabetes was made according to the American Diabetes Association26 criteria (fasting glucose ≥126mg/dl; HbA1C ≥6.5%) in Diabetology Outpatient Department of Haydarpasa Numune Training and Research Hospital. Blood samples from DN patients were collected during their routine control in the clinic by checking their at least two previous 24h urinary albumin excretion (UAE) records and estimated glomerular filtration rate (eGFR mL/min) within 6 months. Patients with UAE<30mg/24h, 30–299mg/24h and ≥300mg/24h have been classified normoalbuminuria, microalbuminuria and macroalbuminuria, respectively.27 According to the latest records, patients, who had microalbuminuria (30.6–295.2mg/24h; mean 137.55mg/24h; n=23), macroalbuminuria (309.96–5876mg/24h; mean 1442.07mg/24h; n=16 (4 of 16 was also dialysis patients)) and normoalbuminuria with eGFR<60mL/min/1.73m2 1.77–27mg/24h; mean 13.29mg/24h; n=11) were diagnosed with diabetic nephropathy. eGFR mL/min of DN patients were calculated using the Cockroft–Gault equation via current serum creatinine levels (creatinine clearance (ml/min)=[(140−age (years)]×weight (kg)/[72×serum creatinine (mg/dl)×(0.85 if female)]; https://www.kidney.org/professionals/KDOQI/gfr_calculatorCoc) and DN was classified into stages (Table 1). The Research protocol was approved by The Ethical Committee of Marmara University Institute of Health Sciences in accordance with the Declaration of Helsinki (ID number: 02.05.2014-7) and written informed consent was obtained from each subject before collecting blood samples. Also, participants answered the standardized health questionnaires relating to family history of T2DM, accompanying diseases, behavioral and lifestyle factors such as smoking, antioxidant usage, etc. as shown in Table 2.
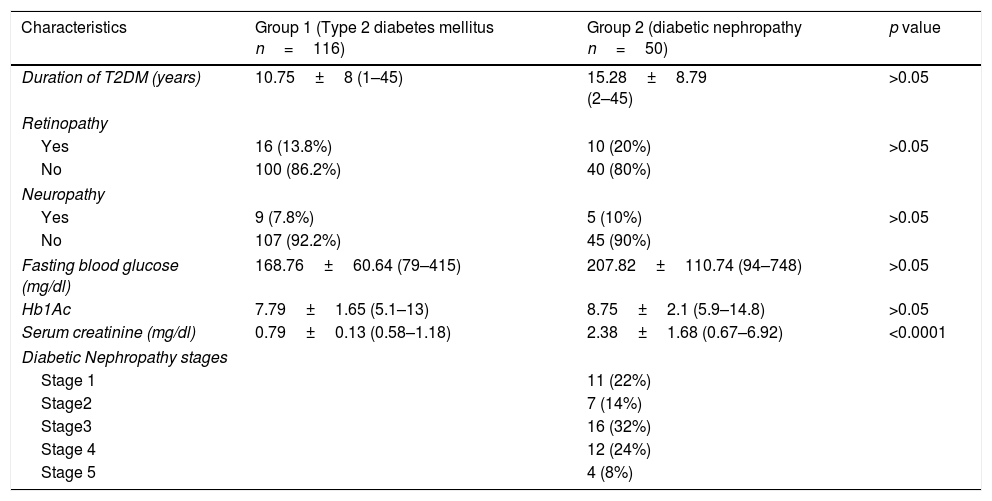
Clinical characteristics of diabetic subjects.
