There is scarce information regarding the performance of a specific, structured education program addressed to patients with type 1 diabetes (T1D) using continuous subcutaneous insulin infusion (CSII) including both routine use of the therapy and patient experience evaluation. We aimed to assess the routine use of CSII and patient's experience and satisfaction regarding a specific structured patient self-management education and care program.
MethodsA retrospective, observational, cross-sectional study collecting CSII routine use downloaded data. Patient experience and satisfaction were evaluated using an anonymous online survey covering different aspects of CSII self-management education and care program.
Results380 T1D subjects were included (aged 45.3±12.17 years, 62.1% women, diabetes duration 27.8±10.3 years, 9.7±4.7 years on CSII, HbA1c 7.7+1.0%; 61.0±7.9mmol/mol). Participants with HbA1c≤7.5% (58mmol/mol, n=178) did more SMBGs per day (4.4±2.1 vs. 3.9±1.9); used more boluses (5.0±1.8 vs. 4.5±2.0); the percentage of insulin given as bolus was higher (50.1±12.8 vs. 44.9±13.2%); the night bolus wizard (BW) high glucose target was lower (125.9±4.4 vs. 130.5±12.8mg/dl) and time on CSII therapy was shorter (8.9±4.6 vs. 10.3±4.6 years. p<0.05 all comparisons). More SMBG/day, shorter duration of CSII treatment, a lower BW low glucose target at night, a lower BW high glucose target at night, total insulin dose per day and total number of carbohydrates per day were related with better HbA1c levels. 60% of 373 patients answered the questionnaire. The response to the different aspects of the educational program was homogeneously highly satisfactory. Seventy-seven percent of patients scored the program as very useful. Ninety-three percent of CSII users would not return to their previous insulin treatment.
ConclusionsThe analysis of routine clinical use of CSII by T1D patients demonstrates that glucose control may be associated with some pump usage and adherence parameters. The overall user experience and satisfaction with our CSII self-management education and care program was remarkably favorable.
Hay poca información sobre la eficacia de un programa educativo estructurado específico dirigido a los pacientes con diabetes tipo 1 (DT1) que utilizan infusión subcutánea continua de insulina (ISCI) que incluye tanto el uso habitual del tratamiento como la evaluación de la experiencia de los pacientes. Nuestro objetivo era valorar el uso habitual de la ISCI, la experiencia y la satisfacción del paciente con un programa educativo y asistencial estructurado específico para autogestión de los pacientes.
MétodosEstudio transversal retrospectivo observacional en el que se recogieron datos descargados sobre el uso habitual de la ISCI. Se evaluaron la experiencia y la satisfacción de los pacientes mediante una encuesta en línea anónima que abarcaba distintos aspectos del programa educativo y asistencial para autogestión de la ISCI.
ResultadosSe incluyó a 380 pacientes con DT1 (45,3±12,17 años de edad, 62,1% mujeres, duración de la diabetes 27,8±10,3 años, 9,7±4,7 años con ISCI, HbA1c 7,7+1,0%; 61,0±7,9mmol/mol). Los participantes con HbA1c<7,5% (58mmol/mol, n=178) practicaron más autocontroles al día (4,4±2,1 vs. 3,9±1,9); usaron más bolos (5,0±1,8 vs. 4,5±2,0); tuvieron un porcentaje de insulina administrada en bolo mayor (50,1±12,8 vs. 44,9±13,2%) y el objetivo de glucosa nocturna alta en el recomendador de bolo (bolus wizard, BW) era más bajo (125,9±4,4 vs. 130,5±12,8mg/dl), y su tiempo con ISCI era menor (8,9±4,6 vs. 10,3±4,6 años, p<0,05 para todas las comparaciones). Más autocontroles al día, la menor duración del tratamiento con ISCI, un objetivo de glucosa baja del BW menor por la noche, un objetivo de glucosa alta del BW por la noche menor, la dosis total diaria de insulina y el número total de hidratos de carbono diarios estaban relacionados con mejores valores de HbA1c. El 60% de 373 pacientes contestaron el cuestionario. La respuesta a los distintos aspectos del programa educativo fue muy satisfactoria en conjunto. El 77% de los pacientes valoraron el programa como muy útil. El 93% de los usuarios de ISCI no volverían el tratamiento de insulina previo.
