Gastrointestinal stromal tumours (GIST) make up 2% of gastrointestinal tumours. Surgery is the only treatment method in localised cases. The laparoscopic approach has increased over the last few years. We present our experience in the treatment of GIST.
Material and methodsA total of 40 patients with 45 GIST had been subjected to surgical treatment between 1997 and 2010. Data was retrospectively collected on, demographic characteristics, location and tumour biology, diagnosis, type of surgery and the results of that surgery.
ResultsA total of 24 males and 16 women, with a mean age of 66.7 years, were treated. The location was gastric in 24 cases (60%), small intestine in 13 (32.5%), colon in 2 (5%) and oesophagus in 1 case (2.5%). Laparotomy was performed in 27 cases, 12 by laparoscopy (1 thoracoscopy), and 1 endoscopic sigmoid tumour resection. Four cases (10%), all after laparotomy, had recurred after a median follow-up of 31 months (2–120), and 2 patients of the laparotomy group died due to their cancer. After a univariate analysis, the prognostic factors for a laparoscopic recurrence were: tumour size (P=.0001), mitosis number (P=.001), being a locally advanced tumour (P=.01) and a ruptured tumour (P=.002). Only size remained as a prognostic factor after the multivariate analysis (P=.029; RR 1.363; 95% CI; 1.033–1.799). The presence of a locally advanced tumour was shown to be significant in the univariate analysis, while there were no significant factors after the multivariate analysis.
ConclusionsCorrect preoperative staging is essential for deciding which surgical approach to employ.
Los tumores del estroma gastrointestinal (GIST) representan el 2% de los tumores digestivos. La cirugía constituye el único método curativo en los casos localizados. El abordaje laparoscópico se ha extendido en los últimos años. Presentamos nuestra experiencia en el tratamiento de los GIST.
Material y métodosEntre 1997–2010 se han intervenido con intención curativa 40 pacientes de un total de 45 diagnosticados de GIST. Recogimos prospectivamente datos referentes a: características demográficas, localización y biología tumoral, diagnóstico, tipo de cirugía y resultados de la misma.
ResultadosSe trataba de 24 varones y 16 mujeres con una edad media de 66,7 años. La localización fue gástrica en 24 casos (60%), intestino delgado 13 (32,5%), colon 2 (5%) y esófago 1 (2,5%). Fueron intervenidos por laparotomía 27 casos, 12 mediante laparoscopia (1 toracoscopia) y 1 resección endoscópica en tumor de sigma. Tras una mediana de seguimiento de 31 meses (2–120) han recidivado 4 casos (10%) todos tras laparotomía. 2 pacientes del grupo de laparotomía han fallecido por la neoplasia. Tras el estudio univariante los factores pronósticos para la RL fueron: tamaño tumoral (p=0,0001), número de mitosis (p=0,001), tratarse de un tumor localmente avanzado (p=0,01) y la rotura tumoral (p=0,002). Tras el estudio multivariante sólo permanece el tamaño (p=0,029; RR 1,363; IC 95% 1,033–1,799). Para la supervivencia, tras el estudio univariante, se muestra significativo la presencia de tumor localmente avanzado, mientras que ningún factor se muestra significativo tras el estudio multivariante.
ConclusionesLa correcta estadificación preoperatoria es básica para la decisión del abordaje quirúrgico a emplear.
Gastrointestinal stromal tumours (GIST) represent 2% of the tumours of the human gastrointestinal tract.1 They can originate in any part of the tract from the oesophagus to the rectum. The most frequent location is gastric (60%), followed by the small intestine (25%–30%), colon and rectum (10%) and abdominal cavity (5%).2 Clinical behaviour at diagnosis is variable, from clearly benign tumours to metastatic tumours. Different classifications have been proposed to predict the behaviour of these tumours. The classifications take into account the size of the tumour, the number of mitoses per 50 high powered fields (HPFs) and the location of the tumour.3,4
Surgery is the only curative method for localised tumours and should be considered in the context of a multidisciplinary approach which includes surgeons, radiologists, pathologists and oncologists.5,6 In spite of there being a lack of consensus about which tumours are eligible for laparoscopic treatment, more and more authors advocate a laparoscopic approach.7–9 The determining factors for laparoscopic access in the past were tumour size and location,10,11 although recent series have been published with excellent results both for large tumours and tumours located in the intestine.12,13
The aim of this study is to review the experience or our Unit in the surgical management of these tumours.
