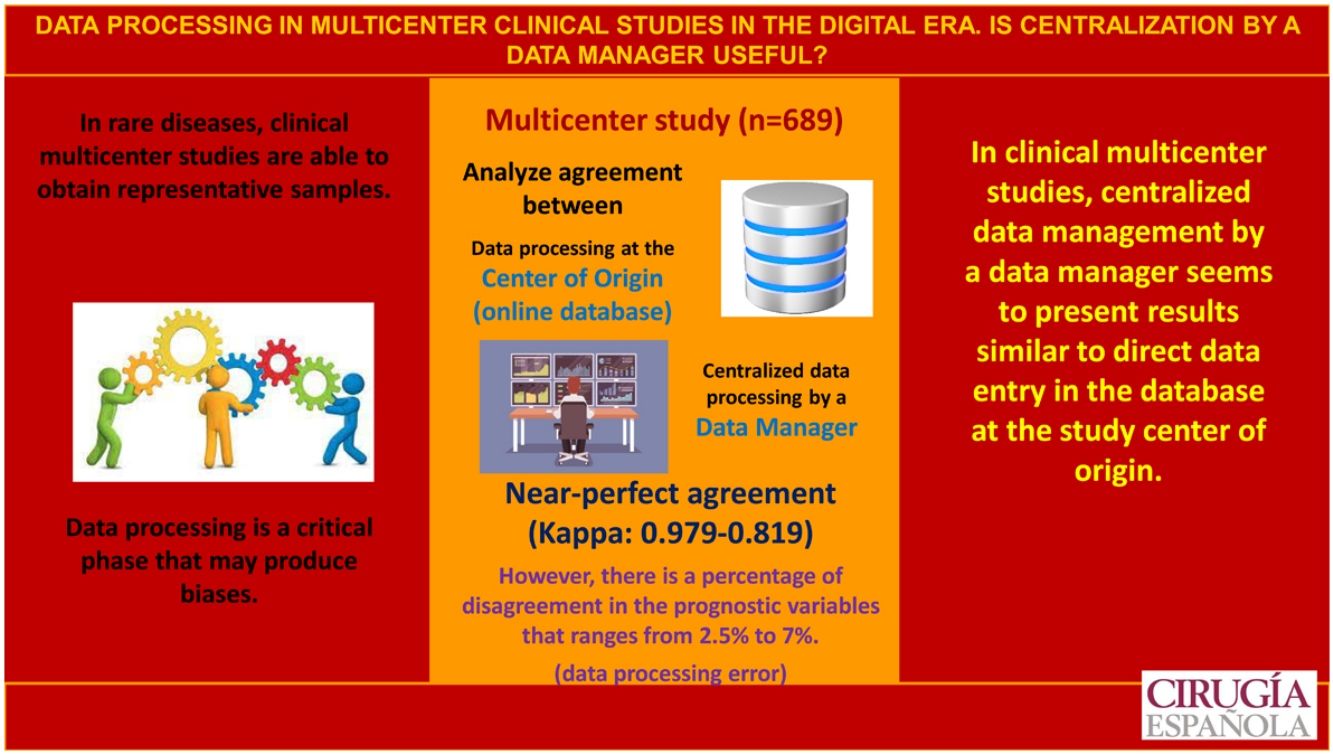
In multicenter studies, the protocolization of data is a critical phase that can generate biases.The objective is to analyze the concordance and reliability of the data obtained in a clinical multicenter study between the protocolization in the center of origin and the centralized protocolization of the data by a data -manager.
MethodsNational multicenter clinical study about an infrequent carcinoma. A double protocolization of the data is carried out: (a) center of origin; and (b) centralized by a data manager: The concordance between the data is analyzed for the global data and for the two groups of the project: (a) study group (Familiar carcinoma, 30 researchers protocolize); (b) control group (Sporadic carcinoma, 4 people protocolize). Interobserver variability is evaluated using Cohen's kappa coefficient.
