Percutaneous ethanol injection (PEI) has been shown to be a valuable treatment for thyroid nodular pathology and metastatic cervical adenopathies.
ObjectiveTo evaluate the effectiveness, safety, and cost-effectiveness of PEI in thyroid nodular pathology and metastatic cervical adenopathies.
MethodsA systematic review (SR) using meta-analysis was conducted on the effectiveness and safety of PEI. A SR on cost-effectiveness was also performed. The SRs were conducted according to the methodology developed by the Cochrane Collaboration with reporting in accordance with the PRISMA statement. A cost-minimization analysis was carried out using a decision tree model. Assuming equal effectiveness between two minimally invasive techniques (PEI and radiofrequency ablation (RFA)), the model compared the costs of the alternatives with a horizon of six months and from the perspective of the Spanish National Health System.
ResultsThe search identified three RCTs (n=157) that evaluated PEI versus RFA in patients diagnosed with benign thyroid nodules: ninety-six patients with predominantly cystic nodules and sixty-one patients with solid nodules. No evidence was found on other techniques or thyroid nodular pathology. No statistically significant differences were observed between PEI and RFA in volume reduction (%), symptom score, cosmetic score, therapeutic success and major complications. No economic evaluations were identified. The cost-minimization analysis estimated the cost per patient of the PEI procedure at €326 compared to €4781 for RFA, which means an incremental difference of −€4455.
ConclusionsThere are no differences between PEI and RFA regarding their safety and effectiveness, but the economic evaluation determined that the former option is cheaper.
La inyección percutánea de etanol (IPE) ha demostrado ser un tratamiento útil para la patología nodular tiroidea y las adenopatías cervicales metastásicas.
ObjetivoEvaluar la efectividad, la seguridad y el coste-efectividad de la IPE en la patología nodular tiroidea y las adenopatías cervicales metastásicas.
MétodosSe realizó una revisión sistemática (RS) mediante un metanálisis sobre la efectividad y la seguridad de la IPE. También se realizó una RS sobre su coste-efectividad. Las RS se llevaron a cabo de acuerdo con la metodología desarrollada por la Colaboración Cochrane con la presentación de informes de acuerdo con la declaración PRISMA. Se realizó un análisis de coste-minimización mediante un modelo basado en un árbol de decisión. Asumiendo igual efectividad entre dos técnicas mínimamente invasivas (IPE y ablación por radiofrecuencia [ARF]), el modelo comparó los costes de las 2 alternativas con un horizonte temporal de seis meses y desde la perspectiva del Sistema Nacional de Salud español.
ResultadosLa búsqueda identificó tres ECA (n=157) que evaluaron la IPE frente a la ARF en pacientes diagnosticados con nódulos tiroideos benignos: 96 pacientes con nódulos predominantemente quísticos y 61 pacientes con nódulos sólidos. No se encontró evidencia sobre otras técnicas ni la patología nodular tiroidea. No se observaron diferencias estadísticamente significativas entre la IPE y la ARF en cuanto a reducción de volumen (%), puntuación de síntomas, puntuación cosmética, éxito terapéutico y complicaciones mayores. No se identificaron evaluaciones económicas. El análisis de coste-minimización estimó el coste por paciente del procedimiento de IPE en 326€ frente a los 4781€ de la ARF, lo que supone una diferencia incremental de −4455€.
ConclusionesNo hay diferencias entre la IPE y la ARF en cuanto a su seguridad y efectividad, pero la evaluación económica determinó que la primera opción es más barata.
Thyroid nodules are one of the most common reasons for an endocrinology consultation today.1,2 Thyroid nodules, often discovered incidentally during a thyroid ultrasound or on physical examination, are common and usually benign. Between 5 and 15% are at risk of becoming malignant (thyroid cancer).3 It is also common to examine the cervical lymph nodes, as suspected lymphadenopathy and metastatic thyroid cancer can be detected.4,5
The incidence of thyroid nodules is estimated at around 0.1% per year and almost 10% throughout life.6 Their prevalence ranges from 4 to 8% through palpation and up to 50–70% by ultrasound.7,8 Thyroid nodules increase linearly with age, with exposure to ionizing radiation, with a family history of thyroid disease or thyroid cancer, as well as in iodine-deficient regions.1,7,9 In addition, thyroid nodules are approximately ten times more common in women than in men.7
Most cases of benign thyroid nodules (BTN) remain asymptomatic and can be treated by clinical follow-up. However, some of them may require treatment related to cosmetic issues, pain or local pressure.6,9 The current standard of care for symptomatic BTN, as well as for thyroid cancer and metastatic cervical adenopathies, is surgery.10,11 However, patients may have problems with surgery or may be unwilling to undergo these procedures.6,8 Surgery is not only expensive, but is also associated with a 2–10% risk of complications, such as hypothyroidism, voice change or hypocalcemia.8,10 In addition, the quality of life and general well-being of the patients can be significantly affected by lifelong dependence on thyroid hormone replacement therapy, metabolic changes, and the presence of a neck scar that can sometimes be unsightly.11
In the last twenty years, other non-surgical image-guided techniques for treatment have been introduced into clinical practice, which are less invasive than surgery and are generally performed on an outpatient basis, such as radiofrequency ablation (RFA) and ultrasound- guided percutaneous ethanol injection (PEI).3,6,12 In PEI, 95–99% ethanol is slowly injected into the cyst cavity, inducing small vessel thrombosis and coagulative necrosis in the cyst wall, followed by fibrosis, contraction and consequent reduction in lesion volume.2,3
PEI is currently used as standard of care in the management of symptomatic BTN (fluid portion>50%), but it is less common in the treatment of both solid and benign parathyroid nodules.6,13
The aim of this study was to identify, critically assess and synthesize the available scientific evidence on the clinical effectiveness and safety, as well as on the cost-effectiveness, of PEI for the therapeutic management of people with thyroid nodular pathology or metastatic cervical adenopathies. A further aim was to conduct an economic evaluation to compare the health outcomes and costs of PEI and RFA in patients with predominantly cystic BTN.
