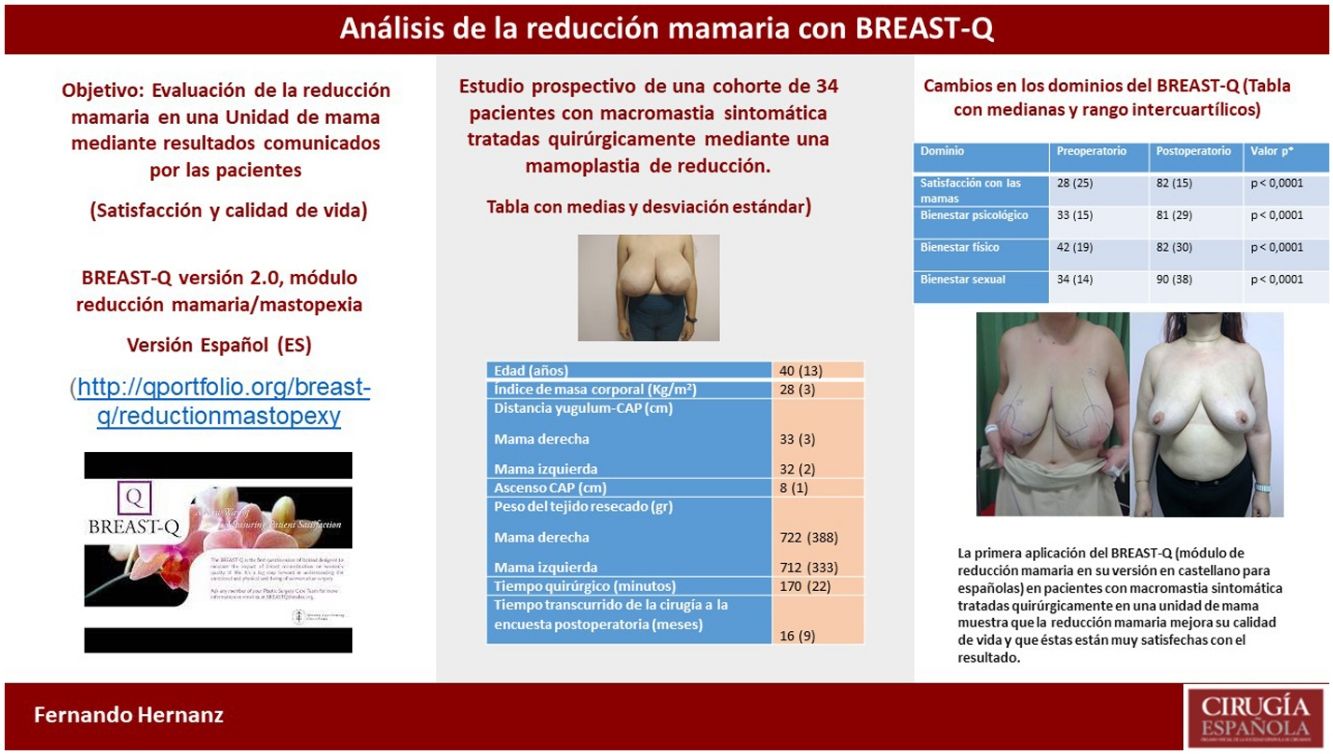
The BREAST-Q (breast reduction module) is a specific and validated questionnaire to evaluate breast reduction in the treatment of symptomatic macromastia, offering information on their quality of life and degree of satisfaction.
MethodsProspective study of a cohort of 34 patients treated by bilateral breast reduction in a breast unit in 2017–2020 surveyed with the Spanish version of BREAST-Q version 2. The statistical study to assess the effect of reduction, changes from the pre to postoperative scores of the domains were performed using the Wilcoxon signed rank test. Statistical significance was determined with p values <0.05.