ConclusionesEl análisis del uso clínico sistemático de la ISCI por pacientes con DT1 demuestra que el control de la glucosa puede relacionarse con algunos parámetros de uso y cumplimiento de la bomba. La experiencia global del usuario y la satisfacción con nuestro programa educativo y asistencial de autogestión de la ISCI fueron notablemente favorables.
Type 1 diabetes (T1D) requires life-long insulin replacement therapy with continuous health care support to achieve optimal blood glucose control and reduce the risk of long-term diabetes-related complications.1,2 Despite remarkable advances in diabetes treatment, highly motivated and everyday life dedicated patients continue to struggle in achieving glucose targets avoiding a high frequency of severe and non-severe hypoglycemia.3 Having been used for nearly 40 years, there is no doubt that continuous subcutaneous insulin infusion (CSII) therapy is an efficient, safe and flexible treatment for improving both glucose control and quality of life of patients with T1D.4,5 Various manuscripts have been published throughout recent years regarding the mid and long-term effects of CSII on glucose control mainly focusing on changes in HbA1c values.6,7 Real-life studies are deemed necessary to complement information retrieved with clinical trials. Both have limitations and should be seen as complementary. Routine use studies add data on effectiveness and safety of therapies in the real world setting.
Patient experience encompasses the personal impact of a wide range of interactions that patients have with the health care system (health plans, communication and care provided by doctors, nurses and other staff in hospitals, physician practices, and health care facilities).8 It is a component of health care quality, at the same level as safety and effectiveness, and it is a key step in moving toward patient-centered care. Evaluating patient experience along with other components such as effectiveness, safety and satisfaction of/with care is essential to provide a comprehensive picture of the quality of health care specific programs.9
The aim of our study was to analyze the real life routine use of CSII therapy and its relationship with blood glucose control, as well as, the patient experience and satisfaction regarding a specific structured patient self-management education program addressed to patients using this modality of insulin therapy.
Material and methodsStudy design and subjectsWe performed a cross-sectional study that involved reviewing electronic medical records and databases of adult individuals with T1D followed at the Diabetes Unit, Endocrinology and Nutrition Department at Hospital Clínic i Universitari of Barcelona. In the present analysis, we used anonymized CareLink-Pro platform (Medtronic-Minimed, Northridge, CA) data collected between January 2016 and December 2016 as a part of a routine visit of all patients with T1D using CSII under the specific indications of the Catalan National Health Service authorities. All patients had been previously using either a Veo® or 640G® Medtronic-Minimed insulin pump linked to a glucometer (Contour Next Link/2.0′, Bayer®) for at last 12 months. Thirty five patients used sensor augmented pump therapy (SAP, 9.2%). Only data on capillary glucose measurements, pump use and carbohydrate intake from 14 consecutive days were collected from uploads. HbA1c (Tosoh G8 Automated HPLC Analyzer – Tosoh Bioscience Inc., South San Francisco, CA, USA – DCCT aligned, normal range 4–6%) was obtained from medical records (average of the last three HbA1c performed during the previous 12 months to the data download). Demographic characteristics and clinical data were also recorded using computerized clinical records.
In our unit, all patients under CSII therapy had previously completed the educational program. Individuals who met the specific indications to start CSII therapy under the funding of the Catalan National Health Service authorities were invited to an informative session in order to explain this therapy. If they agreed to start CSII, they received our specific therapeutic education program for individuals beginning CSII in which nurses and endocrinologists participate. The program consists of 4 weekly visits (first week two visits) in groups of 4 people over the first month. After these visits, they were cited individually every month or every two months as required during the first 6 months, and at 6 and 12 months after the start of the program the participants were revaluated. Thereafter, they continued an ambulatory follow-up specifically designed for individuals on CSII.
Patient experience and satisfaction with the CSII self-management education and care program (12 months duration) were evaluated using an anonymous online survey (LimeSurvey®) performed from November to December 2016 (voluntary sampling). Previously, a group of subjects participated in a focus group led by a psychologist. Prevailing themes based on review of all transcripts were included in the online survey. The questionnaire enclosed questions (score 1–4, useless/very useful) covering different aspects of program in 5 sections:
- (1)
Before starting CSII: group informative session regarding CSII therapy; CSII user participation during informative session; Individual evaluation visit with a diabetes specialist nurse and technical training by CSII device manufacturer expert personnel (4 questions).