MethodsSystematic review on effectiveness, safety and cost-effectivenessA systematic review (SR) of the scientific literature was conducted according to the methodology developed by the Cochrane Collaboration with reporting in accordance with the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) statement.14 The review protocol was registered at the International Prospective Register of Systematic Reviews (PROSPERO) with reference number CRD42022338437.
Information sources and search strategyThe following electronic databases were searched (from database inception to July 2022): Medline (OVID), EMBASE (Elsevier) and the Cochrane Central Register of Controlled Trials (Wiley). The strategy was initially developed for Medline and then adapted for each of the other databases. The search strategy included both controlled vocabulary and text-word terms related to PEI. Searches were restricted to the English or Spanish languages and no time limits were imposed. The search strategies are available in Supplementary Material 1.
Finally, the reference lists of all relevant papers were examined to identify other additional studies that could meet the selection criteria but were not retrieved by means of electronic search.
Selection criteriaStudies were eligible for inclusion if they fulfilled the following criteria:
- a)
Type of study: randomized controlled trials (RCTs) and full economic evaluations were included. Depending on the quality and quantity of the latter, cost-consequences analysis and partial economic evaluations for Spain were considered for inclusion.
- b)
Population: patients of any age with thyroid nodular pathology or metastatic cervical adenopathies.
- c)
Intervention: ultrasound-guided PEI.
- d)
Comparator: standard of care (surgery) or percutaneous thermal procedure (laser, radiofrequency or microwave ablation).
- e)
Outcome measures: outcomes on safety or effectiveness (i.e. volume reduction (%), symptom score, cosmetic score, therapeutic success and major complications), and on cost-effectiveness (i.e. incremental cost-effectiveness ratio (ICER), incremental cost-utility ratio, costs in monetary units, and benefits in quality-adjusted life years (QALYs), life years (LYs) gained, monetary units or in any safety or effectiveness outcomes.
- f)
Language: Spanish or English.
- g)
Publication type: only full original publications.
Titles and abstracts of the references identified by the electronic search were independently screened by two reviewers. Full texts of those studies that met the prespecified selection criteria were read and evaluated for inclusion. Doubts and discrepancies between reviewers were resolved by discussion or consultation with a third reviewer until a consensus was reached.
Data collection process and risk of bias assessmentData extraction and assessment of risk of bias were also conducted independently and in parallel by two reviewers. Discrepancies were consulted with a third reviewer.
A data extraction form (in Excel format) was prepared by the authors, pilot tested on two studies and refined accordingly. Data extracted include general study characteristics (first author, publication year, country, funding, conflict of interests), design and methodology (objective, number of centers, duration of follow-up), sample characteristics (i.e., age, sex, pathology), intervention details and comparator details, outcomes and results.
Risk of bias was assessed according to the Cochrane risk of bias tool for ECAs (RoB 2).15 RoB 2 is structured into five bias domains, focusing on different aspects of trial design, conduct and reporting. A proposed judgment about the risk of bias arising from each domain is generated by an algorithm, based on answers to each domain's signaling questions. The judgment can be ‘Low’ or ‘High’ risk of bias, or can express ‘Some concerns’ for both individual domains and the overall risk-of-bias judgment. The overall risk of bias generally corresponds to the worst risk of bias in any of the domains. A high risk of bias rating in any of the domains assessed or an uncertain risk of bias rating in three or more domains leads to the study being assessed as having a high overall risk of bias.
The Drummond checklist16 and the recommendation guideline developed by López-Bastida,17 for Spanish studies, were considered to evaluate the methodological quality of the economic evaluations.
Publication bias assessmentAccording to the Cochrane Collaboration recommendations,15 publication bias was examined by performing Egger's test, with the statistical significance level set at 0.05, using meta bias commands in Stata Statistical Software (STATA 17. StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC).
Statistical analysisResults were quantitatively synthetized by means of meta-analysis using the Review Manager computer software (RevMan, version 5.4.1. Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2020).
The Mantel–Haenszel method was used to estimate the pooled risk ratio (RR) for each dichotomous variable. Continuity correction was used for studies with zero events in one or both groups. The generic inverse variance method and mean difference (MD) or standardized mean difference (SMD) were used to combine continuous variables.18 Heterogeneity was assessed using Higgins’ I2 statistic. When there was heterogeneity (I2≥50% or p<0.1), meta-analyses were performed using a random-effects model. A sensitivity analysis was conducted by omitting each study individually to determine the stability of the overall estimate of the effect. When there was neither clinical nor statistical heterogeneity, a fixed-effect statistical model was used.19
Subgroup analysis was performed by group when it was possible (predominantly cystic nodules and solid nodules).
Certainty of evidence assessmentThe overall certainty of evidence was graded using the GRADE approach, evaluating the evidence of each key outcome on the following domains: risk of bias, inconsistency, indirectness, imprecision, and publication bias.20 The GRADEpro app was used to rate the evidence and present it in GRADE evidence profiles. Overall certainty of evidence was rated as high (very confident that the true effect is close to that of the estimated effect), moderate, low or very low (very little confidence in the estimated effect).
Economic evaluationA full economic evaluation was carried out for Spain to compare PEI and RFA to treat predominantly cystic BTN requiring treatment in patients for whom surgery was ruled out. The evaluated strategy consisted of the administration of PEI, while the comparator was the use of RFA.
This economic evaluation is based on a cost-minimization analysis due to the fact that PEI does not generate statistically significant benefits, in terms of therapeutic success, compared to RFA, and results on health-related quality of life were not assessed.21 The Spanish National Health System (Sistema Nacional de Salud; SNS) perspective, including only direct costs covered by the SNS, was used, as well as a horizon of six months, which was determined by the follow-up period of the RCTs included in the meta-analysis. Discount rates were not applied given the short-term horizon.