| Characteristics | Group 1 (Type 2 diabetes mellitus n=116) | Group 2 (diabetic nephropathy n=50) | p value |
|---|---|---|---|
| Duration of T2DM (years) | 10.75±8 (1–45) | 15.28±8.79 (2–45) | >0.05 |
| Retinopathy | |||
| Yes | 16 (13.8%) | 10 (20%) | >0.05 |
| No | 100 (86.2%) | 40 (80%) | |
| Neuropathy | |||
| Yes | 9 (7.8%) | 5 (10%) | >0.05 |
| No | 107 (92.2%) | 45 (90%) | |
| Fasting blood glucose (mg/dl) | 168.76±60.64 (79–415) | 207.82±110.74 (94–748) | >0.05 |
| Hb1Ac | 7.79±1.65 (5.1–13) | 8.75±2.1 (5.9–14.8) | >0.05 |
| Serum creatinine (mg/dl) | 0.79±0.13 (0.58–1.18) | 2.38±1.68 (0.67–6.92) | <0.0001 |
| Diabetic Nephropathy stages | |||
| Stage 1 | 11 (22%) | ||
| Stage2 | 7 (14%) | ||
| Stage3 | 16 (32%) | ||
| Stage 4 | 12 (24%) | ||
| Stage 5 | 4 (8%) | ||
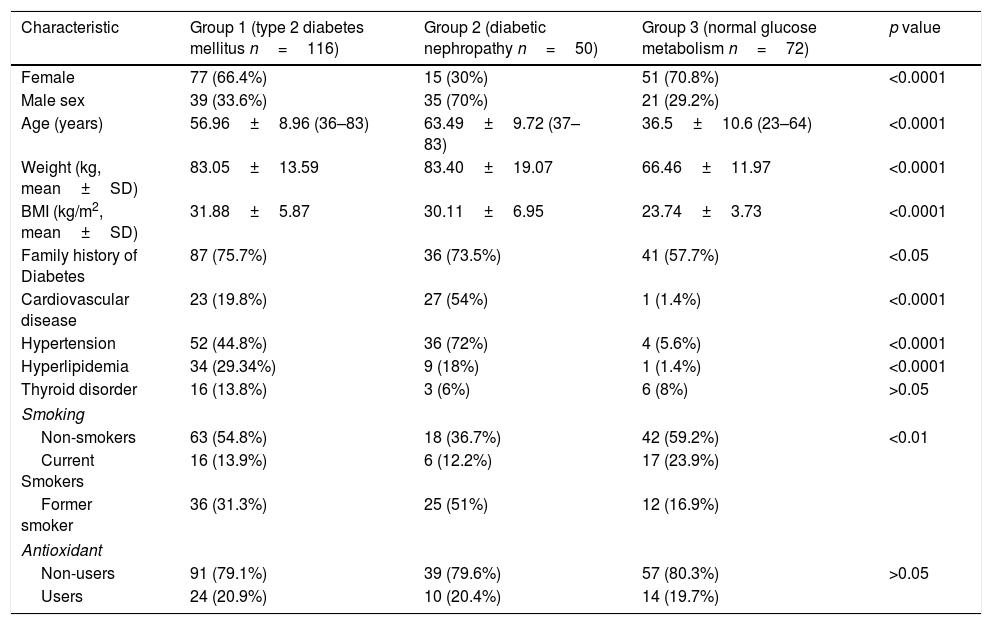
Demographic and clinical characteristics of each group.