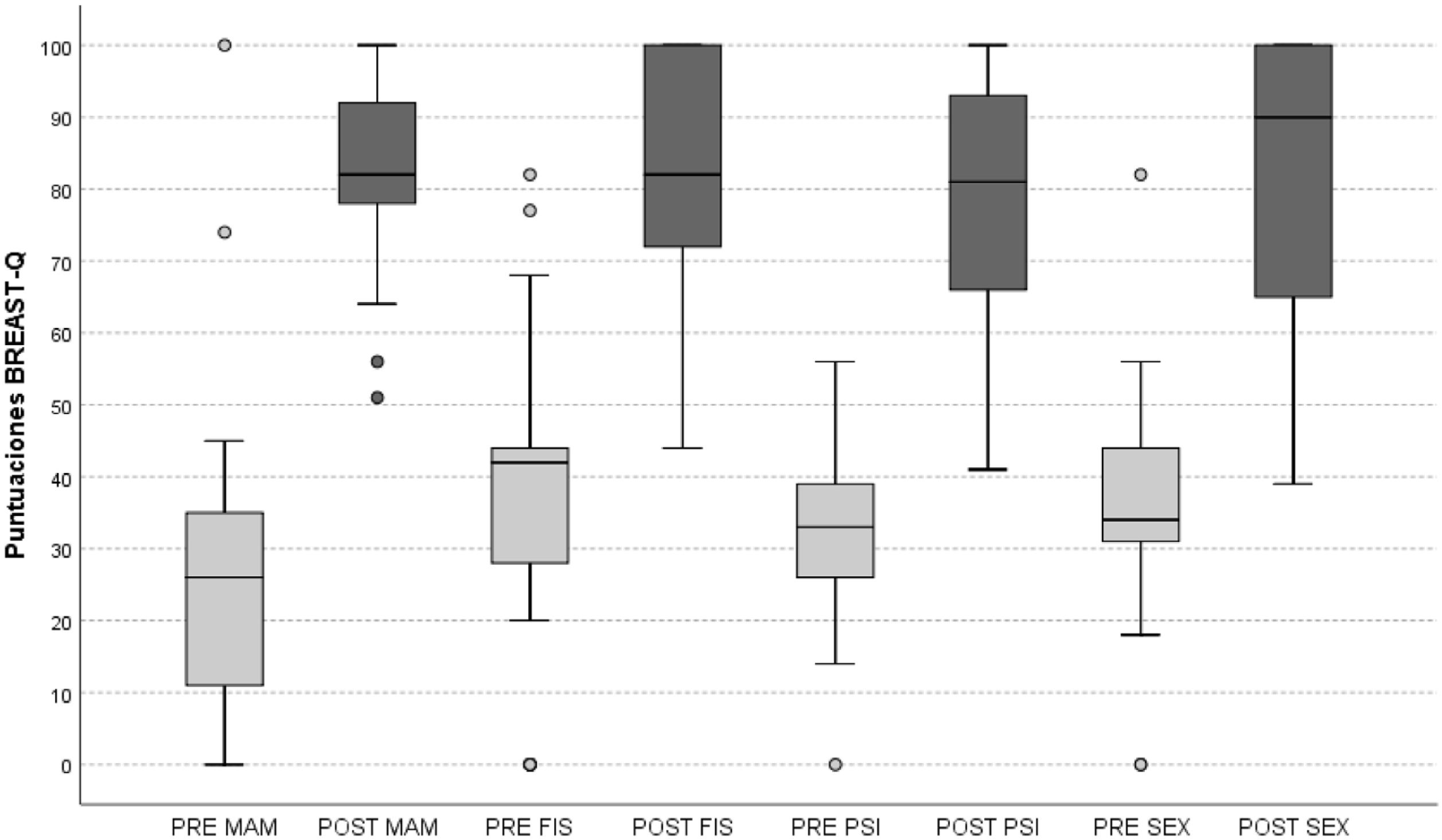
ResultsThe mean time elapsed from surgery to the postoperative survey was 16 (SD 9) months. Post-surgical complications or sequelae occurred in 14 (42%) patients with 23 events. The preoperative scores, median and interquartile range, in satisfaction with the breasts (28, 26), psychological (33, 14), physical (42, 19) and sexual (34, 14) well-being improved in the postoperative survey to (82, 15), (81.29), (82, 30) and (90, 38), respectively. These changes were statistically significant, p < 0.001.