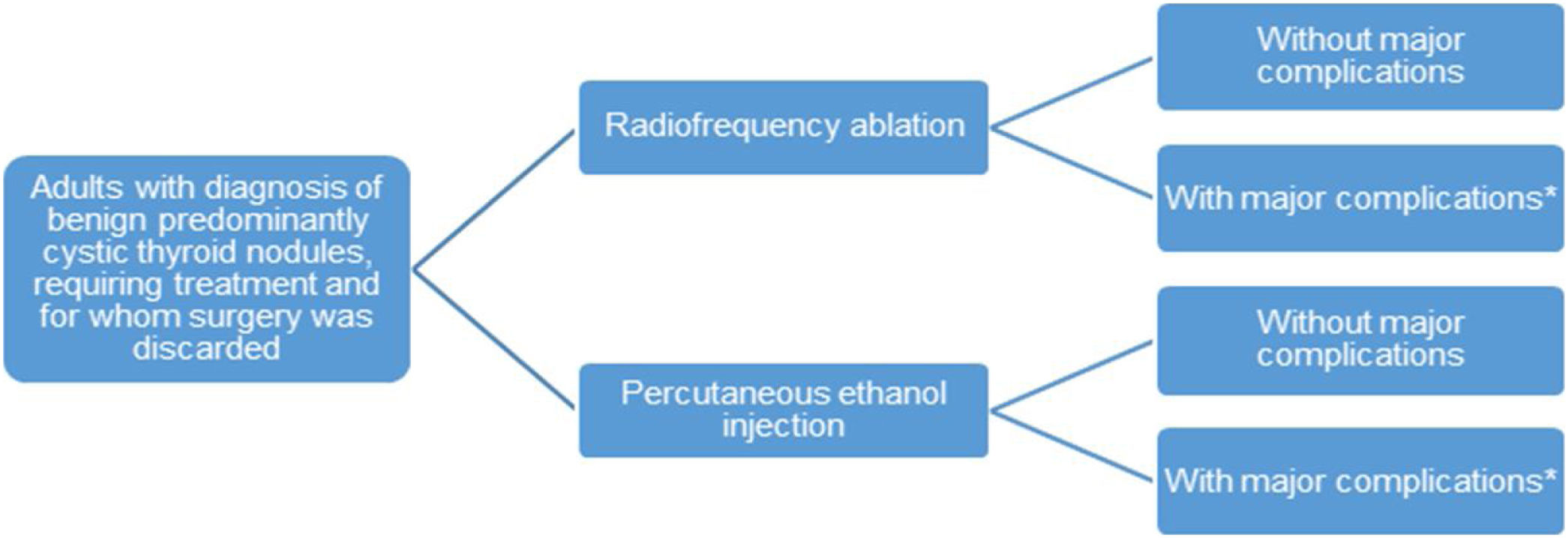
Model structureThe short-term horizon and the lack of observed changes in health status determined the choice of a decision tree model. This is made up of two branches representing the two alternative treatments (Fig. 1).
Patients enter the model when they receive the diagnosis and surgery is ruled out as the first-line treatment, due to medical contraindications or the patient's own decision. Patients can then be treated with PEI or RFA and will be followed over six months. After the intervention, major complications can arise. Nevertheless, even though they are shown in Fig. 1, their costs were not considered in the model because no statistically significant differences in their occurrence were found between the alternatives (see meta-analysis results).
The cost per patient associated with each strategy and the incremental difference between the two were calculated. The model was implemented in Microsoft Excel 2013 (Microsoft Corporation), using the programming language Visual Basic.
ParametersGiven the results of Baek et al.,21 equal effectiveness was assumed for the two alternatives compared in the model.
The direct costs included in the analysis were related to the interventions, radiologists and the observation period after the treatment, and were expressed in 2022 euros. The Spanish consumer price index (CPI) was applied when necessary (http://www.ine.es/calcula/). Supplementary Material 2 contains more information on the parameters.
The model assumed that anesthesia is not used in any of the techniques since both can be performed with or without sedation.
Sensitivity analysisA one-way deterministic sensitivity analysis was performed by varying all the parameters included in the model, using minimum and maximum values, or varying by ±20% from the mean. In addition, a probabilistic sensitivity analysis was carried out by running 1000 second-order Markov simulations. In order to do this, the following probabilistic distributions for each group of parameters were specified: gamma distribution for costs and uniform distribution for resource use.
