To summarize the data on SARS-CoV-2 seroprevalence surveys conducted in Brazil before the introduction of vaccines
MethodsThe authors conducted a systematic review and meta-analysis on the seroprevalence of SARS-CoV-2 infection in Brazil. The present review followed the PRISMA guidelines. The authors searched Medline, Embase, and LILACS databases for serologic surveys conducted in the Brazilian population, in the period from 01/10/2019 to 07/11/2021, without language restrictions. The authors included studies that presented data concerning SARS-CoV-2 antibodies seroprevalence in Brazil and had a sample size ≥50 individuals. Considering the expected heterogeneity between studies, all analyses were performed using the random effects model, and heterogeneity was assessed using the I2 statistic
ResultsOf 586 publications identified in the initial searches, 54 were included in the review and meta-analysis, which contained the results of 135 surveys, with 336,620 participants. The estimated seroprevalence was 11.0%, ranging from 1.0% to 83.0%, with a substantial heterogeneity (I2 = 99.55%). In subgroup analyses, the authors observed that the prevalence of SARS-CoV-2 antibodies was 13.0% in blood donors, 9.0% in the population-based surveys, 13% in schoolchildren, and 11.0% in healthcare workers.
ConclusionsSeroprevalence increases over time. Large differences were observed among the regions of the country. It was higher in the Northern region, decreasing towards the South. The present results may contribute to the analysis of the spread of SARS-CoV-2 infection in the Brazilian population before vaccination, one of the factors that may be influencing the clinical presentation of COVID-19 cases related to the new variants, as well as the effectiveness of the vaccination program.
The COVID-19 pandemic, caused by SARS-CoV-2 was first reported in Wuhan, China, in December 2019 [1]. It quickly spread globally and constitutes the largest pandemic of the last 100 years. In Brazil, the first case of SARS-CoV-2 was confirmed in late February 2020. In the beginning, transmission was restricted to a few large cities where imported cases were detected, and local transmission was established. In late March and April, the disease spread from these original entry points to the whole country. Serologic surveillance is one of the recommended strategies to monitor the spread of SARS-CoV-2 infection in the population, once asymptomatic and moderate cases may be underreported. Serologic surveys provide additional information regarding the spread of SARS-CoV-2 infection in the population and help to understand the spread of infection in the population and their immunity. This knowledge was of great importance in that period, when vaccine trials were still being carried out and real manufacturing and distribution capacity throughout the world were not in place, and just non-pharmacological measures for prevention and control were available.
In April 2020, serological surveys were started for this purpose. A large national seroprevalence survey was undertaken in Brazil, and several others with restricted geographical coverage or convenience samples were carried out. Until December 2020, several studies were carried out with highly variable estimates of seroprevalence that could largely be due to differences in attack rates, but which also feature heterogeneous sampling strategies and assays used.
So far, there is no study summarizing these surveys in Brazil and so the authors conducted a systematic review and meta-analysis with this objective.
The results of the present study may contribute to the analysis of the spread of SARS-CoV-2 infection in the Brazilian population before vaccination, one of the factors that may be influencing the clinical presentation of COVID-19 cases related to the new variants, as well as the effectiveness of the vaccination program.
MethodsThe authors conducted a systematic review of published articles and a manual search, on the seroprevalence of SARS-CoV-2 infection in Brazil. The present review was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement [2].
Search strategiesThe search was carried out in Medline (through the PubMed platform), Embase and Latin American Literature (Lilacs) databases, without language restrictions.
In Medline, the terms COVID, COVID-19, COV, coronavir*, Sars, SARS-CoV-2, 2019-nCoV, prevalence, cross-sectional study, seroepidemiology, serosurvey, serology, serological survey and Brazil, were used, restricting the surveys to humans and in the period from 01/10/2019 to 07/11/2021 (more details on the search strategy in the Supplementary File 1).
In Embase, the terms 'coronavirus disease 2019′:ti,ab,kw AND (prevalence:ti,ab,kw OR seroprevalence:ti,ab,kw) AND brazil:ti,ab,kw, were used, no time or human restrictions.
In Lilacs, the authors used the terms (“SARS-CoV-2”) or “COVID-19” [Descritor de assunto] and “BRASIL” [Descritor de assunto], no time or human restrictions.
The surveys were carried out with different strategies, such as seroprevalence on representative samples of the population, the country as a whole, states, municipalities, or regions, seroprevalence surveys carried out on samples of specific population groups, and surveys on convenience samples.
Through a manual search of references in selected articles and review articles on the topic, the authors sought to identify other relevant studies missed in the searches. The authors also investigated the websites of Municipal and State Health Departments in search of official reports and, considering that the topic of the review is recent and that many researchers are dedicating themselves to its research, the authors also added articles available to the public, but not reviewed by peers (sites: MedRxiv, BioRxiv, Euro PMC Preprint, BMC, SSRN, Wellcome Open Search). Two authors (SRCC and FT) selected articles, examining titles and abstracts, resulting in a list of potentially relevant sources. After reading the full text of the selected references, the articles were selected for inclusion in the review. Disagreements were resolved by discussion and consensus. Study authors were contacted when data were not clear enough.
Selection of studiesOnly articles/documents that contained original data on the seroprevalence of SARS-CoV-2 infection in Brazil carried out in 2020 and whose sample size was greater than or equal to 50, were included. The authors did not include case reports, case series, review articles, comments, studies whose participants did not live in Brazil, or articles that contained the same data. Regarding the latter studies, the article with the most complete data was included in the present review.
The following definition for SARS-CoV-2 infection was used: the presence of anti-SARS-CoV-2 antibodies IgG and/or IgM to SARS-CoV-2 measured by Enzyme Immunoassay (ELISA) or Chemiluminescent Immunoassay (CLIA test) or rapid tests serological Immunochromatography (ICA).