| Characteristic | Group 1 (type 2 diabetes mellitus n=116) | Group 2 (diabetic nephropathy n=50) | Group 3 (normal glucose metabolism n=72) | p value |
|---|---|---|---|---|
| Female | 77 (66.4%) | 15 (30%) | 51 (70.8%) | <0.0001 |
| Male sex | 39 (33.6%) | 35 (70%) | 21 (29.2%) | |
| Age (years) | 56.96±8.96 (36–83) | 63.49±9.72 (37–83) | 36.5±10.6 (23–64) | <0.0001 |
| Weight (kg, mean±SD) | 83.05±13.59 | 83.40±19.07 | 66.46±11.97 | <0.0001 |
| BMI (kg/m2, mean±SD) | 31.88±5.87 | 30.11±6.95 | 23.74±3.73 | <0.0001 |
| Family history of Diabetes | 87 (75.7%) | 36 (73.5%) | 41 (57.7%) | <0.05 |
| Cardiovascular disease | 23 (19.8%) | 27 (54%) | 1 (1.4%) | <0.0001 |
| Hypertension | 52 (44.8%) | 36 (72%) | 4 (5.6%) | <0.0001 |
| Hyperlipidemia | 34 (29.34%) | 9 (18%) | 1 (1.4%) | <0.0001 |
| Thyroid disorder | 16 (13.8%) | 3 (6%) | 6 (8%) | >0.05 |
| Smoking | ||||
| Non-smokers | 63 (54.8%) | 18 (36.7%) | 42 (59.2%) | <0.01 |
| Current Smokers | 16 (13.9%) | 6 (12.2%) | 17 (23.9%) | |
| Former smoker | 36 (31.3%) | 25 (51%) | 12 (16.9%) | |
| Antioxidant | ||||
| Non-users | 91 (79.1%) | 39 (79.6%) | 57 (80.3%) | >0.05 |
| Users | 24 (20.9%) | 10 (20.4%) | 14 (19.7%) | |
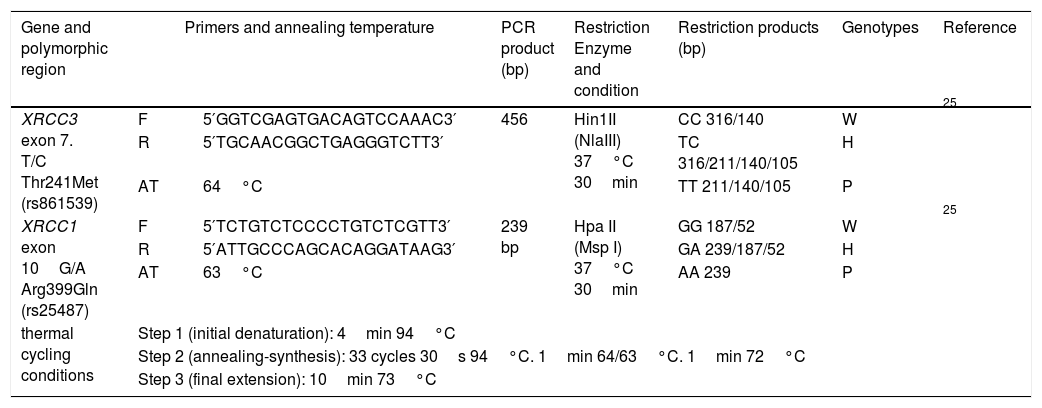
Genomic DNA was extracted from peripheral blood lymphocytes by PureLink® Genomic DNA Mini Kit, Invitrogen, USA. Polymerase chain reaction-based restriction fragment length polymorphism (PCR-RFLP) was performed to identify the distribution of genotypes and frequency of alleles of XRCC3 T241M (rs861539) and XRCC1 A399G (rs25487). The 20μl PCR mix consisted of 10ng genomic DNA, 250μM dNTP mix, 1× PCR buffer, 0.5pmol from each primer, 3.75mM MgCl2, 2.5U Taq DNA polymerase (Thermo Scientific™) and PCR reactions were carried out by thermal cycler (Cleaver Scientific Ltd, model GTC96S). PCR-RFLP procedures are summarized in Table 3 in detail with thermal cycling conditions, the primer sequences,25 annealing temperatures and restriction enzymes.
Summary of PCR-RFLP assay.
| Gene and polymorphic region | Primers and annealing temperature | PCR product (bp) | Restriction Enzyme and condition | Restriction products (bp) | Genotypes | Reference | |
|---|---|---|---|---|---|---|---|
| XRCC3 exon 7. T/C Thr241Met (rs861539) | F | 5′GGTCGAGTGACAGTCCAAAC3′ | 456 | Hin1II (NlaIII) 37°C 30min | CC 316/140 | W | 25 |
| R | 5′TGCAACGGCTGAGGGTCTT3′ | TC 316/211/140/105 | H | ||||
| AT | 64°C | TT 211/140/105 | P | ||||
| XRCC1 exon 10G/A Arg399Gln (rs25487) | F | 5′TCTGTCTCCCCTGTCTCGTT3′ | 239 bp | Hpa II (Msp I) 37°C 30min | GG 187/52 | W | 25 |
| R | 5′ATTGCCCAGCACAGGATAAG3′ | GA 239/187/52 | H | ||||
| AT | 63°C | AA 239 | P | ||||
| thermal cycling conditions | Step 1 (initial denaturation): 4min 94°C | ||||||
| Step 2 (annealing-synthesis): 33 cycles 30s 94°C. 1min 64/63°C. 1min 72°C | |||||||
| Step 3 (final extension): 10min 73°C | |||||||
F: forward primer; R: reverse primer; W: wild genotype; H: heterozygous genotype; P: polymorphic genotype.