ConclusionsThe first application of the BREAST-Q in its version in Spanish for Spanish women in patients with symptomatic macromastia treated surgically in a breast unit shows that breast reduction improves the quality of life of patients and that they are very satisfied with the outcome of the surgery and its surgeon, although the information received should clearly be improved.
El BREAST-Q (módulo reducción mamaria) es un cuestionario específico y validado para evaluar la reducción mamaria en el tratamiento de la macromastia sintomática ofreciendo información sobre la calidad de vida y grado de satisfacción de las pacientes.
MétodosEstudio prospectivo de una cohorte de 34 pacientes tratadas mediante reducción mamaria bilateral, en una unidad de mama en 2017 a 2020, que fueron encuestadas con la versión adaptada al castellano del BREAST-Q. Las pacientes cumplimentaron el cuestionario en el mes previo a la cirugía y después de ésta. Los cambios de las puntuaciones pre y postoperatorias en los diferentes dominios, se analizaron mediante la prueba de rangos con signo de Wilcoxon. La significación estadística fue determinada con valores de p < 0,05.
ResultadosEl tiempo medio desde la cirugía a la encuesta postoperatoria fue 16 (SD 9) meses. Complicaciones o secuelas postquirúrgicas sucedieron en 14 (42%) pacientes con 23 eventos. Las puntuaciones preoperatorias, medianas y rango intercuartílico, en la satisfacción con las mamas (28, 26), bienestar psicológico (33, 14), físico (42, 19) y sexual (34, 14) mejoraron en la encuesta postoperatoria a (82, 15), (81, 29), (82, 30) y (90, 38), respectivamente, con significación estadística, p < 0,001.
ConclusionesLa primera aplicación del BREAST-Q versión en castellano para españolas a pacientes con macromastia tratadas quirúrgicamente en una unidad de mama demuestra que la reducción mamaria mejora la calidad de vida de las pacientes y, que éstas están muy satisfechas con el resultado de la cirugía y su cirujano, aunque la información recibida es mejorable.
The incorporation of breast reduction into a breast unit can facilitate training in oncoplastic breast cancer surgery, as well as offering an effective treatment for patients with symptomatic macromastia that is usually insufficiently offered to meet demand.1 The evaluation of our initial experience in the treatment of symptomatic macromastia showed good results (physical symptoms disappeared or improved significantly in 88% of patients and the degree of satisfaction with the care process and the overall outcome was really high), although the evaluation was made using a non-validated survey.2
In recent years there has been an interest in placing patients at the centre of health care research and evaluation in order to improve and ensure that health care is robust and of maximum value for the use of medicines, products, therapies or health services. Patient-reported outcomes (PROs) are reported directly by the patient without interpretation of the response by a physician or anyone else with reference to their health, quality of life or functional status associated with healthcare or treatment.3 In 2009, A. Pusic published the development of a questionnaire, the BREAST-Q, for measuring the satisfaction and quality of life of patients undergoing different breast surgery techniques,4 including breast reduction or mastopexy; it has become widely used as a tool to assess the results of these techniques through patient-reported outcomes).5
The aim of our study was to evaluate the surgical treatment of symptomatic macromastia, performed in our breast unit, by means of the "patient-reported outcomes" obtained with the BREAST-Q questionnaire.
MethodsProspective cohort study conducted in a single centre to assess the effect of breast reduction in patients with symptomatic macromastia using a validated instrument (BREAST-Q) to measure patient-reported outcomes.