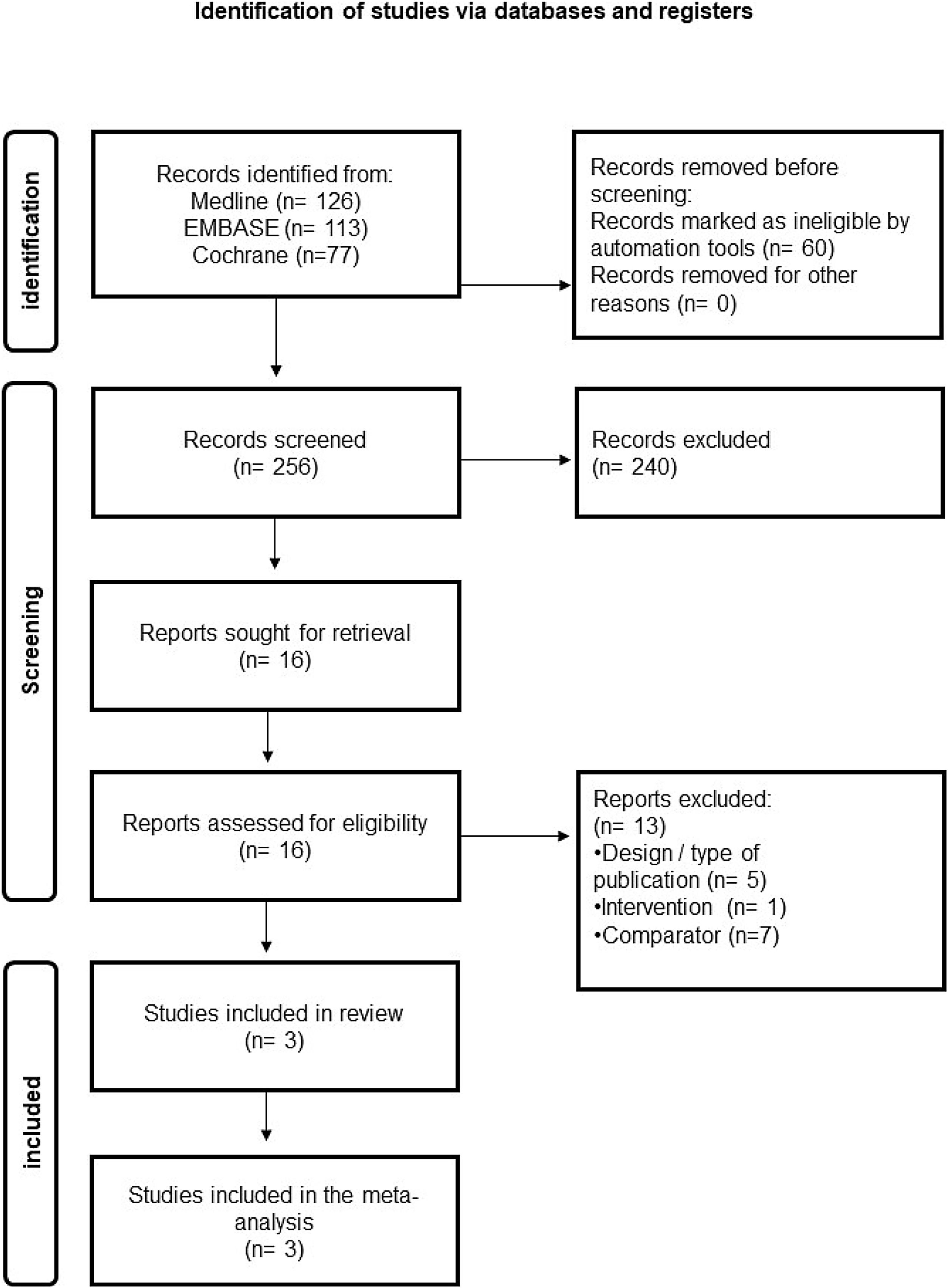
ResultsSystematic review on effectiveness and safetyThe results of the literature search and study selection process related to effectiveness and safety are shown in Fig. 2. From a total of 256 references retrieved in the electronic databases after eliminating duplicates, three studies were ultimately considered eligible for inclusion according to the pre-established selection criteria and assessed PEI vs RFA in patients with BTN. No studies were found on other thyroid pathologies (i.e. metastatic cervical adenopathies) and other comparators (i.e. surgery) according to the pre-established selection criteria. The list of studies excluded at the full-text level and the main reason for exclusion can be found in Supplementary Material 3.
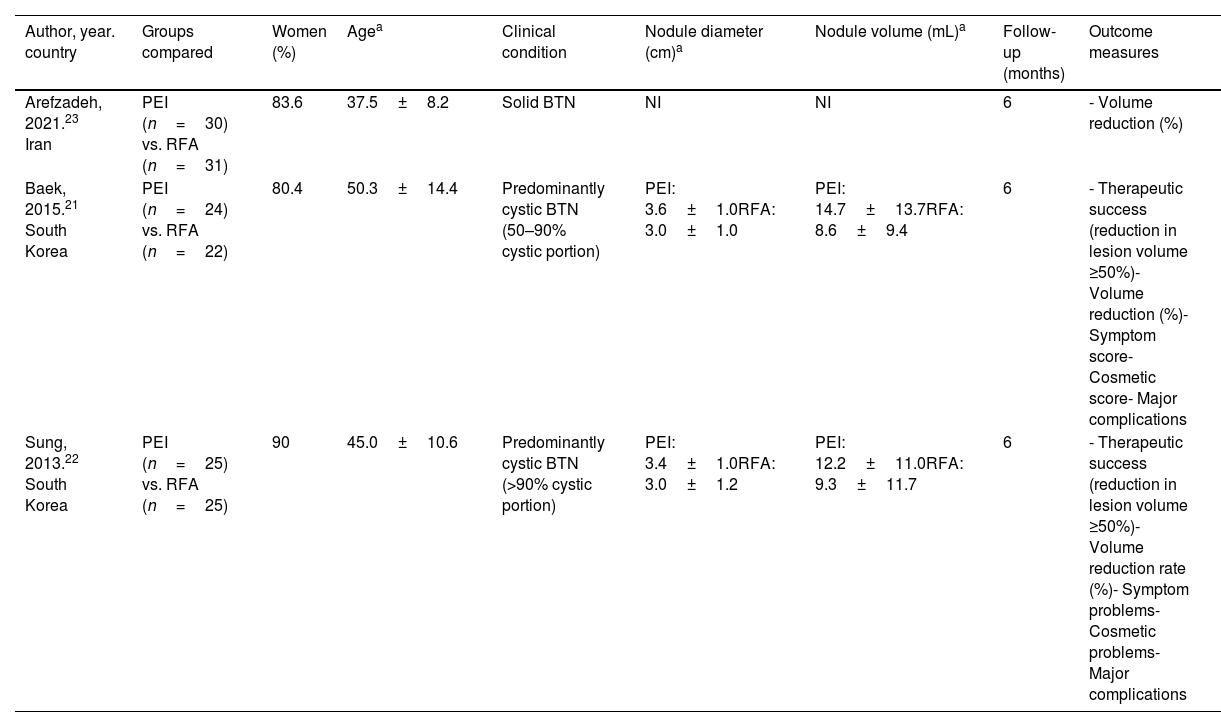
Description of included studiesThe main characteristics of the included studies are summarized in Table 1. All of them are RCTs published in English between 2013 and 2021 and assessed PEI vs RFA in patients with BTN; two of them were predominantly cystic BTN21,22 and one study was solid BTN.23
Main characteristics of the included studies on effectiveness and safety.