Each group was evaluated for Hardy–Weinberg equilibrium (HWE) by Chi-square test. Descriptive statistics are presented via mean±standard deviation for continuous variables and frequencies (%) for categorical variables. The normal distribution of continuous variables was tested by Shapiro–Wilk's test. The mean values were compared by Mann–Whitney U test (between two groups) and Kruskal–Wallis test (between three groups). Pearson Chi-square or Fisher's exact Chi-square test was used for categorical variables. In addition, the association of XRCC3 T241M, XRCC1 A399G and other covariates with T2DM and DN was analyzed by multiple logistic regression (MLR) by computing odds ratio (OR) and their respective 95% confidence interval (CI) after adjusting for potential confounding variables (as described in Tables 4 and 5). XRCC3 T241M, XRCC1 A399G and assumed risk factors were analyzed by multiple logistic regression accepting as T2DM+DN groups as a dependent variable and other factors as independent variables to evaluate presence of T2DM (Table 4) and DN group as a dependent variable to evaluate presence of DN (Table 5). A two tailed p-value of <0.05 was considered statistically significant.
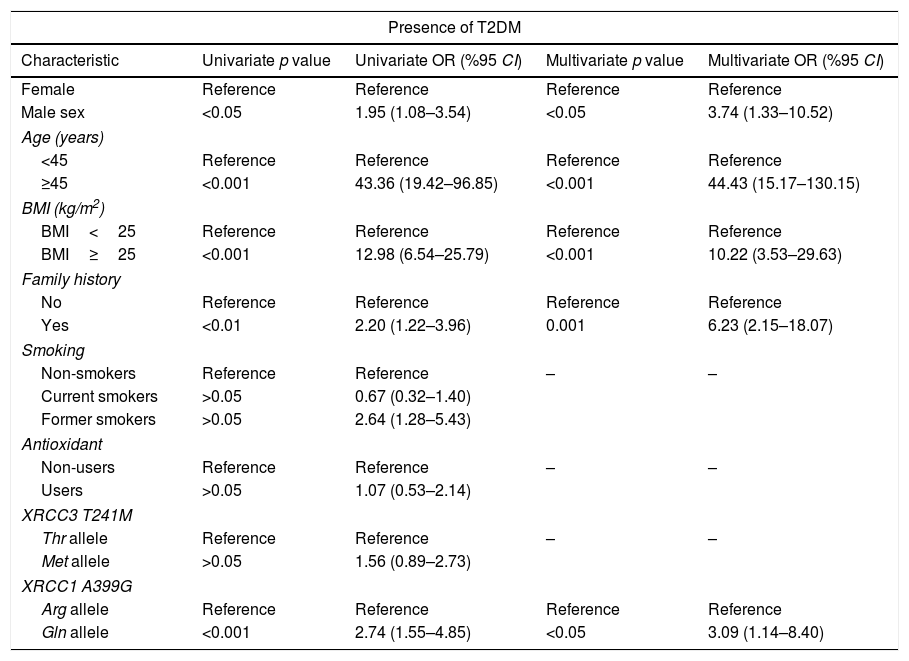
Univariate and multivariate analysis for the association between type 2 diabetes mellitus (T2DM) and XRCC3 T241M polymorphism, XRCC1 A399G polymorphism and other putative risk factors.