The patients to be operated on had to satisfy the selection criteria in force in our Autonomous Health Service for the care of patients requesting breast reduction for treatment of their breast hypertrophy. They had to be over 18 years of age, the estimated amount of breast tissue to be removed had to be greater than 500 g per breast, a body mass index (BMI) of less than 30 kg/m2 and they had no desire to become pregnant in the next 3 years.
All surgical procedures were performed under general anaesthesia, the patients received antibiotic prophylaxis (cephalosporin in a single dose of 2 g intravenously half an hour before anaesthetic induction) and antithrombotic with low molecular weight heparin administered subcutaneously during the first 10 postoperative days. Breast tumescent anaesthetic infiltration was not used, but locoregional nerve blocks were performed under ultrasound control. The incision pattern used was that of an inverted T. Mobilisation of the nipple-areola complex was performed with different flaps, the most commonly used being the superomedial flap; in cases of gigantomastia, the free graft technique was used. We placed low-pressure drains in all patients through the incisions, which we fixed with adhesives that we removed in the first treatment 72 h after surgery. After surgery, the patient's torso was bandaged with a compressive bandage that was removed on the first consultation visit, 3 or 4 days after surgery.
The BREAST-Q questionnaire contains 104 questions divided into 11 parts that assess satisfaction with the breasts, with the nipple result, the overall result, the information given by the surgeon, with the surgeon, the health care team and the administrative team, quality of life and psychosocial, physical and sexual state. The domains or parts such as satisfaction with the breasts, psychosocial, physical and sexual well-being can be assessed before surgery or after surgery. The patient grades their answer in one of the different options, e.g., from highly satisfied to highly dissatisfied or from strongly agree to strongly disagree. Each part of the questionnaire can be used independently. The patient can leave out those questions that they deem appropriate. The estimated average time to complete the questionnaire is 25−30 min.
In our unit, under licence from The Memorial Sloan Kettering Cancer Center, we carried out the linguistic adaptation from English to Spanish of the breast reduction module BREAST-Q version 2.0 (http://qportfolio.org/breast-q/reductionmastopexy; accessed on 8 November 2021) following the recommendations for linguistic and cultural adaptation6 with the aim of evaluating the effects of bilateral breast reduction in patients with symptomatic macromastia treated in our unit in recent years (2017–2020) using a validated and widely used instrument, the BREAST-Q.
The study was approved by the Clinical Research Ethics Committee of Cantabria and patients signed a written informed consent and completed the BREAST-Q questionnaire version 2.0 module reduction/Mastopexy, pre- and postoperative scales Spanish version which was administered on paper. Patients were surveyed within one month prior to surgery and 6 months after surgery. Personal data were considered confidential and were processed in accordance with the provisions of the Organic Law on Data Protection of 10 November 2017 and the European Data Protection Regulation of 25 May 2018. Clinicopathological data were collected from the hospital's electronic patient records and scores were derived for each of the questionnaire domains. These were transformed (conversion of the sum of the scores into their equivalent transformed rash score) on a scale from 0 to 100 according to the BREAST-Q protocol, with a higher value representing a more favourable outcome.
For the calculation of the sample size, the scores obtained in the different domains of the preoperative questionnaire in the linguistic adaptation process were used. With the GRANMO V7.11 programme for paired samples (repeated in one group) with the physical well-being domain scores (mean 36, standard deviation [SD] 19), an alpha risk of 5% and beta of 20% for a bilateral contrast, at least 32 cases were required to detect the smallest significant difference7 (SD 0.5) 9.5. The choice of the physical domain as the main variable to estimate the sample size is justified by the fact that it is the domain with the largest minimal important difference to be detected.