| Author, year. country | Groups compared | Women (%) | Agea | Clinical condition | Nodule diameter (cm)a | Nodule volume (mL)a | Follow-up (months) | Outcome measures |
|---|---|---|---|---|---|---|---|---|
| Arefzadeh, 2021.23 Iran | PEI (n=30) vs. RFA (n=31) | 83.6 | 37.5±8.2 | Solid BTN | NI | NI | 6 | - Volume reduction (%) |
| Baek, 2015.21 South Korea | PEI (n=24) vs. RFA (n=22) | 80.4 | 50.3±14.4 | Predominantly cystic BTN (50–90% cystic portion) | PEI: 3.6±1.0RFA: 3.0±1.0 | PEI: 14.7±13.7RFA: 8.6±9.4 | 6 | - Therapeutic success (reduction in lesion volume ≥50%)- Volume reduction (%)- Symptom score- Cosmetic score- Major complications |
| Sung, 2013.22 South Korea | PEI (n=25) vs. RFA (n=25) | 90 | 45.0±10.6 | Predominantly cystic BTN (>90% cystic portion) | PEI: 3.4±1.0RFA: 3.0±1.2 | PEI: 12.2±11.0RFA: 9.3±11.7 | 6 | - Therapeutic success (reduction in lesion volume ≥50%)- Volume reduction rate (%)- Symptom problems- Cosmetic problems- Major complications |
BTN: benign thyroid nodule; PEI: percutaneous ethanol injection; RFA: radiofrequency ablation; NI: not informed.
Study size ranged from forty-six to sixty-one participants, with an average of fifty-two participants per study. Among the three studies, 157 participants were analyzed: ninety-six participants with predominantly cystic thyroid nodules and sixty-one with solid thyroid nodules. Most of the participants were female (84.7%), with a mean age of 44.3 years (SD=11.1). The follow-up duration was six months.
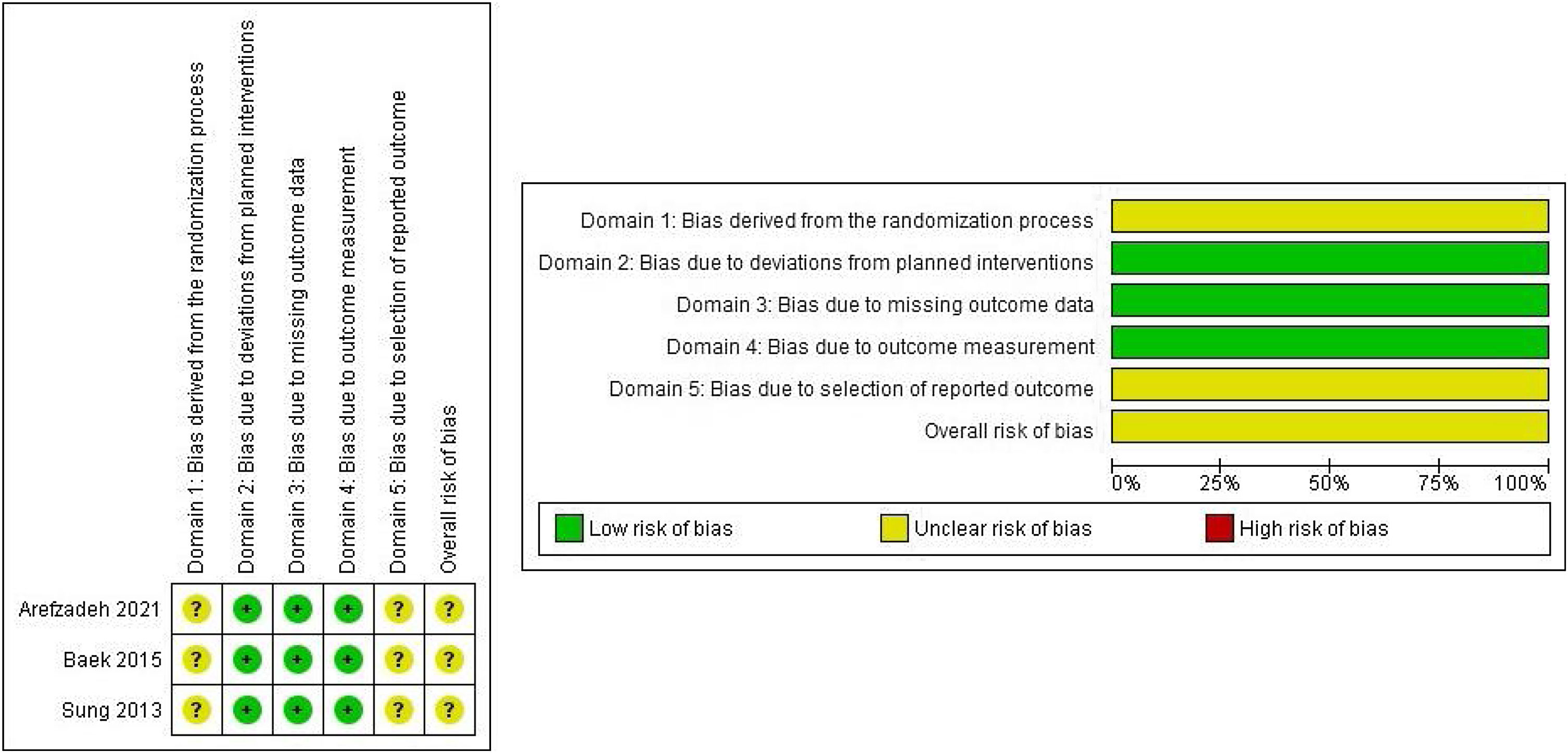
Risk of bias in included studiesThe results of the risk of bias assessment in the included studies are shown in Fig. 3. The risk of bias was considered unclear in the three included studies. In addition, in all cases no information on the randomization process was provided and no trial register record or protocol where the pre-specified intentions of the studies can be checked was available.