Data extractionTwo investigators (SRCC, FMT) collected data independently and disagreements were resolved through discussions and consensus. The following data were collected: name of the first author/document title, State of Brazil where the study was carried out, data collection period, sample size, gender, age, race, number of positive individuals for anti-SARS-CoV-2 and diagnostic method for detecting anti-SARS-CoV-2.
Inclusion criteria: Seroprevalence surveys were conducted in Brazil, with a sample ≥50, without other restrictions.
Exclusion criteria: Reports of clinical trials of therapeutic or preventive products, studies without one of the following data: number of participants; the number of participants with reagent results for SARS-CoV-2 antibodies; studies that did not explicitly state their geographic scope; studies that did not explicitly state the laboratory assay that was used for antibodies detection; studies that did not explicitly state the methods for sample selection.
Statistical analysisConsidering the expected heterogeneity between studies, all meta-analyses were performed using the random effects model, which includes variation among studies. Heterogeneity was assessed using the I2 statistic, which describes the percentage of variation among studies that is due more to heterogeneity than to chance [3]. I2 values greater than 25%, 50%, and 75% are considered evidence of mild, moderate to high heterogeneity among studies. Low values of I2 suggest that variability among estimates is compatible with random variation.
To investigate possible causes of heterogeneity among studies, the authors performed a meta-analysis of the following subgroups.
- 1.
Study groups: The surveys were grouped into the following subgroups: population-based surveys with randomly selected samples, blood donors, schoolchildren, and healthcare workers. The surveys addressing other population groups, such as indigenous people, pregnant women, patients with different chronic conditions, self-selected samples, and others, were included just in the main seroprevalence meta-analysis. The authors decided not to compose other subgroups due to the small number of surveys in each category.
- 2.
Studies carried out by trimester (in the 1st, 2nd, 3rd, and 4th trimester).
- 3.
Studies carried out in each region of Brazil.
Potential sources of heterogeneity were also investigated by regression analysis. The objective of which was to report differences in the size of the effect of the study characteristics. The following factors were examined: study group (population-based or not), sample size (continuous variable), and laboratory method for detecting anti-SARS-CoV-2 (rapid test or not.).
To examine the publication bias, the authors used tests proposed by Begg and Mazumdar [4] and Egger et al [5].
The authors performed four sensitivity analyses, considering only studies with:
- 1.
sample size < 100;
- 2.
sample size < 500;
- 3.
Sample size < 1000;
- 4.
Studies published in scientific journals;
- 5.
Studies that used rapid tests (immunochromatography) to detect anti-SARS-CoV-2 antibodies.
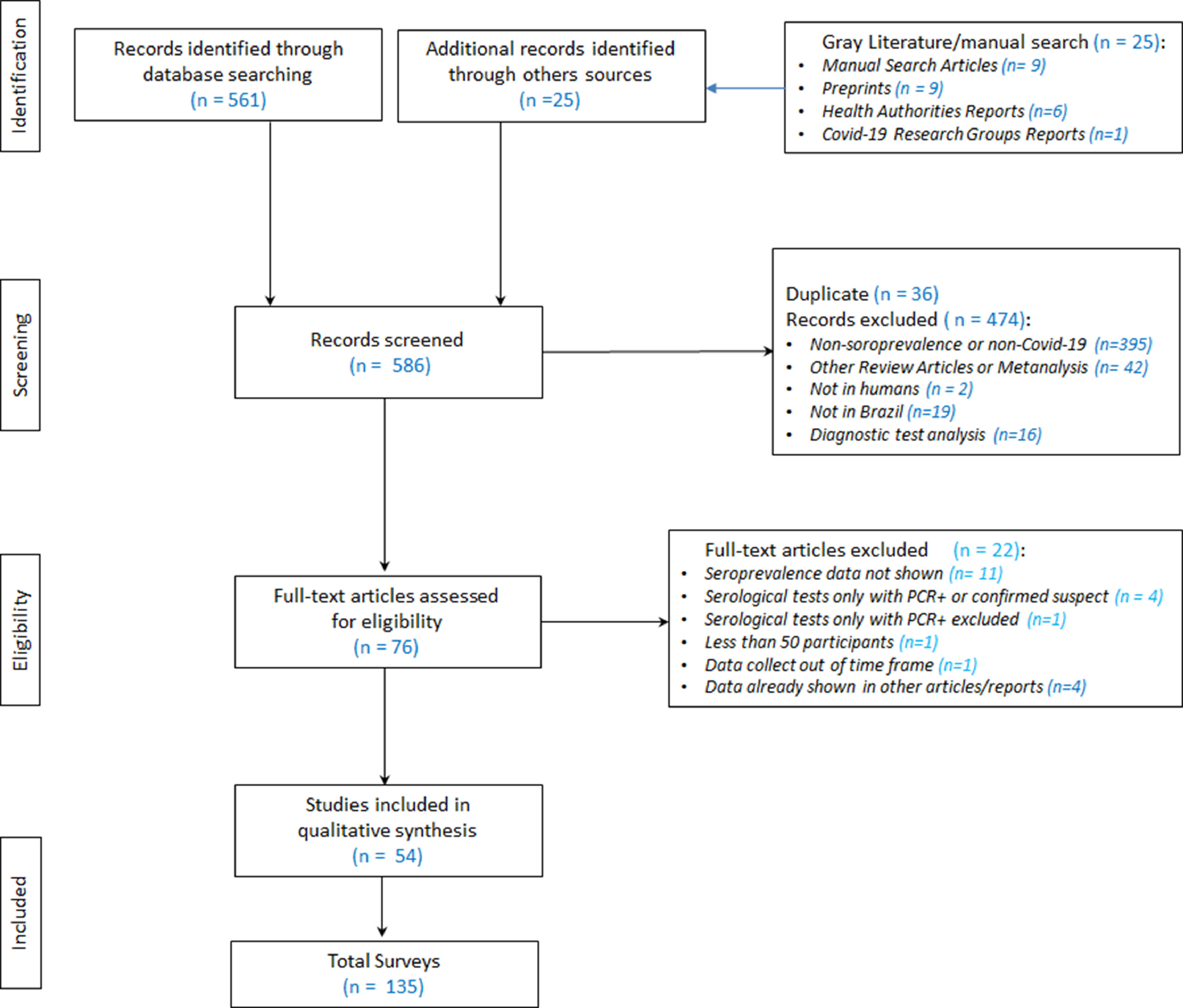
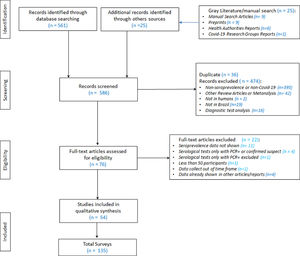
The authors initially identified 586 publications in the databases (MEDLINE, Lilacs and Embase), and in manual searching (Fig. 1Supplementary file 1) After the exclusion of duplicates (36), the authors analyzed 550 references by reading the abstracts. 474 were subsequently excluded, leaving 76 references selected for full-text reading. After reading the full text of the 76 articles, the authors ultimately selected 54 for final inclusion in the review.