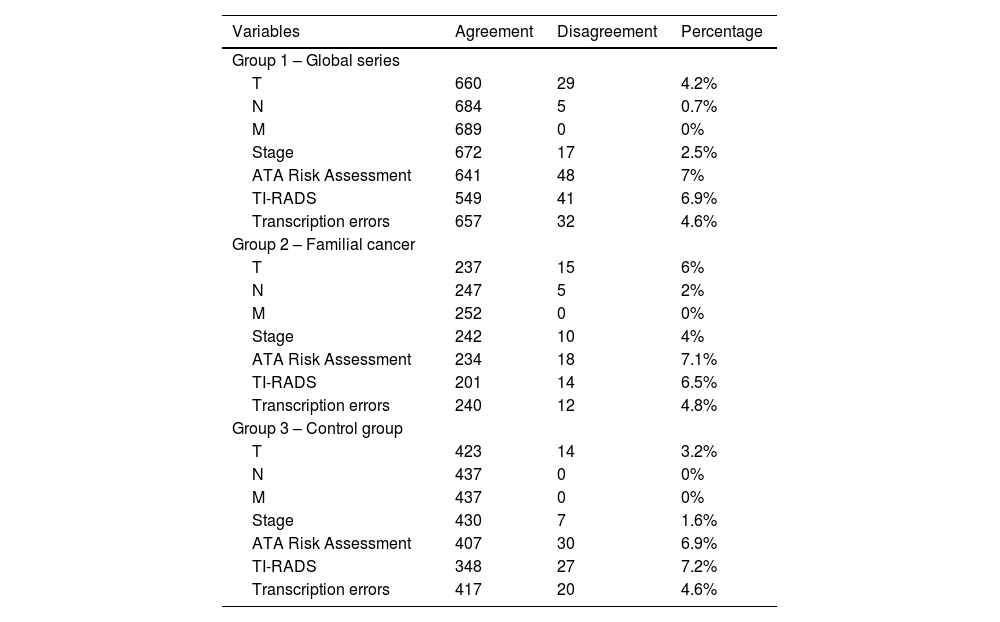
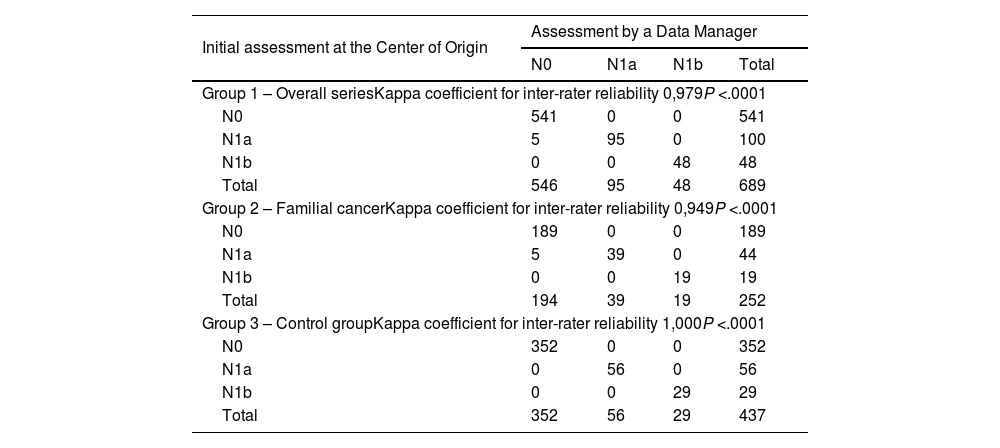
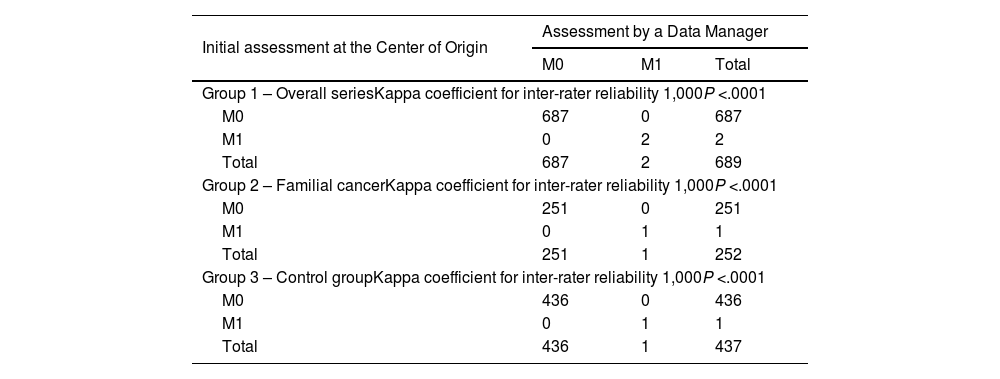
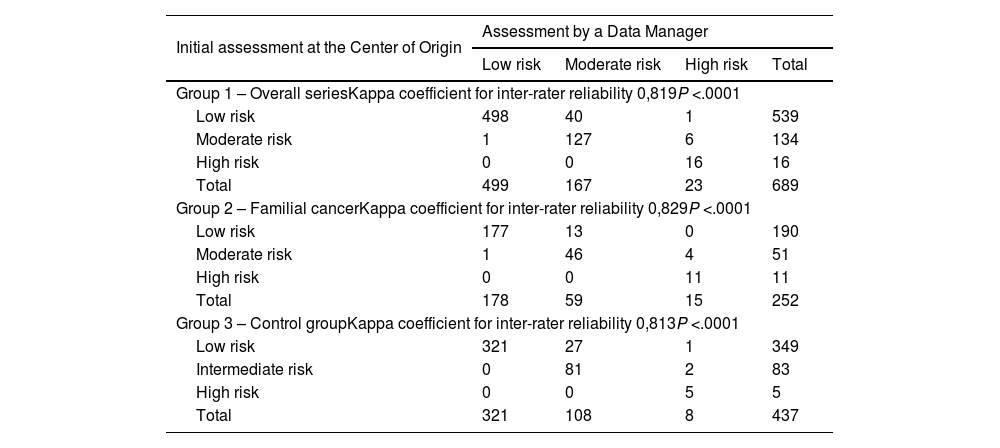
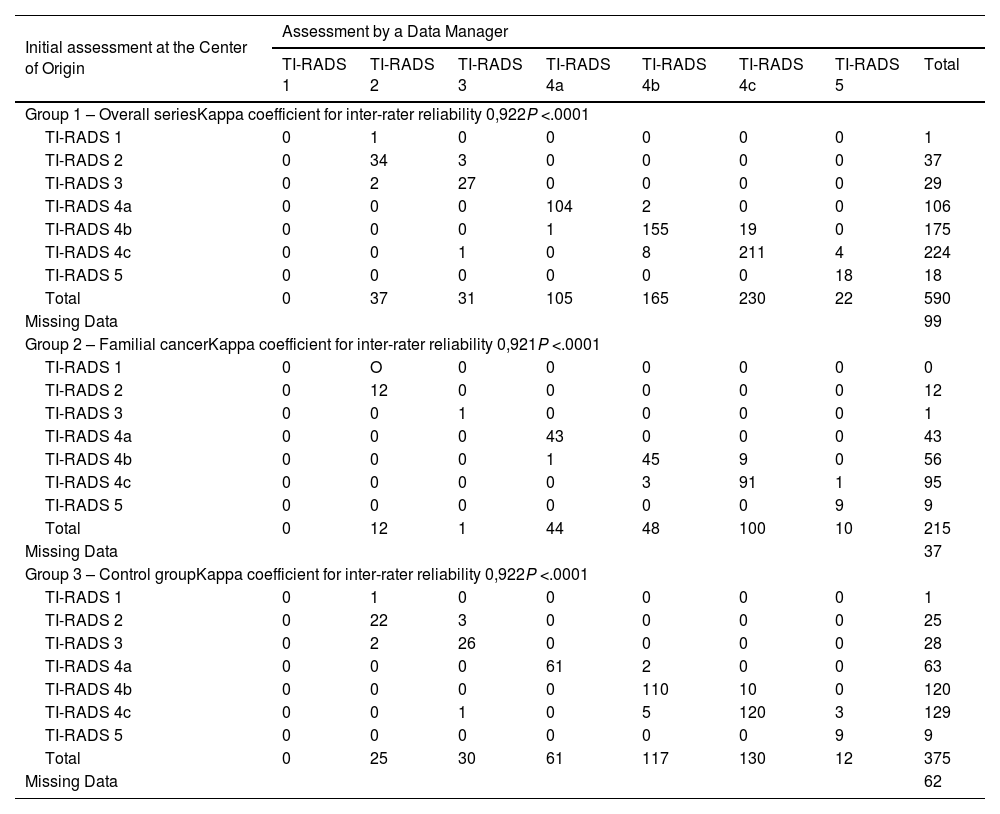
ResultsThe study includes a total of 689 patients with carcinoma, 252 in the study group and 437 in the control group. Regarding the concordance analysis of the tumor stage, 2.5% of disagreements were observed and the concordance between people who protocolize was near perfect (Kappa = 0.931). Regarding the evaluation of the recurrence risk, disagreements occurred in 7% of the cases and the concordance was near perfect (Kappa = 0.819). Regarding the sonography evaluation (TIRADS), the disagreements were 6.9% and the concordance was near perfect (Kappa = 0.922). Also, 4.6% of transcription errors were detected.
ConclusionsIn multicenter clinical studies, the centralized data protocolization o by a data-manager seems to present similar results to the direct protocolization in the database in the center of origin.
En los estudios multicéntricos la protocolización de los datos es una fase crítica que puede generar sesgos, sobre todo en estudios clínicos con presupuesto limitado. El objetivo es analizar la concordancia y cofiabilidad de los datos obtenidos en un estudio multicéntrico clínico entre la protocolización el centro de origen y la protocolización centralizada mediante un data-manager.