Material and MethodsA retrospective analysis was performed of patients diagnosed with GIST in our hospital from 1997 to 2010. Of the 45 cases diagnosed, 40 were operated on with curative intent (R0 or R1 surgery) and they are the basis of our study.
Preoperative diagnosis was performed using abdominal CT scans in all cases, endoscopy in those located in the stomach, and endoscopic ultrasound for those located in the stomach and oesophagus since 2007.
Data analysed included age, sex, location, histological type, immunohistochemistry, size, number of mitoses per 50 HPFs, type of surgery, findings during surgery, tumour rupture during surgery, resection margins, postoperative complications, recurrence and survival.
Patients were classified into groups according to the risk of recurrence using the classification proposed by Fletcher et al.2
We defined tumours as locally advanced when the preoperative studies or findings during operation showed involvement of other organs, but for which a curative surgical resection could be performed.
The laparoscopic approach was first used in 2005 for tumours <5cm located in the stomach. Subsequently, the indication for laparoscopy was extended to tumours located in the small intestine and the oesophagus, and larger tumours that were considered to be localised in the preoperative staging studies. The decision to use a laparoscopic approach was made individually for each patient. We did not perform routine pathology studies on the resection margins.
Since 2009, patients with a high risk of recurrence have received adjuvant treatment with imatinib (400mg/day) for at least one year.
The follow-up was carried out by clinical assessment and CT every 6 months for the first 2 years and then once a year until five years, after which only clinical follow-up was performed. Gastroscopy was performed once a year during the first 2 years after surgery in cases of gastric tumours.
Local recurrence (LR) was defined as new growth of the tumour in the same location as the original tumour or in the abdominal cavity. Metastatic recurrence was defined as the presence of a tumour in the liver or any extra abdominal organ.
To analyse the factors related to recurrence and survival, a Cox logistic regression was carried out. The significance was established at a value of P<.05.
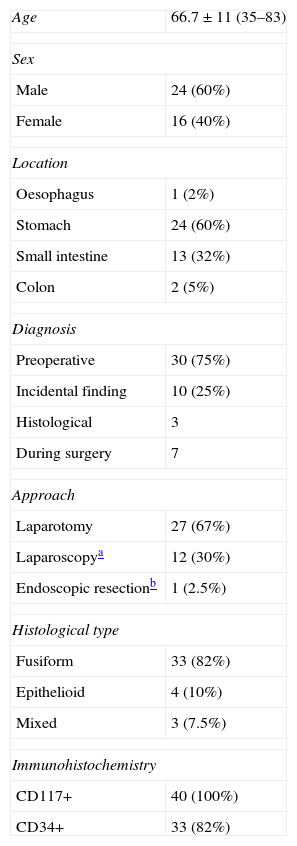
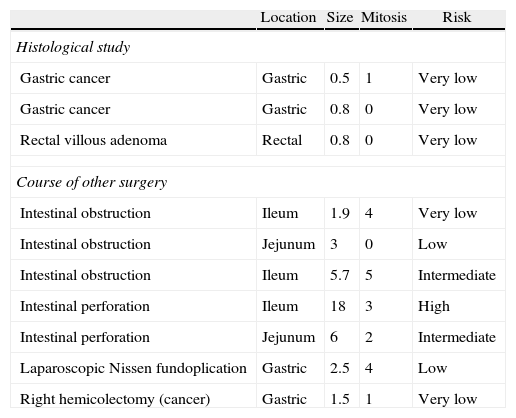
ResultsSurgery with curative intent was performed on a total of 40 patients, 24 male and 16 female, with a mean age of 66.7. Table 1 shows the demographic characteristics of the operated patients, the location of the tumour, diagnosis, approach used and the characteristics of the tumours. In our series, all cases were CD117+. Ten cases were incidental findings, 5 occurred during laparotomy due to intestinal obstruction or perforation, one case during a laparoscopic Nissen fundoplication, one case of right colon resection; one case was diagnosed after the study of a colorectal villous adenoma and 2 after the study of surgical samples from gastrectomies performed for gastric cancer. The characteristics of these tumours are indicated in Table 2.