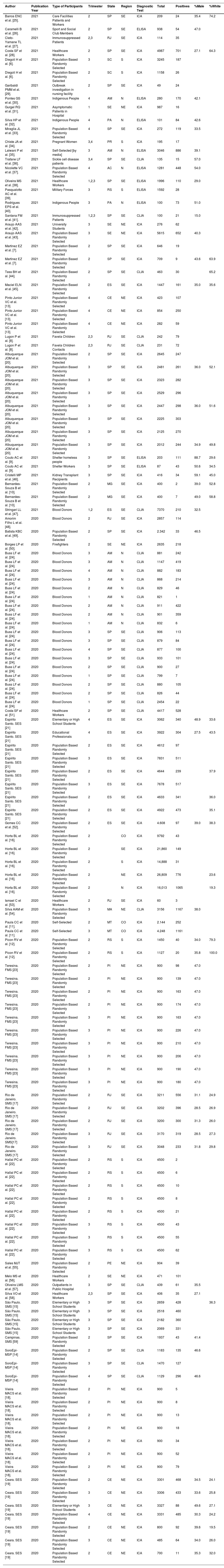
Through the search and selection of articles and/or reports on the prevalence of SARS-CoV-2 infection in Brazil shown in Fig. 1 (Search and Selection Flowchart), the authors identified 54 relevant reference sources for this review, in which 135 serological surveys were identified, with a total of 336,62 participants, about the topic: 7 articles/reports containing two surveys each; [6–12] two articles/reports containing three surveys each [13,14], one report with four surveys [15], two articles containing five surveys each [16,17], two articles/reports containing seven surveys each [18,19], three articles/reports containing eight surveys each [20–22], one report with data from ten surveys [23] and one article with information from 18 surveys [24].
General characteristics of selected surveysThe general characteristics of the selected surveys are shown in Table 1. Of the 135 surveys, 91 (67.4%) were published in the year 2020 and 44 (32.6%) in 2021. Three surveys and studies (2.2%) were carried out in the Central-West region of Brazil, 14 (10.4%) in the North region, 35 (25.9%) in the Northeast region, 15 (11.1%) in the South region and 68 (50.4%) in the Southeast. Data from 5 (3.7%) surveys were collected in the first quarter of 2020, 62 (45.9%) in the second quarter, 46 (34.0%) in the third quarter, 13 (9.6%) in the fourth trimester, and the rest of the survey studies [9] were carried out in more than one quarter, as shown in Table 1. The most frequent surveys were population-based studies (58.5%), in blood donors (14.8%), schoolchildren (4.4%), and health workers (3.7%). The most frequently used diagnostic test for the detection of anti-SARS-CoV-2 was immunochromatography (70.4%), followed by ELISA (22.4%) and CLIA (7.4%).
Estimation of the prevalence of SARS-CoV-2 in studies conducted in Brazil.