- (2)
Initiation of CSII and 1st month of therapy: group session including 3–4 patients; patient's family participation; expert physician and nurse collaborative participation; group interaction in learning process; content of educational program and methodology (5 questions).
- (3)
First year follow-up: individual follow-up visit with a diabetes specialist nurse; telemedicine consultations; 6 and 12 months group evaluation; day hospital consultation facility for emergencies; 24/7 clinical emergency telephone line (endocrinologist); telephone answering service delivered by an expert nurse and 24/7 technical support service delivered by the manufacturer (7 questions).
- (4)
Beyond the 1st year of CSII therapy use: quality of life and satisfaction with CSII therapy, diabetes control and daily CSII self-management (7 questions).
- (5)
Demographics (5 questions: age, gender, disease duration, time on CSII and educational degree) and HbA1c.
The score result in each question was evaluated following the criteria from the Catalan National Health authorities for satisfaction surveys evaluating citizens’ perception of the quality of the service in health policies. Considering these criteria a score >3.6 indicates excellence, ≥3.0 and <3.6 indicates an average result and <3.0 indicates that an improvement is needed.
The study was approved by the Ethical Committee of Hospital Clínic i Universitari de Barcelona, and all subjects gave informed consent.
The results are presented as mean±SD or proportions. The differences between subgroups were analyzed using an unpaired Student t-test. The strengths of association between two variables were calculated using Pearson's correlation coefficient. A multiple linear regression analysis was conducted in order to evaluate factors related with the HbA1c outcome. A p-value <0.05 was considered statistically significant. Data analysis was carried out with SPSS software, version 20.0 (SPSS Inc., Chicago IL, USA).
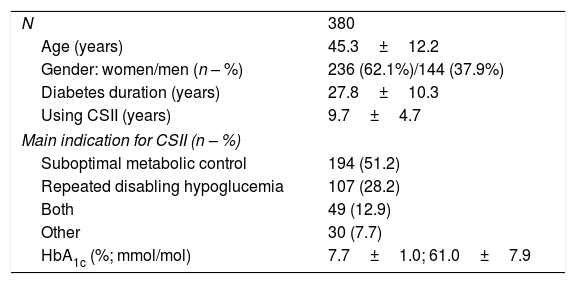
ResultsRoutine use of CSIIData from 380 subjects with T1D were included. Table 1 shows their baseline characteristics. The main indication to start CSII was suboptimal metabolic control in 194 participants (51.2%.) Total daily dose of insulin was 41.9±16.1U/day, 52.6% used as basal and 47.4% as bolus. The total number of carbohydrates introduced per day was 129±62g. Considering the total number of boluses/day (4.8±1.9), 79.1% were bolus wizard and 20.9% manual boluses. In 19.4% of occasions, bolus advice needed to be corrected by patient's judgment. Basal insulin was divided in 6.0±1.9 segments in a day and pump settings included 1.2±0.5 different basal patterns and 3.4±1.5 different insulin/carbohydrate ratios per day. Regarding bolus wizard settings, at night high and low glucose targets were 128.4±13.7 and 104.3±14.4; while during day time were 119.7±14.3 and 95.8±11.2mg/dl, respectively.
Baseline characteristics of patients included in the evaluation of routine use of CSII.
| N | 380 |
| Age (years) | 45.3±12.2 |
| Gender: women/men (n – %) | 236 (62.1%)/144 (37.9%) |
| Diabetes duration (years) | 27.8±10.3 |
| Using CSII (years) | 9.7±4.7 |
| Main indication for CSII (n – %) | |
| Suboptimal metabolic control | 194 (51.2) |
| Repeated disabling hypoglucemia | 107 (28.2) |
| Both | 49 (12.9) |
| Other | 30 (7.7) |
| HbA1c (%; mmol/mol) | 7.7±1.0; 61.0±7.9 |
Values expressed as mean±SD or percentage. CSII: continuous subcutaneous insulin infusion.