Characteristics of Patients and Tumours.
| Age | 66.7±11 (35–83) |
| Sex | |
| Male | 24 (60%) |
| Female | 16 (40%) |
| Location | |
| Oesophagus | 1 (2%) |
| Stomach | 24 (60%) |
| Small intestine | 13 (32%) |
| Colon | 2 (5%) |
| Diagnosis | |
| Preoperative | 30 (75%) |
| Incidental finding | 10 (25%) |
| Histological | 3 |
| During surgery | 7 |
| Approach | |
| Laparotomy | 27 (67%) |
| Laparoscopya | 12 (30%) |
| Endoscopic resectionb | 1 (2.5%) |
| Histological type | |
| Fusiform | 33 (82%) |
| Epithelioid | 4 (10%) |
| Mixed | 3 (7.5%) |
| Immunohistochemistry | |
| CD117+ | 40 (100%) |
| CD34+ | 33 (82%) |
Characteristics of Incidentally Discovered Tumours.
| Location | Size | Mitosis | Risk | |
| Histological study | ||||
| Gastric cancer | Gastric | 0.5 | 1 | Very low |
| Gastric cancer | Gastric | 0.8 | 0 | Very low |
| Rectal villous adenoma | Rectal | 0.8 | 0 | Very low |
| Course of other surgery | ||||
| Intestinal obstruction | Ileum | 1.9 | 4 | Very low |
| Intestinal obstruction | Jejunum | 3 | 0 | Low |
| Intestinal obstruction | Ileum | 5.7 | 5 | Intermediate |
| Intestinal perforation | Ileum | 18 | 3 | High |
| Intestinal perforation | Jejunum | 6 | 2 | Intermediate |
| Laparoscopic Nissen fundoplication | Gastric | 2.5 | 4 | Low |
| Right hemicolectomy (cancer) | Gastric | 1.5 | 1 | Very low |
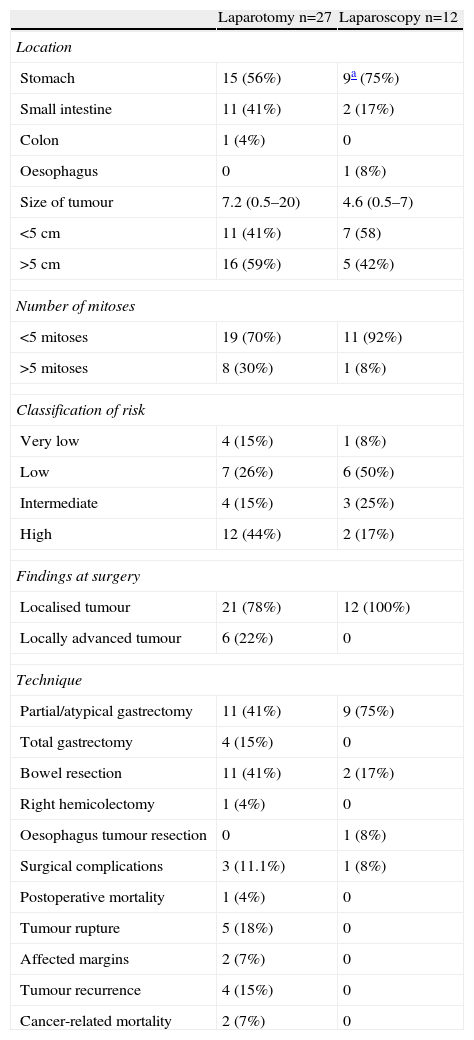
Table 3 shows data relating to the surgical treatment and results. In the group of patients operated on laparoscopically, no conversion to open surgery was needed, and no cases of tumour rupture, resection margin infiltration or implantations in trocar sites were observed. One case of an oesophageal tumour was operated on by thoracoscopy. Two cases of small submucosal tumours of the gastro-oesophageal junction were operated on via laparoscopic intragastric surgery.