| Author | Publication Year | Type of Participants | Trimester | State | Region | Diagnostic Test | Total | Positives | %Male | %White |
|---|---|---|---|---|---|---|---|---|---|---|
| Barros ENC et al. [25]. | 2021 | Care Facilities Patients and Workers | 2 | SP | SE | ICA | 209 | 24 | 35.4 | 74.2 |
| Caramelli B et al. [26]. | 2021 | Sport and Social Club Members | 2 | SP | SE | ELISA | 938 | 54 | 47.0 | |
| Cleto-Yamane TL et al. [27]. | 2021 | Immunosuppressed Patients | 2,3 | RJ | SE | ICA | 114 | 35 | ||
| Costa SF et al. [28]. | 2021 | Healthcare Workers | 2 | SP | SE | ICA | 4987 | 701 | 27.1 | 64.3 |
| Diegoli H et al. [6]. | 2021 | Population Based Randomly Selected | 3 | SC | S | ICA | 3245 | 187 | ||
| Diegoli H et al. [6]. | 2021 | Population Based Randomly Selected | 2 | SC | S | ICA | 1158 | 26 | ||
| Garibaldi PMM et al. [29]. | 2021 | Outbreak investigation in nursing facility | 2 | SP | SE | ICA | 49 | 24 | ||
| Pontes GS et al. [30]. | 2021 | Indigenous People | 4 | AM | N | ELISA | 280 | 170 | 42.1 | |
| Gurgel RQ et al. [31]. | 2021 | Asymptomatic Patients in Hospital | 1 | SE | NE | ICA | 987 | 16 | ||
| Silva HP et al. [32]. | 2021 | Indigenous People | 4 | PA | N | ELISA | 101 | 84 | 42.6 | |
| Miraglia JL et al. [33]. | 2021 | Population Based Randomly Selected | 4 | SP | SE | ICA | 272 | 119 | 33.5 | |
| Chiste JA et al. [34]. | 2021 | Pregnant Women | 3,4 | PR | S | ICA | 195 | 17 | ||
| Lalwani P et al. [35]. | 2021 | Self-Selected [by media] | 3 | AM | N | ELISA | 3046 | 886 | 39.1 | |
| Trafane LF et al. [36]. | 2021 | Sickle cell disease patients | 3,4 | SP | SE | CLIA | 135 | 15 | 57.0 | |
| Nicolette VC et al. [37]. | 2021 | Population Based Randomly Selected | 4 | AC | N | ELISA | 1281 | 448 | 54.0 | |
| Oliveira MS et al. [38]. | 2021 | Healthcare Workers | 1,2,3 | SP | SE | ELISA | 1996 | 110 | 29.0 | |
| Pasqualotto AC et al. [39]. | 2021 | Military Forces | 3 | RS | S | ELISA | 1592 | 28 | ||
| Rodrigues EPS et al. [40]. | 2021 | Indigenous People | 3 | PA | N | ELISA | 100 | 73 | 51.0 | |
| Santana FM et al. [41]. | 2021 | Immunosuppressed Patients | 1,2,3 | SP | SE | CLIA | 100 | 21 | 15.0 | |
| Araujo AAS et al. [42]. | 2021 | University Students | 3 | SE | NE | ICA | 276 | 62 | ||
| Araujo AAS et al. [43]. | 2021 | Population Based Randomly Selected | 3 | SE | NE | ICA | 5615 | 652 | 40.3 | |
| Martinez EZ et al. [7]. | 2021 | Population Based Randomly Selected | 2 | SP | SE | ICA | 646 | 19 | ||
| Martinez EZ et al. [7]. | 2021 | Population Based Randomly Selected | 2 | SP | SE | ICA | 709 | 9 | 43.6 | 63.9 |
| Tess BH et al. [44]. | 2021 | Population Based Randomly Selected | 2 | SP | SE | CLIA | 463 | 30 | 65.2 | |
| Maciel ELN et al. [45]. | 2021 | Population Based Randomly Selected | 2 | ES | SE | ICA | 1447 | 161 | 35.0 | 35.6 |
| Pinto Junior VC et al. [13]. | 2021 | Population Based Randomly Selected | 4 | CE | NE | ICA | 423 | 107 | ||
| Pinto Junior VC et al. [13]. | 2021 | Population Based Randomly Selected | 4 | CE | NE | ICA | 854 | 250 | ||
| Pinto Junior VC et al. [13]. | 2021 | Population Based Randomly Selected | 4 | CE | NE | ICA | 282 | 59 | ||
| Lugon P et al. [8]. | 2021 | Favela Children | 2,3 | RJ | SE | CLIA | 242 | 79 | ||
| Lugon P et al. [8]. | 2021 | Favela Children Contacts | 2,3 | RJ | SE | CLIA | 231 | 72 | ||
| Albuquerque JOM et al. [20]. | 2021 | Population Based Randomly Selected | 2 | SP | SE | ICA | 2645 | 247 | ||
| Albuquerque JOM et al. [20]. | 2021 | Population Based Randomly Selected | 3 | SP | SE | ICA | 2481 | 261 | 36.0 | 52.1 |
| Albuquerque JOM et al. [20]. | 2021 | Population Based Randomly Selected | 3 | SP | SE | ICA | 2323 | 282 | ||
| Albuquerque JOM et al. [20]. | 2021 | Population Based Randomly Selected | 3 | SP | SE | ICA | 2529 | 296 | ||
| Albuquerque JOM et al. [20]. | 2021 | Population Based Randomly Selected | 3 | SP | SE | ICA | 2447 | 298 | 36.0 | 51.6 |
| Albuquerque JOM et al. [20]. | 2021 | Population Based Randomly Selected | 3 | SP | SE | ICA | 2225 | 303 | ||
| Albuquerque JOM et al. [20]. | 2021 | Population Based Randomly Selected | 3 | SP | SE | ICA | 2125 | 270 | ||