| Presence of T2DM | ||||
|---|---|---|---|---|
| Characteristic | Univariate p value | Univariate OR (%95 CI) | Multivariate p value | Multivariate OR (%95 CI) |
| Female | Reference | Reference | Reference | Reference |
| Male sex | <0.05 | 1.95 (1.08–3.54) | <0.05 | 3.74 (1.33–10.52) |
| Age (years) | ||||
| <45 | Reference | Reference | Reference | Reference |
| ≥45 | <0.001 | 43.36 (19.42–96.85) | <0.001 | 44.43 (15.17–130.15) |
| BMI (kg/m2) | ||||
| BMI<25 | Reference | Reference | Reference | Reference |
| BMI≥25 | <0.001 | 12.98 (6.54–25.79) | <0.001 | 10.22 (3.53–29.63) |
| Family history | ||||
| No | Reference | Reference | Reference | Reference |
| Yes | <0.01 | 2.20 (1.22–3.96) | 0.001 | 6.23 (2.15–18.07) |
| Smoking | ||||
| Non-smokers | Reference | Reference | – | – |
| Current smokers | >0.05 | 0.67 (0.32–1.40) | ||
| Former smokers | >0.05 | 2.64 (1.28–5.43) | ||
| Antioxidant | ||||
| Non-users | Reference | Reference | – | – |
| Users | >0.05 | 1.07 (0.53–2.14) | ||
| XRCC3 T241M | ||||
| Thr allele | Reference | Reference | – | – |
| Met allele | >0.05 | 1.56 (0.89–2.73) | ||
| XRCC1 A399G | ||||
| Arg allele | Reference | Reference | Reference | Reference |
| Gln allele | <0.001 | 2.74 (1.55–4.85) | <0.05 | 3.09 (1.14–8.40) |
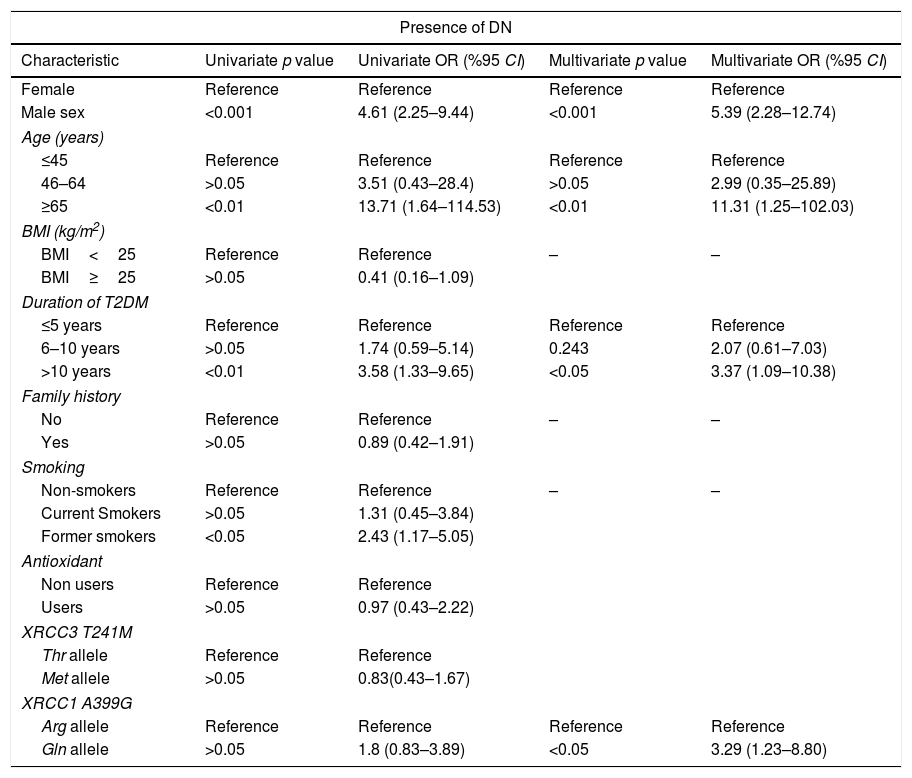
Univariate and multivariate analysis for the association between diabetic nephropathy (DN) and XRCC3 T241M polymorphism, XRCC1 A399G polymorphism and other putative risk factors.