Statistical analysis was performed with the SPSS v25 (IBM Corp. Released 2017 programme. IBM SPSS Statistics for Windows, version 25.0. Armonk, New York: IBM Corp.). The Kolmogorov-Smirnov test was used to test the normal distribution of the variables. To describe the descriptive statistics, we used mean and SD and median and interquartile range (25th–75th percentile) and percentages. Comparisons between scores were performed by Wilcoxon signed-rank tests and Student’s t-tests. Statistical significance was determined when p-values were <.05.
ResultsThe characteristics of the patient cohort are set out in Table 1. Fourteen patients (42%) had at least one postsurgical complication or sequela; complications were in order of highest to lowest frequency: wound dehiscence (6), scar dog-ears (6), hypertrophic scarring (3), wound infection (2), haematoma (2), and one in the following: adiponecrosis, depigmentation of the areola-nipple complex, partial necrosis of the areola-nipple complex, and areolar fistula. Seven patients (21%) underwent reoperation for haematoma evacuation, dog-ear correction and fistulous tract excision. The BREAST-Q domain scores obtained pre- and postoperatively are shown in Table 2 and Fig. 1.
Characteristics of the 34 patients with symptomatic macromastia treated with reduction mammoplasty.
| Variable | Mean (standard deviation) |
|---|---|
| Age (years) | 40 (13) |
| Body mass index (kg/m2) | 28 (3) |
| Jugulum-ANC distance (cm) | |
| Right breast | 33 (3) |
| Left breast | 32 (2) |
| ANC rise (cm) | 8 (1) |
| Weight of resected tissue (g) | |
| Right breast | 722 (388) |
| Left breast | 712 (333) |
| Time in surgery (minutes) | 170 (22) |
| Time from surgery to postoperative survey (months) | 16 (9) |
| Number (percentage) | |
|---|---|
| Smokers | 5 (15) |
| Background of obesitya | 16 (47) |
| Background of psychological help | 11 (32) |
| Comorbidityb | 15 (44) |
| ASA (American Society of Anesthesiologists) classification | |
| I | 15 (44) |
| II | 18 (53) |
| II | 1 (3) |
| Surgeon | |
| General | 23 (68) |
| Plastic | 11 (32) |
| Pedícle or technique used to transport the ANC | |
| Superomedial | 27 (79) |
| Inferior | 3 (1) |
| Bipediculate | 3 (9) |
| Free graft of the ANC | 3 (9) |
| Complication or postoperative sequela | 14 (41) |
| Reintervention | 7 (21) |
ANC: areola-nipple complex.
Comparison of pre- and post-operative BREAST-Q scores of 34 operated patients with symptomatic macromastia expressed as medians and interquartile range by Wilcoxon signed-rank test.
| Domain | Preoperative | Postoperative | p Vvalue* |
|---|---|---|---|
| Satisfaction with breasts (MAM) | 28 (25) | 82 (15) | p < 0.0001 |
| Psychological well-being (PSI) | 33 (15) | 81 (29) | p < 0.0001 |
| Physical well-being (FIS) | 42 (19) | 82 (30) | p < 0.0001 |
| Sexual well-being (SEX) | 34 (14) | 90 (38) | p < 0.0001 |
| Nipple satisfactiona | |||
| Situation | 4 (0) | ||
| Symmetry | 4 (0) | ||
| Shape of the ANC | 4 (1) | ||
| Appearance of the ANC | 4 (1) | ||
| Sensitivity | 3 (2) | ||
| Satisfaction with the overall result | 100 (24) | ||
| Satisfaction with the information | 67 (33) | ||
| Satisfaction with the surgeon | 96 (25) | ||
| Satisfaction with the team | 100 (0) | ||
| Satisfaction with the administrative personnel | 100 (0) | ||
ANC: areola-nipple complex.