Based on downloaded data, CSII users performed on average 4.3±2.1 self monitoring blood glucose (SMBG) measurements per day. Average SMBG value was 161±30mg/dl; 37.5±15.8% of values were >180mg/dl, 11.2±9.1% were <70mg/dl. The mean HbA1c was 7.7±1.0% (61.0±7.9mmol/mol). Nine patients out of 380 (2.4%) had an HbA1c>10% (86mmol/mol), 259 (68.2%) <8% (64mmol/mol), 162 (42.6%) <7.5% (58mmol/mol) and 75 out of 380 (19.7%) patients had an HbA1c <7% (53mmol/mol). A multiple linear regression was conducted in order to evaluate factors related with the HbA1c outcome. More SMBG/day (β −0.286, p<0.001), shorter duration of CSII treatment (β 0.107, p=0.041), a lower BW low glucose target at night (β 0.145, p=0.006), a lower BW high glucose target at night (β 0.128, p=0.012, total insulin dose per day (β 0.114, p=0.046) and total number of carbohydrates per day (β −0.205, p=0.001) were related with better HbA1c levels (other variables included in the analysis were total number of boluses per day, percentage of boluses used as BW and BW high/low glucose target at daytime).
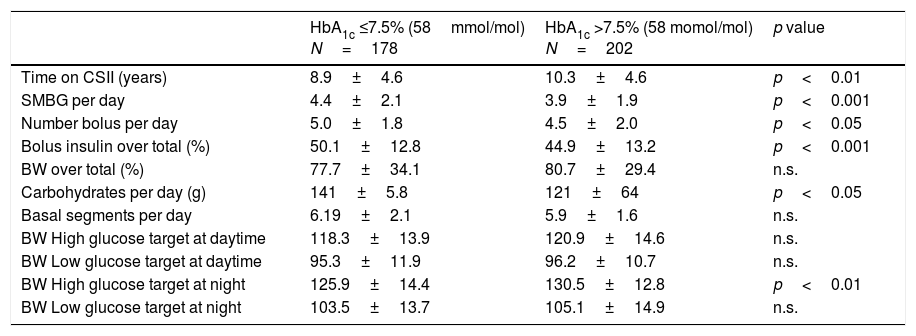
We compared the characteristics of routine use of CSII in two different subgroups of patients; those with an HbA1c ≤7.5 and those with a value >7.5% (58mmol/mol) (cut-off value defining suboptimal metabolic control and CSII indication by the Catalan National Health Service authorities) (Table 2). The proportion of patients using SAP was equally distributed between groups. In comparison with participants with an HbA1c >7.5% (58mmol/mol), participants with an HbA1c ≤7.5% (58mmol/mol) performed more SMBG measurements per day (4.4±2.1 vs. 3.9±1.9; p<0.001); used a higher number of boluses per day (5.0±1.8 vs. 4.5±2.0; p<0.05); the percentage of total insulin used as bolus was higher (50.1±12.8 vs 44.9±13.2%; p<0.001); the bolus wizard high glucose target at night was lower (125.9±14.4 vs 130.5±12.8mg/dl; p<0.01); total number of carbohydrates per day was higher (141±58 vs. 121±64; g per day p<0.05) and time on CSII therapy was shorter (8.9±4.6 vs. 10.3±4.6 years; p<0.01). In terms of glucometrics, as expected the percentage of SMBG values <70mg/dl were lower in the group with an HbA1c >7.5% (9.6±8.10%) in comparison with the group with an HbA1c ≤7.5% (13.3±9.8%, p<0.05).
Routine use of CSII in patients with an HbA1c ≤7.5 and >7.5%.
| HbA1c ≤7.5% (58mmol/mol) N=178 | HbA1c >7.5% (58 momol/mol) N=202 | p value | |
|---|---|---|---|
| Time on CSII (years) | 8.9±4.6 | 10.3±4.6 | p<0.01 |
| SMBG per day | 4.4±2.1 | 3.9±1.9 | p<0.001 |
| Number bolus per day | 5.0±1.8 | 4.5±2.0 | p<0.05 |
| Bolus insulin over total (%) | 50.1±12.8 | 44.9±13.2 | p<0.001 |
| BW over total (%) | 77.7±34.1 | 80.7±29.4 | n.s. |
| Carbohydrates per day (g) | 141±5.8 | 121±64 | p<0.05 |
| Basal segments per day | 6.19±2.1 | 5.9±1.6 | n.s. |
| BW High glucose target at daytime | 118.3±13.9 | 120.9±14.6 | n.s. |
| BW Low glucose target at daytime | 95.3±11.9 | 96.2±10.7 | n.s. |
| BW High glucose target at night | 125.9±14.4 | 130.5±12.8 | p<0.01 |
| BW Low glucose target at night | 103.5±13.7 | 105.1±14.9 | n.s. |
Values expressed as mean±SD or percentage. n.s.: non significant; CSII: continuous subcutaneous insulin infusion; BW: bolus wizard.