Tumour Characteristics According to the Approach.
| Laparotomy n=27 | Laparoscopy n=12 | |
| Location | ||
| Stomach | 15 (56%) | 9a (75%) |
| Small intestine | 11 (41%) | 2 (17%) |
| Colon | 1 (4%) | 0 |
| Oesophagus | 0 | 1 (8%) |
| Size of tumour | 7.2 (0.5–20) | 4.6 (0.5–7) |
| <5cm | 11 (41%) | 7 (58) |
| >5cm | 16 (59%) | 5 (42%) |
| Number of mitoses | ||
| <5 mitoses | 19 (70%) | 11 (92%) |
| >5 mitoses | 8 (30%) | 1 (8%) |
| Classification of risk | ||
| Very low | 4 (15%) | 1 (8%) |
| Low | 7 (26%) | 6 (50%) |
| Intermediate | 4 (15%) | 3 (25%) |
| High | 12 (44%) | 2 (17%) |
| Findings at surgery | ||
| Localised tumour | 21 (78%) | 12 (100%) |
| Locally advanced tumour | 6 (22%) | 0 |
| Technique | ||
| Partial/atypical gastrectomy | 11 (41%) | 9 (75%) |
| Total gastrectomy | 4 (15%) | 0 |
| Bowel resection | 11 (41%) | 2 (17%) |
| Right hemicolectomy | 1 (4%) | 0 |
| Oesophagus tumour resection | 0 | 1 (8%) |
| Surgical complications | 3 (11.1%) | 1 (8%) |
| Postoperative mortality | 1 (4%) | 0 |
| Tumour rupture | 5 (18%) | 0 |
| Affected margins | 2 (7%) | 0 |
| Tumour recurrence | 4 (15%) | 0 |
| Cancer-related mortality | 2 (7%) | 0 |
In the laparotomy group, 2 scheduled splenectomies were associated to total gastrectomy, in one of them, resection of the tail of the pancreas was also performed. In another patient with distral gastrectomy partial removal of the transverse mesocolon was performed. In the tumours of the small intestine, 2 extended resections were performed: one case of bladder dome resection, and another case with partial cystectomy and en bloc resection of a sigmoid loop.
In 2 cases a resection margin infiltrated by the tumour was found. One was located in the jejunum (low risk tumour) and the other was gastric (high risk tumour), neither of which had further surgery. The patient with a high risk gastric tumour underwent postoperative treatment with imatinib for one year. Neither of the 2 cases recurred after a follow-up of 95 months (jejunal) and 20 months (gastric).
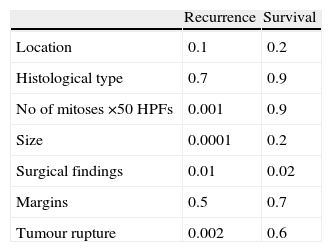
After a median follow-up of 31 (2–120) months, 4 cases recurred (10%): all had undergone laparotomic surgery. The location of the recurrence was peritoneal and liver in 3 cases and exclusively in the liver in one case. The recurrence free period was 42 months. Univariate analysis showed that the predictive factors for recurrence were: size, number of mitoses, locally advanced tumours and the presence of tumour rupture during surgery (Table 3). After multivariate analysis, only size is shown as a significant factor (P=.029; RR 1.363 CI 95% 1.033–1.799).
There were 2 cancer-related deaths, and thus a mortality rate of 5%. The factors that have shown to be significant for survival following univariate analysis are displayed in Table 4. No factor was significant after multivariate analysis.