| Albuquerque JOM et al. [20]. | 2021 | Population Based Randomly Selected | 3 | SP | SE | ICA | 2012 | 244 | 34.9 | 49.8 |
| Couto AC et al. [9]. | 2021 | Shelter homeless people | 3 | SP | SE | ELISA | 203 | 111 | 88.7 | 29.6 |
| Couto AC et al. [9]. | 2021 | Shelter Workers | 3 | SP | SE | ELISA | 87 | 43 | 50.6 | 34.5 |
| Cristelli MP et al. [46]. | 2021 | Kidney Transplant Recipients | 3 | SP | SE | ICA | 416 | 34 | 59.1 | 45.0 |
| Bernardes-Souza B et al. [10]. | 2021 | Population Based Randomly Selected | 2 | MG | SE | ICA | 400 | 2 | 39.0 | 52.8 |
| Bernardes-Souza B et al. [10]. | 2021 | Population Based Randomly Selected | 2 | MG | SE | ICA | 400 | 7 | 49.0 | 58.8 |
| Stringari LL et al. [47]. | 2021 | Blood Donors | 1,2 | ES | SE | CLIA | 7370 | 210 | 32.5 | |
| Amorim Filho L et al. [48]. | 2020 | Blood Donors | 2 | RJ | SE | ICA | 2857 | 114 | ||
| Batista KBC et al. [49]. | 2020 | Population Based Randomly Selected | 2 | SP | SE | ICA | 2.342 | 33 | 46.5 | |
| Borges LP et al. [50]. | 2020 | Firefighters | 2 | SE | NE | ICA | 2635 | 218 | ||
| Buss LF et al. [24]. | 2020 | Blood Donors | 3 | AM | N | CLIA | 881 | 242 | ||
| Buss LF et al. [24]. | 2020 | Blood Donors | 3 | AM | N | CLIA | 1147 | 419 | ||
| Buss LF et al. [24]. | 2020 | Blood Donors | 4 | AM | N | CLIA | 882 | 183 | ||
| Buss LF et al. [24]. | 2020 | Blood Donors | 3 | AM | N | CLIA | 868 | 214 | ||
| Buss LF et al. [24]. | 2020 | Blood Donors | 2 | AM | N | CLIA | 829 | 46 | ||
| Buss LF et al. [24]. | 2020 | Blood Donors | 1 | AM | N | CLIA | 821 | 1 | ||
| Buss LF et al. [24]. | 2020 | Blood Donors | 2 | AM | N | CLIA | 911 | 422 | ||
| Buss LF et al. [24]. | 2020 | Blood Donors | 2 | AM | N | CLIA | 901 | 359 | ||
| Buss LF et al. [24]. | 2020 | Blood Donors | 1 | AM | N | CLIA | 832 | 6 | ||
| Buss LF et al. [24]. | 2020 | Blood Donors | 3 | SP | SE | CLIA | 906 | 113 | ||
| Buss LF et al. [24]. | 2020 | Blood Donors | 3 | SP | SE | CLIA | 879 | 84 | ||
| Buss LF et al. [24]. | 2020 | Blood Donors | 4 | SP | SE | CLIA | 877 | 100 | ||
| Buss LF et al. [24]. | 2020 | Blood Donors | 3 | SP | SE | CLIA | 933 | 101 | ||
| Buss LF et al. [24]. | 2020 | Blood Donors | 2 | SP | SE | CLIA | 900 | 27 | ||
| Buss LF et al. [24]. | 2020 | Blood Donors | 1 | SP | SE | CLIA | 799 | 7 | ||
| Buss LF et al. [24]. | 2020 | Blood Donors | 2 | SP | SE | CLIA | 880 | 105 | ||
| Buss LF et al. [24]. | 2020 | Blood Donors | 2 | SP | SE | CLIA | 826 | 44 | ||
| Buss LF et al. [24]. | 2020 | Blood Donors | 1 | SP | SE | CLIA | 2454 | 22 | ||
| Costa SF et al. [51]. | 2020 | Healthcare Workers | 2 | SP | SE | CLIA | 4417 | 528 | ||
| Espírito Santo. SES [21] | 2020 | Elementary or High School Students | 4 | ES | SE | ICA | 3062 | 340 | 48.9 | 33.6 |
| Espírito Santo. SES [21] | 2020 | Educational Professionals | 4 | ES | SE | ICA | 3922 | 304 | 27.5 | 43.5 |
| Espírito Santo. SES [21] | 2020 | Population Based Randomly Selected | 2 | ES | SE | ICA | 4612 | 97 | ||
| Espírito Santo. SES [21] | 2020 | Population Based Randomly Selected | 3 | ES | SE | ICA | 7831 | 511 | ||
| Espírito Santo. SES [21] | 2020 | Population Based Randomly Selected | 2 | ES | SE | ICA | 4644 | 239 | 37.9 | |
| Espírito Santo. SES [21] | 2020 | Population Based Randomly Selected | 3 | ES | SE | ICA | 7678 | 517 | ||
| Espírito Santo. SES [21] | 2020 | Population Based Randomly Selected | 2 | ES | SE | ICA | 4633 | 341 | 36.0 | |
| Espírito Santo. SES [21] | 2020 | Population Based Randomly Selected | 2 | ES | SE | ICA | 4922 | 473 | 35.1 | |
| Gomes CC et al. [52]. | 2020 | Population Based Randomly Selected | 2 | ES | SE | ICA | 4.608 | 97 | 39.0 | 38.3 |
| Horta BL et al. [16]. | 2020 | Population Based Randomly Selected | 2 | CO | ICA | 9792 | 43 | |||
| Horta BL et al. [16]. | 2020 | Population Based Randomly Selected | 2 | SE | ICA | 21,860 | 149 | |||
| Horta BL et al. [16]. | 2020 | Population Based Randomly Selected | 2 | S | ICA | 14,888 | 31 | |||
| Horta BL et al. [16]. | 2020 | Population Based Randomly Selected | 2 | NE | ICA | 26,809 | 776 | 23.6 | ||