| Presence of DN | ||||
|---|---|---|---|---|
| Characteristic | Univariate p value | Univariate OR (%95 CI) | Multivariate p value | Multivariate OR (%95 CI) |
| Female | Reference | Reference | Reference | Reference |
| Male sex | <0.001 | 4.61 (2.25–9.44) | <0.001 | 5.39 (2.28–12.74) |
| Age (years) | ||||
| ≤45 | Reference | Reference | Reference | Reference |
| 46–64 | >0.05 | 3.51 (0.43–28.4) | >0.05 | 2.99 (0.35–25.89) |
| ≥65 | <0.01 | 13.71 (1.64–114.53) | <0.01 | 11.31 (1.25–102.03) |
| BMI (kg/m2) | ||||
| BMI<25 | Reference | Reference | – | – |
| BMI≥25 | >0.05 | 0.41 (0.16–1.09) | ||
| Duration of T2DM | ||||
| ≤5 years | Reference | Reference | Reference | Reference |
| 6–10 years | >0.05 | 1.74 (0.59–5.14) | 0.243 | 2.07 (0.61–7.03) |
| >10 years | <0.01 | 3.58 (1.33–9.65) | <0.05 | 3.37 (1.09–10.38) |
| Family history | ||||
| No | Reference | Reference | – | – |
| Yes | >0.05 | 0.89 (0.42–1.91) | ||
| Smoking | ||||
| Non-smokers | Reference | Reference | – | – |
| Current Smokers | >0.05 | 1.31 (0.45–3.84) | ||
| Former smokers | <0.05 | 2.43 (1.17–5.05) | ||
| Antioxidant | ||||
| Non users | Reference | Reference | ||
| Users | >0.05 | 0.97 (0.43–2.22) | ||
| XRCC3 T241M | ||||
| Thr allele | Reference | Reference | ||
| Met allele | >0.05 | 0.83(0.43–1.67) | ||
| XRCC1 A399G | ||||
| Arg allele | Reference | Reference | Reference | Reference |
| Gln allele | >0.05 | 1.8 (0.83–3.89) | <0.05 | 3.29 (1.23–8.80) |
The demographic and clinical characteristics (body mass ındex – BMI, family history of T2DM, accompanying diseases, smoking status and antioxidant usage, etc.) of each participant has been questioned in detail and presented in Table 2. Serum creatinine (mg/dl) levels of T2DM patients with DN were significantly higher than T2DM patients without DN as demonstrated in Table 1 with all other clinical characteristics.
Genotype distribution of XRCC3 T241M among T2DM patients were in accordance with HWE, however, DN patients and subjects with normal glucose metabolism deviated significantly from expected HWE. Otherwise, genotype distribution of XRCC1 A399G was not in accordance with HWE among all groups. Allele frequency distributions of genes among groups were evaluated. The percentage of polymorphic Met allele (CT+TT) distribution of XRCC3 gene was observed 52.6% for T2DM, 48% for DN and 40.3% for normal glucose metabolism and there was no statistically significant difference between groups (p>0.05). However, statistically significant difference (p=0.01) between groups was observed for polymorphic Gln allele (GA+AA) distribution of XRCC1 gene (66.4% for T2DM, 78% for DN and 45.8% for normal glucose metabolism). Followed by univariate and multivariate analysis; the association between disease risk and XRCC1 gene allele frequency is shown by odd ratios in Tables 4 and 5.
XRCC3 T241M had no significant impact on T2DM or DN development (OR 1.56, 95%CI 0.89–2.73 and OR 0.83 95% CI 0.43–1.67, respectively). However, polymorphic 399Gln variant of XRCC1 was found to be related with increased T2DM (OR 3.09, 95% CI 1.14–8.40) and DN (OR 3.29, 95% CI 1.23–8.80) risk. According to MLR results, male sex (p<0.05), age ≥45 years (p<0.001), BMI≥25 (p<0.001) and family history (p<0.001) demonstrated a significant association with T2DM. Also, male sex (p<0.001), age ≥65 (p<0.05) and duration of T2DM more than 10 years had a significant association with DN as demonstrated in Tables 4 and 5.