The patient experience and satisfaction questionnaire was sent by email to all patients with an email address (381 out of 420 patients actively using CSII) in our computerized clinical records. Email was successfully sent to 373 patients (8 email delivery errors) and 223 of them (60%) answered the questionnaire. Regarding demographics, 61% percent of them were women, 66% reported a university educational degree, aged of 45.0±12.0 years, with a disease duration of 27.1±10.2 years and 9.1±3.8 years using CSII therapy. Self-reported last HbA1c was 7.4+1.4%.
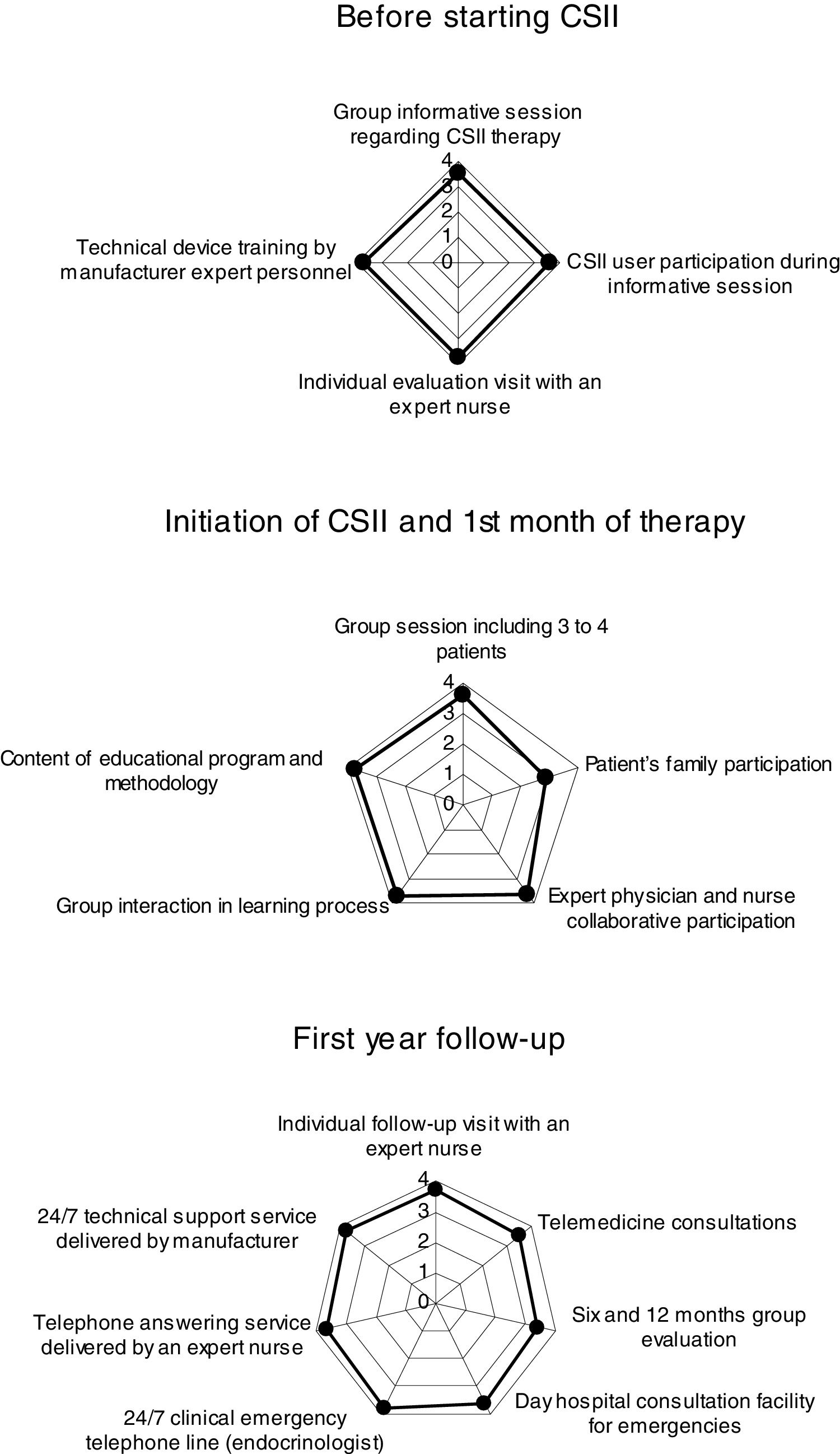
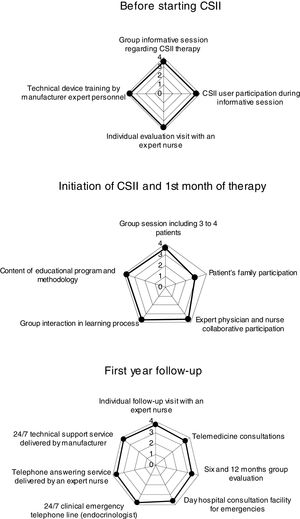
Results of the questionnaire regarding aspects1: before starting CSII,2 initiation of CSII and 1st month of therapy and3 first year follow-up are illustrated in Fig. 1. On average 1 and 3 scored 3.6 and 2 scored 3.5. Related to quality of life, satisfaction with CSII therapy and daily CSII self-management beyond the 1st year of CSII therapy use, 77% of patients scored the CSII self-management education program as very useful and 16% as useful. Ninety three percent of CSII users would not return to previous treatment using multiple doses of insulin. Regarding daily CSII self-management, 79% of patients answered that they use bolus wizard every day. Only 32, 41, 21 and 21% of patients reported that they download pump data in a web based Carelink Personal platform, adjust basal patterns of insulin, modify insulin to carbohydrate ratios and change insulin sensitivity factor every one or two months, respectively. These percentages increased to 43, 36, 54 and 54%, respectively, just before (downloading) or during the visit at Hospital. Thus, patients use to download data more frequently when medical visit approaches.
Results of the questionnaire regarding aspects1: before starting CSII,2 initiation of CSII and 1st month of therapy and3 first year follow-up. In the chart each spoke represents one of the variables. The data length of a spoke is proportional to the magnitude of the variable for the data point relative to the maximum magnitude of the variable across all data points.
The present results demonstrate that some pump usage and adherence parameters may be associated with better glucose control in terms of HbA1c. The evaluation of patient experience is feasible and has shown us that overall user experience with our CSII self-management education and care program was largely and remarkably favorable.