| Horta BL et al. [16]. | 2020 | Population Based Randomly Selected | 2 | N | ICA | 16,013 | 1065 | 19.3 | ||
| Ismael C et al. [53]. | 2020 | Healthcare Workers | 2 | RJ | SE | ICA | 60 | 3 | ||
| Silva AAM et al. [54]. | 2020 | Population Based Randomly Selected | 3 | MA | NE | CLIA | 3156 | 1167 | 38.0 | |
| Paula CC et al. [11]. | 2020 | Self-Selected | 2 | MT | CO | ICA | 2.144 | 252 | ||
| Paula CC et al. [11]. | 2020 | Self-Selected | 3 | MT | CO | ICA | 4.248 | 1161 | ||
| Picon RV et al. [12]. | 2020 | Population Based Randomly Selected | 2 | RS | S | ICA | 1450 | 40 | 34.0 | 79.3 |
| Picon RV et al. [12]. | 2020 | Population Based Randomly Selected | 2 | RS | S | ICA | 1127 | 20 | 35.8 | 100.0 |
| Teresina. FMS [23] | 2020 | Population Based Randomly Selected | 2 | PI | NE | ICA | 900 | 98 | 47.0 | |
| Teresina. FMS [23] | 2020 | Population Based Randomly Selected | 2 | PI | NE | ICA | 900 | 139 | 47.0 | |
| Teresina. FMS [23] | 2020 | Population Based Randomly Selected | 2 | PI | NE | ICA | 900 | 163 | 47.0 | |
| Teresina. FMS [23] | 2020 | Population Based Randomly Selected | 2 | PI | NE | ICA | 900 | 174 | 47.0 | |
| Teresina. FMS [23] | 2020 | Population Based Randomly Selected | 3 | PI | NE | ICA | 900 | 163 | 47.0 | |
| Teresina. FMS [23] | 2020 | Population Based Randomly Selected | 3 | PI | NE | ICA | 900 | 226 | 47.0 | |
| Teresina. FMS [23] | 2020 | Population Based Randomly Selected | 3 | PI | NE | ICA | 900 | 210 | 47.0 | |
| Teresina. FMS [23] | 2020 | Population Based Randomly Selected | 3 | PI | NE | ICA | 900 | 206 | 47.0 | |
| Teresina. FMS [23] | 2020 | Population Based Randomly Selected | 3 | PI | NE | ICA | 900 | 190 | 47.0 | |
| Teresina. FMS [23] | 2020 | Population Based Randomly Selected | 3 | PI | NE | ICA | 900 | 180 | 47.0 | |
| Rio de Janeiro. SMS [17] | 2020 | Population Based Randomly Selected | 2 | RJ | SE | ICA | 3211 | 556 | 31.1 | 24.9 |
| Rio de Janeiro. SMS [17] | 2020 | Population Based Randomly Selected | 2 | RJ | SE | ICA | 3202 | 396 | 28.5 | 26.9 |
| Rio de Janeiro. SMS [17] | 2020 | Population Based Randomly Selected | 2 | RJ | SE | ICA | 3200 | 300 | 31.3 | 26.0 |
| Rio de Janeiro. SMS[17] | 2020 | Population Based Randomly Selected | 3 | RJ | SE | ICA | 3170 | 319 | 28.5 | 27.3 |
| Rio de Janeiro. SMS [17] | 2020 | Population Based Randomly Selected | 3 | RJ | SE | ICA | 3048 | 233 | 31.8 | 29.8 |
| Hallal PC et al. [22]. | 2020 | Population Based Randomly Selected | 2 | RS | S | ICA | 4500 | 2 | ||
| Hallal PC et al. [22]. | 2020 | Population Based Randomly Selected | 2 | RS | S | ICA | 4500 | 6 | ||
| Hallal PC et al. [22]. | 2020 | Population Based Randomly Selected | 2 | RS | S | ICA | 4500 | 10 | ||
| Hallal PC et al. [22]. | 2020 | Population Based Randomly Selected | 2 | RS | S | ICA | 4500 | 8 | ||
| Hallal PC et al. [22]. | 2020 | Population Based Randomly Selected | 2 | RS | S | ICA | 4500 | 21 | ||
| Hallal PC et al. [22]. | 2020 | Population Based Randomly Selected | 3 | RS | S | ICA | 4500 | 43 | ||
| Hallal PC et al. [22]. | 2020 | Population Based Randomly Selected | 3 | RS | S | ICA | 4500 | 55 | ||
| Hallal PC et al. [22]. | 2020 | Population Based Randomly Selected | 3 | RS | S | ICA | 4500 | 62 | ||
| Sales MJT et al. [55]. | 2020 | Population Based Randomly Selected | 2 | PE | NE | ICA | 904 | 39 | ||
| Melo MS et al. [56]. | 2020 | Healthcare Workers | 2 | SE | NE | ICA | 471 | 101 | ||
| Oliveira LMS et al. [57]. | 2020 | Outpatients in Public Hospital | 3 | SP | SE | CLIA | 439 | 61 | 35.5 | |
| Silva VO et al. [58]. | 2020 | Healthcare Workers | 2,3 | SP | SE | ICA | 406 | 35 | 27.1 | |
| São Paulo. SMS [15] | 2020 | Elementary or High School Students | 3 | SP | SE | ICA | 2659 | 428 | 36.3 | |
| São Paulo. SMS [15] | 2020 | Elementary or High School Students | 3 | SP | SE | ICA | 2518 | 460 | ||
| São Paulo. SMS [15] | 2020 | Elementary or High School Students | 3 | SP | SE | ICA | 2182 | 360 | ||
| São Paulo. SMS [15] | 2020 | Elementary or High School Students | 3 | SP | SE | ICA | 2069 | 331 | ||
| Campinas. SMS [59] | 2020 | Population Based Randomly Selected | 2 | SP | SE | ICA | 1937 | 43 | 41.4 | |