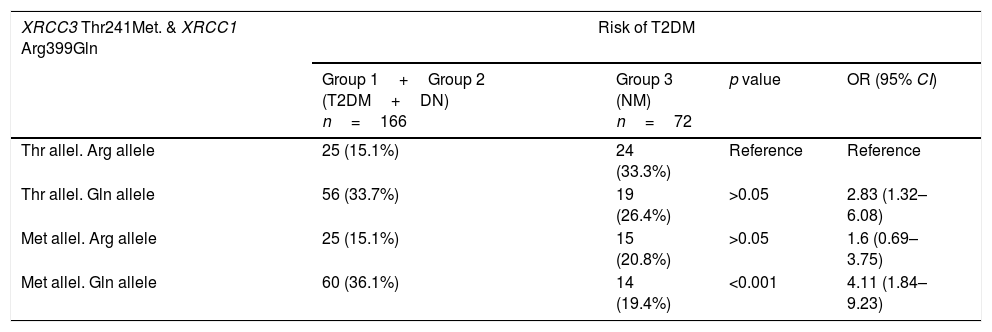
Table 6 shows the combined effects of XRCC1 A399G and XRCC3 T241M in T2DM and DN risk. For this purpose, all diabetic patients (group 1+group 2) were compared with healthy donors (group 3) to evaluate combined effects of genes on T2DM and in addition diabetic patients with DN (group 2) were compared with diabetic patients without DN (group 1) to evaluate combined effects of genes on DN. The wildtype genotype of each gene was taken as reference to analyze the combined effect of XRCC1 A399G and XRCC3 T241M genotypes. Met allele of XRCC3 gene and Gln allele of XRCC1 gene were found to increase T2DM risk synergistically (p<0.001, OR 4.11, 95% CI 1.84–9.23). On the other hand, Thr allele of XRCC3 gene and Gln allele of XRCC1 gene were associated with a joint effect on DN risk (p=0.01, OR 5.11, 95% CI 1.37–19.11) (Table 6).
Combined effect of XRCC3 Thr241Met and XRCC1 Arg399Gln in T2DM and DN risk.
| XRCC3 Thr241Met. & XRCC1 Arg399Gln | Risk of T2DM | |||
|---|---|---|---|---|
| Group 1+Group 2 (T2DM+DN) n=166 | Group 3 (NM) n=72 | p value | OR (95% CI) | |
| Thr allel. Arg allele | 25 (15.1%) | 24 (33.3%) | Reference | Reference |
| Thr allel. Gln allele | 56 (33.7%) | 19 (26.4%) | >0.05 | 2.83 (1.32–6.08) |
| Met allel. Arg allele | 25 (15.1%) | 15 (20.8%) | >0.05 | 1.6 (0.69–3.75) |
| Met allel. Gln allele | 60 (36.1%) | 14 (19.4%) | <0.001 | 4.11 (1.84–9.23) |
| XRCC3 Thr241Met. & XRCC1 Arg399Gln | Risk of DN | |||
|---|---|---|---|---|
| Group 1 (T2DM) n=116 | Group 2 (DN) n=50 | p value | OR (95% CI) | |
| Thr allel. Arg allele | 22 (19%) | 3 (6%) | Reference | Reference |
| Thr allel. Gln allele | 33 (28.4%) | 23 (46%) | 0.01 | 5.11 (1.37–19.11) |
| Met allel. Arg allele | 17 (14.7%) | 8 (16%) | >0.05 | 3.45 (0.79–15.01) |
| Met allel. Gln allele | 44 (37.9%) | 16 (32%) | >0.05 | 2.67 (0.70–10.13) |
DM is one of the most common metabolic diseases, the incidence of DM and its complications is increasing day by day. With regard to future of public health and health economics, it is necessary to develop emerging rationale strategies to manage and prevent the disease.