Reduction mammoplasty is an effective treatment for symptomatic macromastia; due to its effect, patients’ symptomatology, physical and psychological well-being are improved.7 In addition, breast reduction is a cost-effective treatment8; in contrast, its alternative, conservative treatment, is expensive, with an annual cost per patient of more than 1500 euros according to a study of 76 German patients, and does not solve the problem.9
Patients with symptomatic macromastia have a lower quality of life.7 In our patients, BREAST-Q scores in the week prior to surgery were lower in all domains – breast satisfaction, psychological, sexual and physical well-being – than those of 2 reference populations; one composed of 1205 white American women (91%) with a mean age of 55 years (SD 13), a mean BMI of 27 kg/m2 (SD 6) and with a bra D-cup in 40%, who were questioned with the breast reduction module of the BREAST-Q10 questionnaire and the other of 1334 Dutch women, with a mean age of 50.4 years (SD 17) and mean BMI of 25 kg/m2 (SD 4.7) who were questioned with the BREAST-Q questionnaire preoperative breast reconstruction module11 (Table 3).
| Domain | Patients in our series (n = 34) | American reference population (Mundy et al.) (n = 1205) | Dutch reference population (Sadok et al.) (n = 1334) | *Comparisons between the series with each of the reference populations P values |
|---|---|---|---|---|
| Satisfaction with breasts | 276 (20) | 57 (16) | 68 (19) | 0.0001 |
| Psycho-social well-being | 344 (144) | 68 (19) | 72 (17) | 0.0001 |
| Sexual well-being | 372 (163) | 55(19) | 80 (14) | 0.0001 |
| Physical well-being | 396 (217) | 76 (11) | 58 (19) | 0.0001 |
The evaluation of breast reduction as, a treatment for symptomatic macromastia with a specific, validated instrument suchthe BREAST-Q in our unit offers results that show a statistically significant (differences of more than 40 points out of 100), clinical improvement in the quality of life of the patients who underwent surgery. This experience, the first in the application of the BREAST-Q questionnaire in Spanish women, is similar in the scores obtained, in the postoperative changes after breast reduction to those published by other authors who used the BREAST-Q as, an assessment instrument.6,8,12–17
Comparison with these studies is difficult due to the differences in the period between surgery and the survey, in the characteristics of the patients (age, BMI, comorbidities, etc.) and in the breast reduction techniques used. Possibly the best comparison we can make with our results is with those of the published study with 238 patients, the largest number of patients published to date.14 In this one, the survey was performed a mean of 7 months after surgery and the characteristics of age (45 years, SD 13) and BMI (31 kg/m2, SD 5) do not differ too much with those of our series, as well as in the use of the superomedial flap technique (74.4%). Our work obtained very similar mean preoperative scores in all domains to those published in this paper: breast satisfaction 24.5 (SD 11.32) vs 28, (SD 25); psychosocial well-being 39.33, (SD 46.3) vs 33 (SD 15); sexual well-being 38.81, (SD 17.1) vs 34, (SD 14) and physical well-being 46.22, (SD 13.3) vs 42, (SD 19) also showing very clear differences between pre- and post-surgery scores.
The results reported by the patients allow the surgeon to monitor and improve their work. As was observed in the application of the BREAST-Q breast cancer conservative treatment module, the scores on the information given by the surgeon are much lower than the scores given by the patients themselves on how satisfied they are with the treatment.18,19 This significant difference in scores, 18 points in this study, should alert us to the need to detect aspects that need to be improved in the field of doctor-patient information.
Although the sample size is sufficient to detect clinically and statistically significant or relevant differences in the BREAST-Q domains, the number of patients is limited to make comparisons between groups of patients (for example, with or without a history of obesity, between different surgical techniques, surgeons, etc.) or to analyse which factors determine the results.
In conclusion, we can say that the application of the BREAST-Q (breast reduction module in its Spanish version for Spanish) in patients with symptomatic macromastia treated surgically, in a breast unit, shows that breast reduction improves the quality of life of patients and that they are very satisfied with the outcome of surgery and their surgeon, although the information received is clearly improvable.
FundingThis paper did not receive any type of funding.
Conflict of interestsNone.
Our thanks to all those women who selflessly worked with us and made this study possible.