Certainty of evidenceThe overall quality of evidence was moderate. The evidence profile for PEI vs RFA-related outcomes showed that the quality of evidence was moderate (Supplementary Material 4).
Summary of resultsThe results of the meta-analysis, subgroup analysis and sensitivity analysis are available in the Supplementary Material 5.
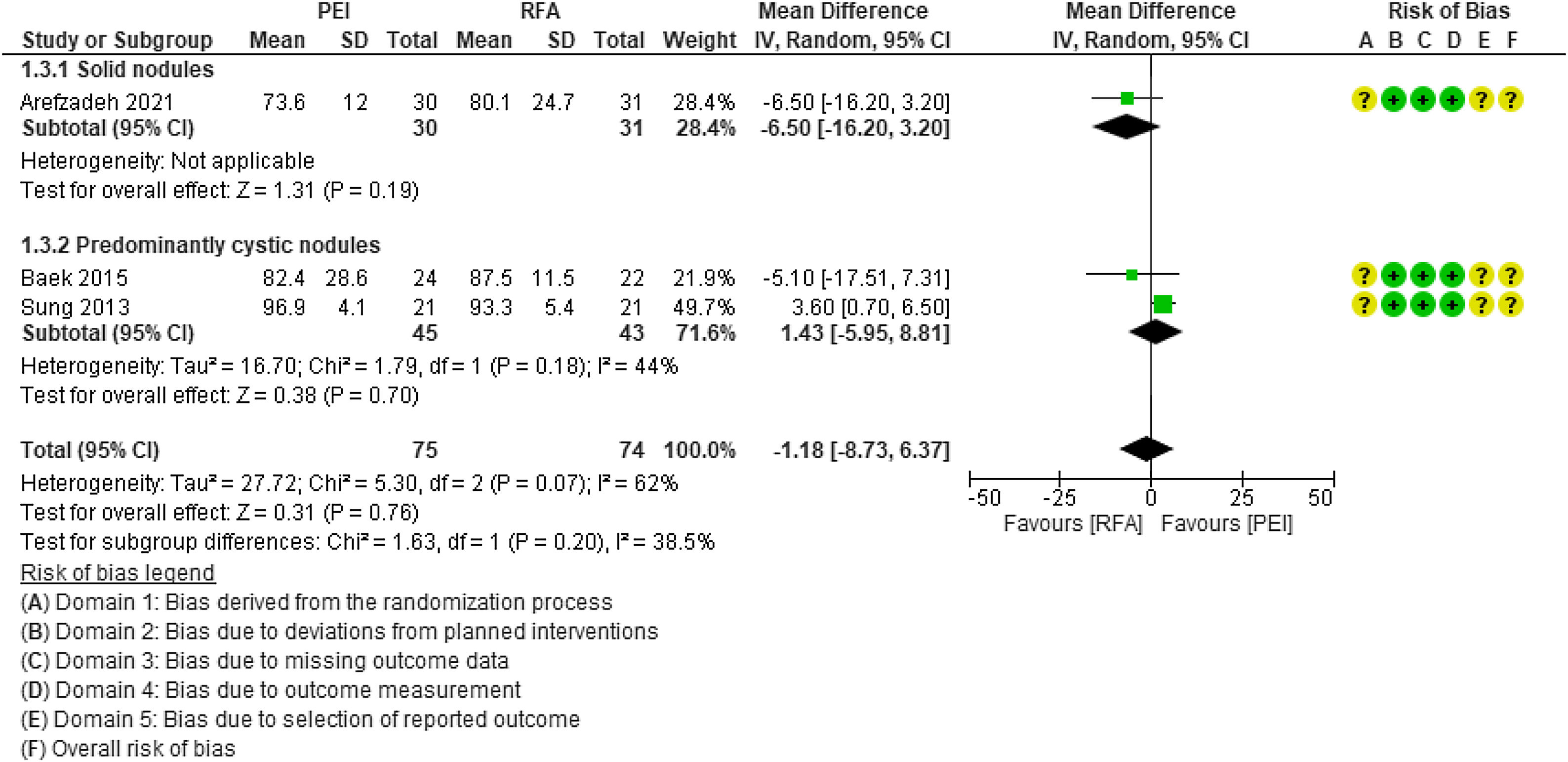
Volume reduction (%). The three studies assessed the volume reduction of the thyroid lesion, two of them in patients with predominantly cystic BTN21,22 and one in patients with solid BTN.23 No statistically significant differences were observed between PEI and RFA (MD=−1.18; 95% CI: −8.73 to 6.37; p=0.76; I2=62%) (Fig. 4). There were also no statistically significant differences between solid and predominantly cystic BTN. In the sensitivity analysis, the elimination of the study by Sung et al. (2013) from the analysis reduced heterogeneity, but the differences remained non-significant (MD=−5.97, 95% CI: −13.61 to 1.67, p=0.13, I2=0%).
Therapeutic success. Two of the identified RCTs21,22 compared the therapeutic success of PEI versus RFA at 6-month follow-up for the treatment of predominantly cystic BTN. No statistically significant differences were observed between PEI and RFA (RR=0.96; 95% CI: 0.88 to 1.04; p=0.33; I2=14%).
Symptom score. The intensity of symptoms in thyroid nodular pathology and metastatic cervical adenopathies was assessed using a symptom score, which measures pressure symptoms through a 10-cm visual analog scale (ranging from 0: no symptoms to 10: many symptoms).24 Two of the identified RCTs21,22 compared the symptoms of patients who underwent PEI versus RFA for the treatment of predominantly cystic BTN. No statistically significant differences were observed between PEI and RFA (RR=0.23; 95% CI: −0.26 to 0.71; p=0.36; I2=47%).