DiscussionSince the first description of GIST tumours by Mazur and Clark,14 the number of diagnoses of these tumours has been increasing due to a more precise diagnosis. In the past, the diagnosis was established by histological criteria and determination by immunohistochemistry of CD117.15 Likewise, in doubtful cases, the genetic study of PDGRFA or mutations in c-kit are useful for confirming the diagnosis and for determining the prognosis of GIST.16
There has also been an increase in cases of incidental diagnosis during the study of other diseases, as well as incidental findings in laparotomies and autopsies. In our series, 24% of cases were incidental findings at laparotomy during the treatment of other processes, or in the histological study of resection samples for other diseases, which corresponds to the data referred to by Goettsch et al.17
Surgery is the only curative treatment for localised GISTs. The goal is to obtain a total resection of the tumour with the entire pseudocapsule with negative microscopic margins. The peritoneal cavity should be examined with care to identify potential metastases or peritoneal dissemination of the tumour.18 In spite of an R0 resection, there is a wide variation in the behaviour of the GIST depending on the different characteristics of the tumour, with recurrence rates that vary between 0 and 90%.19,20 Recurrences are located most frequently in the peritoneum, tumour bed or in the liver, or as a metastatic liver disease. Lymph node involvement is exceptional, and as such lymphadenectomy is not indicated except in cases of macroscopic involvement of regional lymph nodes.19,21
Surgery is also the treatment of choice in the cases in which with an extended resection could achieve the complete elimination of the tumour. In cases in which surgery would mean an “invalidating” resection (oesophagectomy, cephalic duodenopancreatectomy, Miles’ operation), or in some cases in which total resection or obtaining free margins is impossible, it would be justifiable to start preoperative treatment with imatinib to reduce the tumour and allow a complete or less mutilating surgery.5,22
Surgical treatment should be planned after cancer staging by image tests in collaboration with an expert radiologist in order to verify the resectability of the tumour.23 Our group considers that a preoperative CT scan is obligatory to assess resectability and rule out the presence of metastasis or carcinomatosis. In unclear cases a PET scan is indicated to diagnose liver metastases or peritoneal carcinomatosis.24 A proper preoperative staging is fundamental to decide the surgical approach and technique and it should provide us with information about the location, the size and the potential infiltration of neighbouring structures (local extension). Likewise, the characteristics of the patient and experience of the surgeon in laparoscopic surgery are important in the decision approach.
In the last few years, publications9,25–27 in which the viability and security of a minimally invasive approach for the treatment of GIST have increased. The laparoscopic approach obtains the same results in terms of recurrence and survival with the advantages of minimally invasive surgery.9,28,29 All techniques in accordance with the location of the tumour can be performed by laparoscopy in selected cases.11,30 The development of the laparoscopic approach should be gradual in the context of multidisciplinary treatment and after adequate training of surgeons in laparoscopy. In our series, there were no cases of recurrence or mortality due to neoplasms in operations performed laparoscopically and the development of postoperative complications was lower than after open surgery.
Prognostic factors accepted for recurrence-free survival are the size and number of mitoses, to which tumour location has been added.2,3 In our series, the factors that have been significant in the development of LR following univariate analysis were size, the number of mitoses, locally advanced tumours and tumour rupture during surgery. Only the characteristic of locally advanced tumour remains significant after multivariate analysis.
Tumour rupture has been described as a determining factor in the appearance of locoregional recurrences.5,31 In our series, the presence of tumour rupture is significantly associated, in univariate analysis, with the development of local recurrence (P=.002). The main theoretical disadvantage to laparoscopic surgery is the possibility of tumour rupture during the manipulation of the tumour with the resulting risk of tumour recurrence. In our series, all cases of tumour rupture occurred during laparotomies. This was the case for large tumours (mean size 10.4cm; range 5.7–18), in which the size of the tumour and the ease of dissection of the tumour as a whole seems more important than the approach itself, and a series of measures have been described to avoid rupture during laparoscopic surgery. As such, Novitsky et al. advocate abstaining from direct manipulation of the tumour with laparoscopic instruments, in order to avoid tumour rupture.24
We have not verified recurrence in the trocar sites, in agreement with other authors.28,32 The extraction of the resected organ following laparoscopic surgery is always carried out in a plastic device after performing a minilaparotomy in accordance with the size of the tumour.
The management of involved resection margins is yet to be defined. In our series the 2 cases with involved resection margins did not present relapse after 20 and 95 months of follow-up, without undergoing further surgery. As previously mentioned, the main goal of surgery is the complete removal of the tumour with free resection margins (R0), but when involved resection margins (R1) are found, the indications for re-resection are conditioned by the technical possibilities of an adequate resection and the general condition of the patient to undergo further surgery.5,12,33 In our case of the gastric tumour with involved margins the patient had multiple comorbidities; and further surgery would have required a total gastrectomy. Other authors have stated5,20,33 the benefit of further surgery is not fully demonstrated in the case of involved margins. Factors such as size, the number of mitoses and the location are more important for the development of recurrence.
ConclusionsProper preoperative staging is essential to decide the surgical approach. A laparoscopic approach in these patients obtains the same oncological results as conventional surgery.
Conflicts of InterestThe authors have no conflicts of interest to declare.
Please, cite this article as: Martí Obiol R, et al. Tratamiento quirúrgico de los tumores del estroma gastrointestinal. Análisis de nuestra experiencia. Cir Esp. 2013;91:38–43.