Reported studies suggest that oxidative stress, DNA damage and inefficient repair may play a crucial role for progression and severity of diabetes and trigger complications.7,9,28–30 In addition, epidemiologic studies remark an increased risk of cancer among patients with T2DM.31 Therefore, this study clarifies susceptibility to T2DM and DN with XRCC3 T241M and XRCC1 A399G. To the best of our knowledge, this is the first study that investigates the association between T241M polymorphism of XRCC3 gene and T2DM and DN in T2DM patients. Our results suggest T241M polymorphic variant of XRCC3 gene is not associated with an increased susceptibility to T2DM or DN in the studied population.
Comparison of allele frequency of XRCC1 gene among groups (1, 2 and 3) showed statistically significant differences. Polymorphic 399 Gln variant of XRCC1 gene seems to increase T2DM and DN risk as evaluated by MLR analysis. Our data are consistent with previous findings of Majsterek et al. in which susceptibility were reported in T2DM and diabetic retinopathy.32 However, in contrast to our results, no association have been reported by Kasznicki et al. between 399Gln polymorphism of XRCC1 gene and T2DM33 and by Narne et al. between 399Gln polymorphism of XRCC1 gene and DN.34 Besides, a number of studies reported relationship between A399G polymorphism of XRCC1 gene and other vascular complications of T2DM, association between 399Gln allele and diabetic retinopathy, coronary artery disease in T2DM and severity of these complications.32,35,36 As DN is a major cause of ESRD, we directed our research to reported studies related with A399G polymorphism in ESRD patients. In 2012, Trabulus et al. studied ESRD risk in Turkish population and reported that 399Gln variant had an association with an increased ESRD risk.19 Same observation have been reported by Radwan et al. in 2015 that 399Gln variant leads to ESRD risk in Egyptian population.37 In addition, DNA BER pathway gene OGG1 polymorphism have been studied by Gönül et al. and a significant association have been reported in Turkish diabetes mellitus patients.37
In this study, Met allele of XRCC3 gene and Gln allele of XRCC1 gene were found to increase T2DM risk synergistically. On the other hand, Thr allele of XRCC3 gene and Gln allele of XRCC1 gene had a joint effect on DN risk.
Both polymorphisms had no statistical significant association with Hb1Ac levels of T2DM patients with/without DN (data not shown). These findings suggest that there is no relationship between hyperglycemic state and polymorphisms of related DNA repair genes.
Several studies have evaluated the expressions of DNA repair genes in T2DM and vascular complications but results are inconsistent. Certain studies report downregulation of selected DNA repair genes, others speculate an upregulation in consideration of increased DNA damage level in T2DM.38,39
This manuscript with limited sample size focuses on 2 main genes that encode proteins which are considered to play major role in homolog recombination of DSB and BER pathways. Confounding factors have been controlled by MLR due to different distribution of variables such as age, BMI, sex and our findings are consistent with reported literatures.40,41 Interestingly, although the current smoking had no impact on developing DN risk, the former smokers were found at high risk (Tables 2 and 3) which may relate to patients quitting smoking after diagnosis.
In conclusion, we believe that further studies are needed to evaluate the expressions of DNA repair genes and epigenetic modifications, that may be useful to elucidate association between diabetes and cancer also the mechanism underlying the increased DNA damage and inefficient repair in T2DM. The results of our study suggest that 399Gln polymorphic variant of XRCC1 may increase T2DM and DN risk. On the other hand, genotype distribution of XRCC1 A399G among all groups were not in accordance with HWE, further meta-analysis of gene-disease studies are needed to clarify whether deviation from HWE affects the situation. Therefore, these findings need to be confirmed in different population with larger sample size.
This study was supported by Research Foundation of Marmara University [grant no. SAG-C-DRP-100914-0320].
We thank Dr. Mehmet Tepe from Haydarpasa Numune Training and Research Hospital Internal Diseases Policlinics for his precious efforts in supplying blood samples and Prof. Dr. Pınar Ay from Marmara University, Faculty of Medicine, Department of Public Health for her rigorous statistical evaluations.
Conflict of interestThe authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.