MétodoEstudio clínico multicéntrico de prevalencia nacional sobre un carcinoma familiar infrecuente, realizándose una doble protocolización de los datos: (a) en el centro de origen; y, (b) centralizada con un data-manager: La concordancia se analiza para el global de los datos y para los dos subgrupos del proyecto: (a) grupo a estudio (Carcinoma familiar. Protocolizan 30 investigadores); y (b) grupo control (Carcinoma esporádico. Protocolizan 4). Las diferencias interobservador se evalúan mediante el Indice de Kappa de Cohen.
ResultadosSe incluyen 689 pacientes, 252 del grupo a estudio y 437 del grupo control. Respecto al análisis de concordancia del estadio tumoral se han objetivado un 2,5% de discordancias, siendo alta la concordancia entre protocolizadores (Kappa = 0,931). Respecto a la valoración del riesgo de recidiva las discordancias fueron del 7% de los casos, siendo alta la concordancia (Kappa = 0,819). Respecto a la clasificación ecográfica TIRADS las discordancias son del 6,9% y la concordancia es alta (Kappa = 0,922). Se han detectado un 4,6% de errores de transcripción.
ConclusionesEn los estudios multicéntricos clínicos la protocolización centralizada de los datos por un data-manager parece presentar resultados similares a la protocolización directa en la base de datos en el centro de origen.