Information regarding the use of CSII and related devices can be precisely obtained after downloading it from specific software.10 This gave to us the opportunity to analyze which features and parameters of insulin pump use are routinely more used by T1D patients and which were associated with better glucose control. Amongst other parameters (usage and adherence) the number of SMBG was found to be independently associated with better metabolic control. This relationship has been found in previous studies and could be considered a crucial adherence factor in order to predict CSII success in terms of effectiveness.11 Shorter time on CSII treatment, a lower BW low/high glucose target at night, a lower total insulin dose per day and higher total number of carbohydrates per day were also independently related to a lower HbA1c. The association of a higher carbohydrate count with better glucose control has been previously described, which probably indicates better skills in diabetes management rather than reflecting real carbohydrate intake.11 It is well known that in the majority of studies evaluating the mid and long term efficacy of CSII there is a gradual deterioration of HbA1c. Target blood glucose represents one of the parameters to set in the BW. This feature of current insulin pumps helps the patient to adjust the bolus insulin dose based on current SMBG measurement, amount of carbohydrate consumed, insulin to carbohydrate ratio, insulin sensitivity factor and target blood glucose. The use of BW has been previously described to be associated with better glucose control in patients with T1D using CSII.11,12 In fact, in our study the proportion of BW over the total boluses was numerically higher in those with better metabolic control (81% of boluses). However, none of the previous studies separately analyzed data concerning BW settings. The amount of boluses per day was also higher in patients with an HbA1c <7.5%. This finding may indicates that CSII more active self-adjustments could be beneficial in terms of metabolic control.11,13
Regarding efficacy, on average, an HbA1c of 7.7%, with a value <7.5% in 43% of patients and 11% of SMBG<70mg/dl could be considered satisfactory in comparison with previous reports of patients with T1D using CSII.14,15
Concerning patient experience and satisfaction evaluation it is noteworthy that we obtained a response to survey from 2 out of 3 patients who received our email invitation. In addition to this, demographic and clinical characteristics, including age, gender, disease duration, time on CSII therapy and reported HbA1c were closely similar to the group of patients in which routine use of CSII was analyzed (only HbA1c was significantly different). Patients who answered the questionnaire were not exactly the same group of patients whose data were downloaded from Carelink, although most of them would have been included in both groups. Thus, this is a limitation, although we still think the results derived from the online survey could apply to both groups of patients.