Cosmetic score. Cosmetic complaints in thyroid nodular pathology and metastatic cervical adenopathies were assessed using a cosmetic score25 (1: non-palpable mass; 2: non-cosmetic problem but palpable mass; 3: cosmetic problem when swallowing only; 4: cosmetic problem easy to detect). Two of the identified RCTs21,22 compared the cosmetic problems of patients who underwent PEI versus RFA for the treatment of predominantly cystic BTN. No statistically significant differences were observed (RR=0.12; 95% CI: −0.09 to 0.34; p=0.26; I2=0%).
Major complications. Two of the identified RCTs21,22 compared major complications in patients who underwent PEI versus RFA for the treatment of predominantly cystic BTN. Major complications are defined according to the Interventional Radiology Society: (1) require treatment, minor hospitalization (48h); (2) require significant treatment, prolonged hospitalization; (3) permanent adverse sequelae; and (4) death.26 No statistically significant differences were observed between PEI and RFA (RR=2.76; 95% CI: 0.12–64.41; p=0.53).
Publication biasA funnel plot analysis and Egger's test could not be performed because the minimum number of studies necessary to be able to assess the publication bias in any of the outcomes was not attained (n=10).
Systematic review on cost-effectivenessThe results of the literature search and study selection process related to cost-effectiveness are shown in Supplementary Material 2. The electronic databases retrieved sixty-four references without duplicates. After reading titles and abstracts, three studies were selected for a full text reading. All of them were excluded due to the design27,28 or the evaluated comparator.29 Therefore, no economic evaluations comparing PEI with an alternative treatment (surgery or another minimally invasive technique) that met the established inclusion criteria were identified.
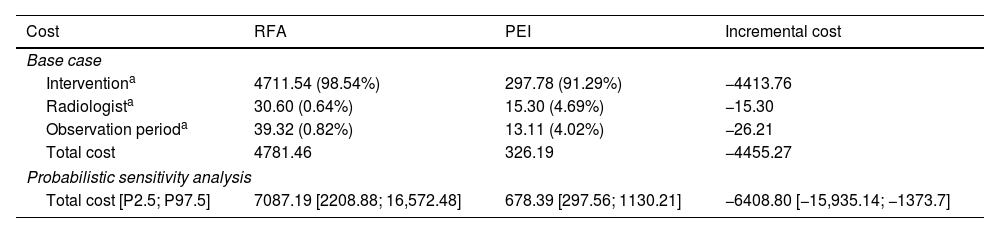
Economic evaluationThe results of the cost-minimization analysis show that PEI-based treatment is less costly compared to RFA, from an SNS perspective (Table 2): the incremental difference between alternatives is −€4455.27 per patient, in favor of PEI. By disaggregating the total costs into three groups (intervention, radiologists and observation period), the highest cost corresponds to the intervention costs in both strategies.
Results of the cost-minimization analysis: base case (disaggregated total costs), deterministic and probabilistic sensitivity analysis. Costs per patient (2022 euros).
| Cost | RFA | PEI | Incremental cost |
|---|---|---|---|
| Base case | |||
| Interventiona | 4711.54 (98.54%) | 297.78 (91.29%) | −4413.76 |
| Radiologista | 30.60 (0.64%) | 15.30 (4.69%) | −15.30 |
| Observation perioda | 39.32 (0.82%) | 13.11 (4.02%) | −26.21 |
| Total cost | 4781.46 | 326.19 | −4455.27 |
| Probabilistic sensitivity analysis | |||
| Total cost [P2.5; P97.5] | 7087.19 [2208.88; 16,572.48] | 678.39 [297.56; 1130.21] | −6408.80 [−15,935.14; −1373.7] |
| One-way deterministic sensitivity analysis | |||
|---|---|---|---|
| Parameter | Value in base case | New value | Incremental cost |
| RFA session | 1 | 2 [experts] | −9236.74 |
| PEI session | 1 | 3 [experts] | −3802.90 |
| Duration of an RFA session (h) | 1 | 0.8 [assumption, −20%] | −4449.15 |
| 0.5 | 1.2 [assumption, +20%] | −4461.39 | |
| Duration of a PEI session (h) | 0.5 | 0.4 [assumption, −20%] | −4458.33 |
| 1.5 | 0.67 [experts] | −4450.17 | |
| Radiologist per session (RFA and PEI) | 1 | 2 [Baek et al.21] | −4470.57 |
| Observation time per patient treated with RFA (h) | 1.5 | 1 [Baek et al.21], from Sung et al.22 | −4442.17 |
| 2 [experts] | −4468.38 | ||
| Observation time per patient treated with PEI (h) | 0.5 | 0.4 [assumption, −20%] | −4457.90 |
| 0.6 [assumption, +20%] | −4452.65 | ||
| RFA intervention cost | 4711.54 | 2671 [min, Oblikue34] | −2414.73 |
| 6752.08 [max, Oblikue34] | −6495.82 | ||
| PEI intervention cost | 297.78 | 273.02 [min, Oblikue34] | −4480.04 |
| 322.55 [max, Oblikue34] | −4430.51 | ||
| Labor cost of radiologists (€/h) | 30.60 | 24.48 [assumption, −20%] | −4452.21 |
| 36.72 [assumption, +20%] | −4458.33 | ||
| Cost of the observation period (€/h) | 26.21 | 5.17 [min, Oblikue34] | −4434.23 |
| 46.58 [max, Oblikue34] | −4475.64 | ||
RFA: radiofrequency ablation; PEI: percutaneous ethanol injection; P2.5: percentile 2.5; P97.5: percentile 97.5; min: minimum; max: maximum.
The sensitivity analysis, both deterministic and probabilistic, confirmed the results estimated in the base case. The variation of the parameters used in the model did not significantly affect the incremental cost, showing a lower cost of PEI compared to RFA in all evaluated cases (Supplementary Material 2).