| SoroEpi-MSP [14] | 2020 | Population Based Randomly Selected | 2 | SP | SE | CLIA | 1183 | 135 | 46.6 | |
| SoroEpi-MSP [14] | 2020 | Population Based Randomly Selected | 3 | SP | SE | CLIA | 1470 | 127 | ||
| SoroEpi-MSP [14] | 2020 | Population Based Randomly Selected | 4 | SP | SE | CLIA | 1129 | 296 | 46.6 | |
| Vieira MACS et al. [18]. | 2020 | Population Based Randomly Selected | 2 | PI | NE | ICA | 900 | 5 | ||
| Vieira MACS et al. [18]. | 2020 | Population Based Randomly Selected | 2 | PI | NE | ICA | 900 | 8 | ||
| Vieira MACS et al. [18]. | 2020 | Population Based Randomly Selected | 2 | PI | NE | ICA | 900 | 13 | ||
| Vieira MACS et al. [18]. | 2020 | Population Based Randomly Selected | 2 | PI | NE | ICA | 900 | 18 | ||
| Vieira MACS et al. [18]. | 2020 | Population Based Randomly Selected | 2 | PI | NE | ICA | 900 | 34 | ||
| Vieira MACS et al. [18]. | 2020 | Population Based Randomly Selected | 2 | PI | NE | ICA | 900 | 52 | ||
| Vieira MACS et al. [18]. | 2020 | Population Based Randomly Selected | 2 | PI | NE | ICA | 900 | 79 | ||
| Ceara. SES [19] | 2020 | Population Based Randomly Selected | 2 | CE | NE | ICA | 3301 | 468 | 34.5 | 24.1 |
| Ceara. SES [19] | 2020 | Population Based Randomly Selected | 3 | CE | NE | ICA | 3306 | 433 | 33.6 | 25.8 |
| Ceara. SES [19] | 2020 | Elementary or High School Students | 3 | CE | NE | ICA | 3327 | 88 | 49.6 | 27.1 |
| Ceara. SES [19] | 2020 | Population Based Randomly Selected | 4 | CE | NE | ICA | 3331 | 485 | 30.3 | 24.2 |
| Ceara. SES [19] | 2020 | Population Based Randomly Selected | 2 | CE | NE | ICA | 800 | 92 | 39.6 | 19.5 |
| Ceara. SES [19] | 2020 | Population Based Randomly Selected | 3 | CE | NE | ICA | 485 | 64 | 34.0 | 26.0 |
| Ceara. SES [19] | 2020 | Population Based Randomly Selected | 2 | CE | NE | ICA | 700 | 11 | 35.3 | 32.0 |
Notes: ICA, Immunocromatography Assays; ELISA, Enzyme-Linked Immunosorbent Assay; CLIA, Chemiluminescence Immunoassay.
- a)
General: The authors found a general estimated prevalence of 11.0% (95% CI 11.0‒12.0), ranging from 1.0% (95% CI 0.0‒1.0) to 83.0% (95% CI 75.0‒89.0), with substantial heterogeneity (I2 = 99.55%) (Supplementary File 2).
- b)
Subgroups:
- i.
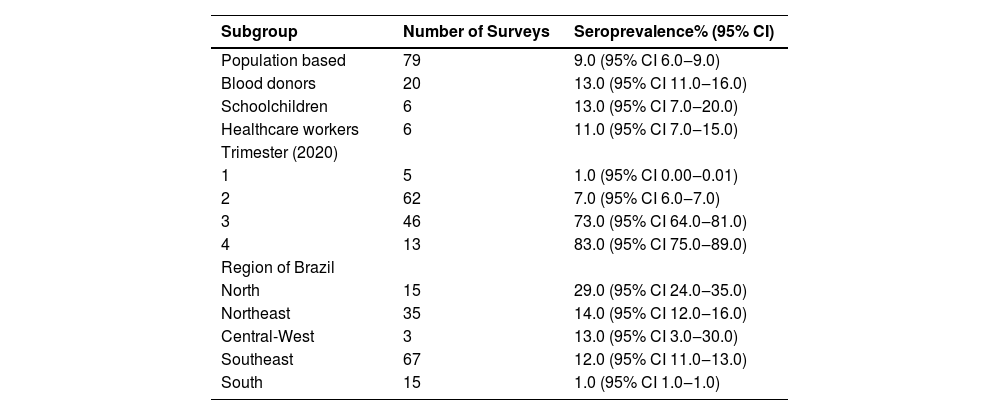
In population-based surveys (Table 2) the estimated prevalence was 9.0% (95% CI 6.0%‒9.0%), in blood donors it was 13.0% (95% CI 11.0‒13.0), in schoolchildren it was 13.0% (95% CI 7.0‒20.0) and in health workers it was 11.0% (95% CI 7.0‒15.0) (more details in the Supplementary File 3, 4, 5, 6).
Table 2.Seroprevalence of anti-SARS-CoV-2 antibodies in Brazil, in selected subgroup [9]..
- ii.
Yet, in Table 2, the authors can see the estimates of anti-SARS-CoV-2 prevalence, separating the surveys according to the period (a quarter of 2020) in which the data were collected. The highest prevalence was observed in the 3rd and 4th trimesters (73.0% and 83.0%, respectively).
- iii.
Finally, analyzing by regions of Brazil, the North region had the highest prevalence rate (Table 2) 32.0% (followed by the Northeast (13.0%), Central-Western (13.0%), Southeast (12.0%) and, finally, the South region (1.0%).
- c)
Sensitivity analysis:
- iv.
Excluding surveys with sample sizes ≤ 100, ≤ 500 and ≤ 1000, the prevalence estimates found was 11.0% (95% CI 11.0‒12.0), 10.0% (9.0‒12.0), 11.0% and 9.0% (9.0‒10.0), respectively. Supplementary File 7, 8, 9).
- v.
The estimated prevalence of published and peer-reviewed surveys was 10.0% (10.0–11.0) Supplementary File 10.
- lowerRoman%1
When analyzing just the surveys that used the rapid test to detect the anti-SARS-CoV-2 antibody (immunochromatography), the authors observed a prevalence of 9.0% (95% CI 9.0‒10.0). Supplementary File 11.
- d)
Others analysis:
- vi.
The estimated prevalence of -SARS-CoV-2 antibodies in male participants was 18.0% (95% CI 17.0‒20.0) and 22.0% (95% CI 20.0‒25.0) in females; in white participants it was 8.0% (7.0‒9.0) and in non-white participants, it was 11.0% (9.0‒13.0). It should be noted that the number of articles that presented these variables was small.
- vii.