The response to aspects included in the “before starting CSII” part of the questionnaire was homogenously satisfactory. This part of the program addressed at the shared decision making process before the initiation of CSII could be considered critical from a future global success perspective. Four out of the five aspects included in the second part of the questionnaire, “initiation of CSII and 1st month of therapy” scored above the excellence threshold. However, patient's family participation in the group sessions was considered far from useful. At the moment of writing this manuscript and after discussion within our Diabetes Unit we have not arrived to a consensual explanation for this finding. However, in light of this result the composition of group sessions has to be reconsidered in the future.16 The response to the questions dealing with the aspects included in the “first year follow-up” scored on average >3.6. It should be mentioned that the availability of 24/7 clinical emergency telephone line supervised by an Endocrinologist obtained the highest score. Undoubtedly, this service favors patient's sense of safety using this modality of therapy. This result suggests that the provision of this service by a specific and structured self-management education and care program addressed to patients using CSII would be highly recommended.
More than 90% of patients consider our structured program, at the very least, useful and the more remarkable finding was that almost all the patients with T1D using CSII would not return to previous treatment using multiple insulin injections. Both findings indicate the high perceived quality of the program and the high level of treatment satisfaction provided by CSII therapy. The potential impact on diabetes self-care, emotional reactions to the insulin pump, body image, social acceptance and complexity of the device, have been suggested as possible explanations for the reluctance to initiate CSII therapy.17 Our experience indicates that if the clinical indication exists, the decision to start CSII is shared with the patient and a structured and specific program accompanies the whole process, the reluctance rate to start CSII therapy could be minimized. The results concerning routine daily CSII self-management indicated there is still room for improvement. Few patients routinely adjust basal insulin patterns, modify insulin to carbohydrate ratios, change insulin sensitivity or download pump data in specific, web-based platforms. The management of CSII in a diligently way over time requires knowledge, and expertise and it is time consuming. It may be difficult even for the most compliant patients, thus preferring to do the adjustments with the help of diabetes health provider.
The low rate of regular use of CareLink Personal therapy management web based software for data downloading could be related to some issues raised by patients: software compatibility with different operating systems, continuous and time consuming complementary software updates, driver installation, connectivity, perceived quality of informative report among others. All of them have been previously forwarded to the web provider.16
Our study has limitations. Due to its cross-sectional design, it cannot be used to analyze behavior over a period of time and does not help determine cause and effect. It has been performed in a single center and by a reference Diabetes Unit with a high expertise in the management of CSII therapy. Thus, the findings could not be considered representative for other centers and different clinical scenarios. Several of the analyzed parameters are based on self report (reported carbohydrate intake). Our conclusions regarding the real life use of CSII were drawn from a cohort of patients who have been using this therapy for 10 years, on average (13 months to 19 years). To determine the influence of the time on CSII, a stratified analysis was not conducted. Regarding the strength of the results from the patient experience and satisfaction questionnaire it is possible that mostly those patients with a positive perception of the CSII self-management education and care program answered the questionnaire. However, from our point of view our study includes some strengths. To the best of our knowledge, this is the first large cross-sectional effort trying to combine patient experience with data obtained from routine daily life use of CSII creating an overall picture of the performance of a structured education and care specific program addressed to patients using this modality of insulin therapy.18 As has been previously argued, asking data to patients is not enough and, furthermore, it is unethical to ask and not take action.16 This evaluation of the educational program for CSII users is a good example of a complete cycle of quality: identify the key questions (focus groups), ask the patients (questionnaire) and take action.19
In summary, the analysis of routine clinical use of CSII by T1D patients demonstrates that glucose control may be associated with some pump usage and adherence parameters. The additional evaluation of patient experience and satisfaction is feasible and helps to obtain a comprehensive picture of the performance of our structured education and care specific program which in our case was considered remarkably favorable.
Conflict of interestAll authors have no relevant conflict of interest to disclose related with the content of the manuscript.
We would like to thank the altruistic participation of the patients.