DiscussionThis SR on effectiveness and safety identified three RCTs (n=157), published between 2013 and 2022 comparing PEI with RFA in patients with BTN.21–23 The evidence obtained was rated as of moderate quality, and no statistically significant differences were observed in terms of therapeutic success, volume reduction of the thyroid lesion, pressure symptoms, cosmetic discomfort and major complications between PEI and RFA. In the subgroup analysis, no statistically significant differences were observed in the percentage of volume reduction between solid nodules and predominantly cystic nodules.
The results here are similar to those of previous SRs assessing the use of PEI for the treatment of BTN.3,30 Both Bandeira et al. (2014)3 and Yang et al. (2021)30 reported no statistically significant differences between the therapeutic success of PEI and that of RFA for the treatment of this pathology. No statistically significant differences were reported regarding the other outcomes considered (pressure symptoms, cosmetic inconveniences, and complications).
The educitiveness and safety of PEI compared to other interventions for the treatment of BTN or for pathologies other than BTN could not be evaluated due to the lack of available published studies concerning the matter. Nevertheless, in He et al. (2021),31 where a network meta-analysis of RCTs evaluating the efficacy and safety of different thermal/chemical ablation treatments for the management of cystic or cyst-predominant BTN was conducted, RFA significantly reduced nodule volume compared to PEI, but PEI was the most effective treatment when considering only cystic nodules. This network meta-analysis also found that both PEI and RFA showed significant symptom score advantage when compared with control and that PEI was the most effective at reducing symptoms.
Additionally, regarding the effectiveness and safety of PEI in pathologies other than BTN, previous SRs of observational studies conducted in patients with thyroid cancer observed a lower rate of therapeutic success of PEI compared with surgery32 and with RFA.33
No economic evaluations comparing PEI with an alternative treatment were identified in the SR. The cost-minimization analysis, based on a decision tree model from an SNS perspective, was carried out to compare PEI-based treatment with RFA for patients with predominantly cystic BTN, requiring treatment and for whom surgery was ruled out. Even though surgery is the first-line treatment in routine clinical practice in Spain, RFA was chosen as the comparator, due to the available evidence from RCTs, which allowed us to compare only these two minimally invasive techniques for this specific population.
Assuming equal effectiveness between the alternatives, PEI is less costly than RFA.
Experts pointed out that the number of required sessions may be higher depending on the response to the intervention and the nodule size, therefore this parameter was varied in the sensitivity analysis. However, this did not change the base case conclusions. Sensitivity analyses showed the robustness of the results. The incremental cost is so high that unrealistically large variations in the parameters are required to observe changes in the conclusions of the economic model.
Study limitationsThe strength of the SR of the literature carried out on safety, effectiveness, and cost-effectiveness, is related to the fact that it was carried out in accordance with the fundamental principles to ensure its transparency, replicability and ease of updating. The explicit information on the methodology used and the availability of the extracted data mean that it can also be used as the object of a critical evaluation.
One of the main limitations of SRs, derived from the methodology, is the possibility that relevant studies are not included in the analysis as a result of their not having been published, because they are published in a language other than English or Spanish or because they have been published in unindexed journals.
Despite the existence of several thyroid pathologies, the economic model focused only on predominantly cystic BTN in order to conform, as far as possible, to current clinical practice, also bearing in mind the available evidence, although the consulted experts pointed out that RFA is not usually applied as first-line treatment in this type of thyroid nodules in Spain. Instead, RFA is only recommended after a first treatment of PEI. Despite this, the authors assumed that all patients in each branch received only the technology represented in it because these alternatives were those evaluated by Baek et al. (2015).21
Regarding costs, it was assumed that anesthesia is not used in either of the two treatment strategies. Considering its application for PEI but not for RFA means that the cost of anesthesia would need to be greater than €4455 for the results to be reversed in favor of RFA, which seems unlikely according to consultation of the Oblikue database.34 If sedation were considered only for RFA, the conclusion would not change (the difference in costs would be higher). Finally, if anesthesia were applied in both alternatives, it would not be necessary to include it in the model due to its being a cost common to both treatments. Therefore, the inclusion or otherwise of the anesthesia cost does not affect the conclusion derived from the economic model. In addition, although the costs could be different over a longer time horizon, as a longer horizon would allow us to observe nodule recurrences (and their additional costs), the economic evaluation is conditioned by the available evidence in terms of clinical efficacy and safety.
Despite these limitations, experts validated the model and the assumptions (face validity), and the methods and tools (such as the TECH-VER checklist) needed to perform the identification and correction of model errors were applied (internal validity).
ConclusionThe available evidence on PEI for the treatment of thyroid nodular pathology is quite limited, and non-existent for metastatic cervical adenopathies and other comparators such as surgery. The present study suggests that PEI is similar to RFA in effectiveness and safety for the treatment of BTN. The economic evaluation found that the PEI option is cheaper.
Well-designed and executed RCTs are needed to confirm the findings here and to have scientific evidence available for other comparators and thyroid pathologies for which the evidence is extremely scarce.
Authors’ contributionsBLS, DIV, AAC, YGH and MTM made substantial contributions to the research design, analysis, interpretation of data and writing the manuscript; AHY and RL developed the economic evaluation and made substantial contributions to the research design of the economic evaluation, analysis, interpretation of data and writing the economic evaluation aspects of the manuscript; all authors critically reviewed the paper, and read and approved the final manuscript.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
International Prospective Register of Systematic Reviews (PROSPERO) numberCRD42022338437 (https://www.crd.york.ac.uk/PROSPERO/).
Availability of data and materialsAll relevant data are within the manuscript and its additional files.
FundingThis work was supported by the Spanish Ministry of Health in the framework of activities developed by Spanish Network of Agencies for Health Technology Assessment for the National Health Service (RedETS).
Conflict of interestsThe authors have declared that no competing interests exist.
The authors would like to thank Carlos González Rodríguez and Leticia Rodríguez Rodríguez for their support in the documentation and editing tasks. We are also grateful to Patrick Dennis for native English language editing support with the final manuscript.