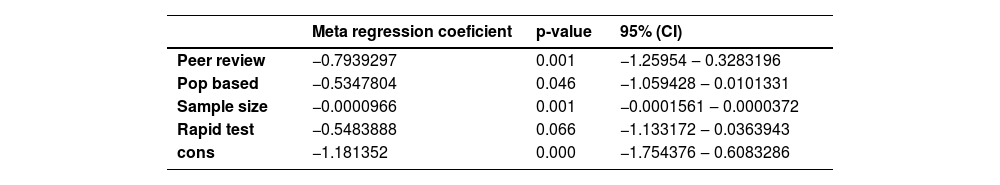
Meta-regression: The authors tested the variables “sample size” (continuous variable), rapid test or not, population-based study or not, and whether published after peer review or not. The first 3 variables showed a significant contribution to the outcome (Table 3).
- viii.
There was evidence of bias using the Egger (p = 0.000) and Begg (p = 0.001) tests.
- e)
Additional studies:
Additional studies of interest included in this review, as the authors identified at least two surveys in these groups, were in indigenous populations, in immunosuppressed patients (i.e., diagnosed with cancer or undergoing solid organ transplantation), and people who self-requested the test for diagnose SARS-CoV-2 infection, as shown in Table 1. The prevalence of SARS-CoV-2 antibodies in indigenous populations ranged from 60.71% to 83.17%; in immunosuppressed patients, the variation was from 8.17% to 30.70%, and in people who self-requested the test, the variation was from 11.75% to 27.33%.
DiscussionThe authors systematically reviewed seroprevalence studies of SARS-CoV-2 antibodies conducted in Brazil and identified fifty-four studies from all Brazilian states. The present review indicated that the overall seroprevalence of SARS-CoV-2 in Brazil was 11.0% (95% CI 11.0‒12.0) and the heterogeneity among the studies was substantial (99.54%%). In subgroup analyses, the authors observed that the prevalence of SARS-CoV-2 antibodies was 13.0% in blood donors, 9.0% in the population-based surveys, 13% in schoolchildren, and 9.0% in the studies that used the commercial immunochromatographic assays to identify the presence of anti-SARS-CoV-2 antibodies.
As expected, seroprevalence increased over time, from very low figures in the first trimester of 2020, to high proportions in the second half of the year. Seroprevalence in Brazil followed similar trends as observed in other countries, such as Spain, where the prevalence was estimated at 5% after the first epidemic wave [60], and the United States, 3.5% among blood donors [61,62], in July 2020. A large increase in the proportion of infected people during 2020 was also observed, both in developed countries, such as the United States and in developing countries, such as India. In the first, the seroprevalence among blood donors rose to 83.3% in May 2021, when combining natural infection and vaccine-induced seroconversion (Jones et al. 2020). In the latter, seroprevalence in the general population was 0.73% in May‒June and increased to 24.1% in December 2020 [63].
Large differences in seroprevalence were observed amongst the different regions of Brazil. Seroprevalence increased from the South to the Northern region, where the Amazon rainforest is located. This region was particularly hit by the second pandemic wave, being the probable emergence of the gama variant of SARS-CoV-2 in Brazil [64], more transmissible than the previous ones.
The Northern Region of Brazil showed high seroprevalence already in the first seroepidemiological surveys, carried out in the second quarter of 2020. In the first national survey, whose data collection was carried out in May 2020, nine of the ten municipalities with the highest seroprevalence in the country were in this region [16]. In the city of Manaus, one of the largest metropolises in the Amazon region, the seroprevalence among blood donors reached values above 40% in the same period. [24] At the other extreme, the Central-West region had a lower seroprevalence, even considering that this region had a lower number of surveys carried out, compared to other regions. The Northeast region, which includes nine states in the country, ranked second in terms of seroprevalence, followed by the Southeast region. Necessary care when interpreting aggregate seroprevalence estimates is that some regions, and within them, some states, carried out a much larger number of surveys than others, and may be overrepresented in the analysis.
Some surveys pointed out a high seroprevalence among the Indigenous peoples, with the Amazon region being the one that concentrates the largest number of indigenous people in the country. Unfortunately, the number of surveys in which data were stratified by skin color/ethnicity was small, which made it impossible to calculate an estimate of seroprevalence according to this variable.
Part of the differences observed in seroprevalence may be related to the type of assay used in the surveys. In the first months of the pandemic, only immunochromatographic assays were available in Brazil. These assays have lower sensitivity than the enzyme immunoassay and chemiluminescence methods [65], Its sensitivity also depends on the type of sample collected, and its performance is worse in samples collected by finger prick. These assays, with this type of biological sample, were the most used in Brazil during the first months of the pandemic, which may have contributed to the underestimation of seroprevalence, although the Northern Region of Brazil showed high seroprevalence already in the first seroepidemiological surveys.
Although the authors detected considerable heterogeneity and publication bias, we could observe that the available data are robust, even including only surveys with a sample size greater than one hundred or only greater than 500; the same would happen if the authors included only surveys published in peer-reviewed scientific journals, with overlapping confidence intervals of prevalence estimates in these cases.
This is the first systematic review of the seroprevalence of SARS-CoV-2 carried out in Brazil before the implementation of the vaccine in the country, which started in January 2021. It was intended to present this methodology of high robustness in an unprecedented infection in the world, with Brazil presenting a very high disease burden. The study also presented the spread of the infection and in which scenarios the effectiveness of vaccination in the country could be estimated.
Authors’ contributionsGerusa M. Figueiredo: Conceptualization; writing original draft; writing review & editing.
Fátima M. Tengan: Conceptualization; writing, original draft; writing review & editing, selected the eligible studies by reading the titles and abstracts, and a list of potentially relevant studies was generated.
Expedito J.A. Luna: Conceptualization; writing original draft; writing review & editing.
Sergio R. Campos: Writing review & editing, selected the eligible studies by reading the titles and abstracts, and a list of potentially relevant studies were generated.
Jadher Percio.