The objective of this study is to create a predictive model of prolonged postoperative length of stay (PLOS) in patients undergoing anatomic lung resection, to validate it in an external series and to evaluate the influence of PLOS on readmission and 90-day mortality.
MethodsAll patients registered in the GEVATS database discharged after the intervention were included. We define PLOS as the postoperative stay in days above the 75th percentile of stay for all patients in the series. A univariate and multivariate analysis was performed using logistic regression and the model was validated in an external cohort. The possible association between PLOS and readmission and mortality at 90 days was analyzed.
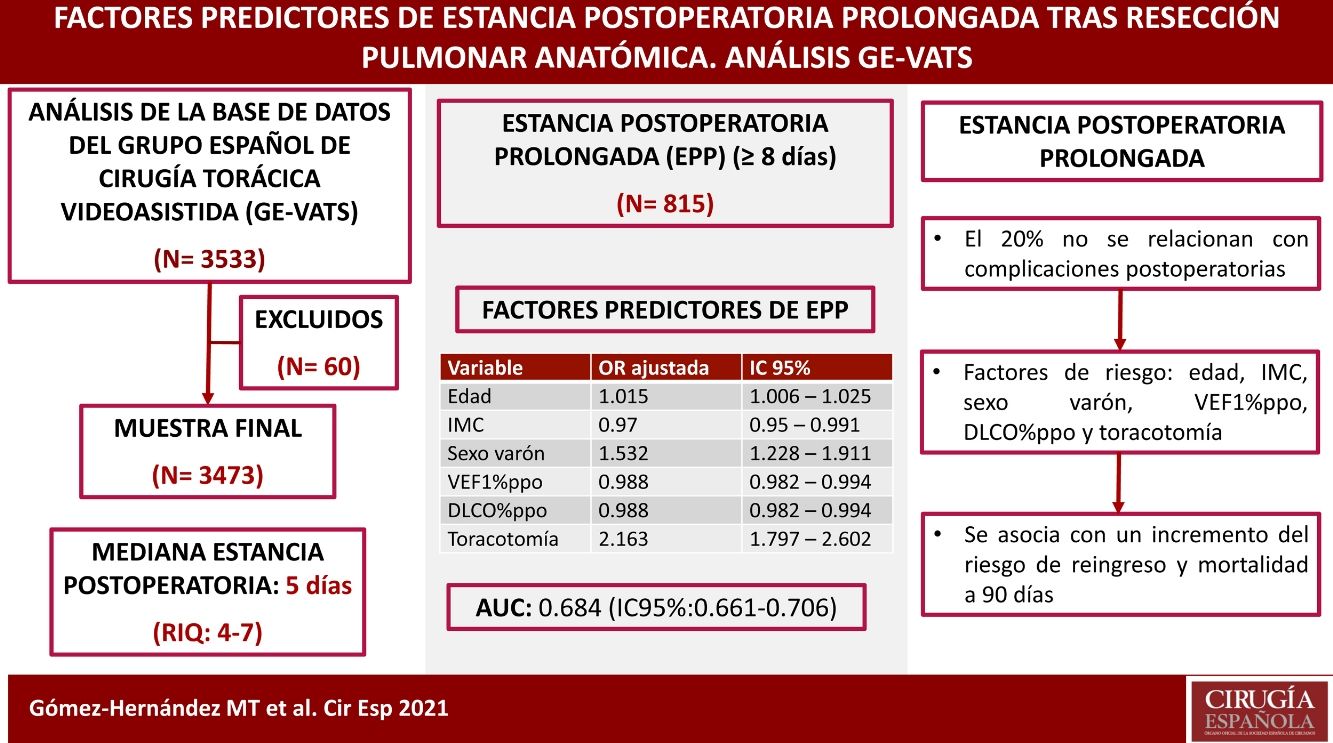
Results3473 patients were included in the study. The median postoperative stay was 5 days (IQR: 4–7). 815 patients had PLOS (≥8 days), of which 79.9% had postoperative complications. The final model included as variables: age, BMI, male sex, ppoFEV1%, ppoDLCO% and thoracotomy; the AUC in the referral series was 0.684 (95% CI: 0.661–0.706) and in the validation series was 0.73 (95% CI: 0.681–0.78). A significant association was found between PLOS and readmission (p < .000) and 90-day mortality (p < .000).
ConclusionsThe variables age, BMI, male sex, ppoFEV1%, ppoDLCO% and thoracotomy affect PLOS. PLOS is associated with an increased risk of readmission and 90-day mortality. 20% of PLOS are not related to the occurrence of postoperative complications.
El objetivo de este estudio es crear un modelo predictivo de estancia postoperatoria prolongada (EPP) en pacientes sometidos a resección pulmonar anatómica, validarlo en una serie externa y evaluar la influencia de la EPP en el reingreso y la mortalidad a 90 días.
MétodosSe incluyeron todos los pacientes registrados en la base de datos del GE-VATS dados de alta tras la intervención. Definimos la EPP como la permanencia postoperatoria en días por encima del percentil 75 de estancia de todos los pacientes de la serie. Se realizó un análisis univariable y multivariable mediante regresión logística y el modelo fue validado en una cohorte externa. Se analizó la posible asociación entre la EPP y el reingreso y la mortalidad a 90 días.
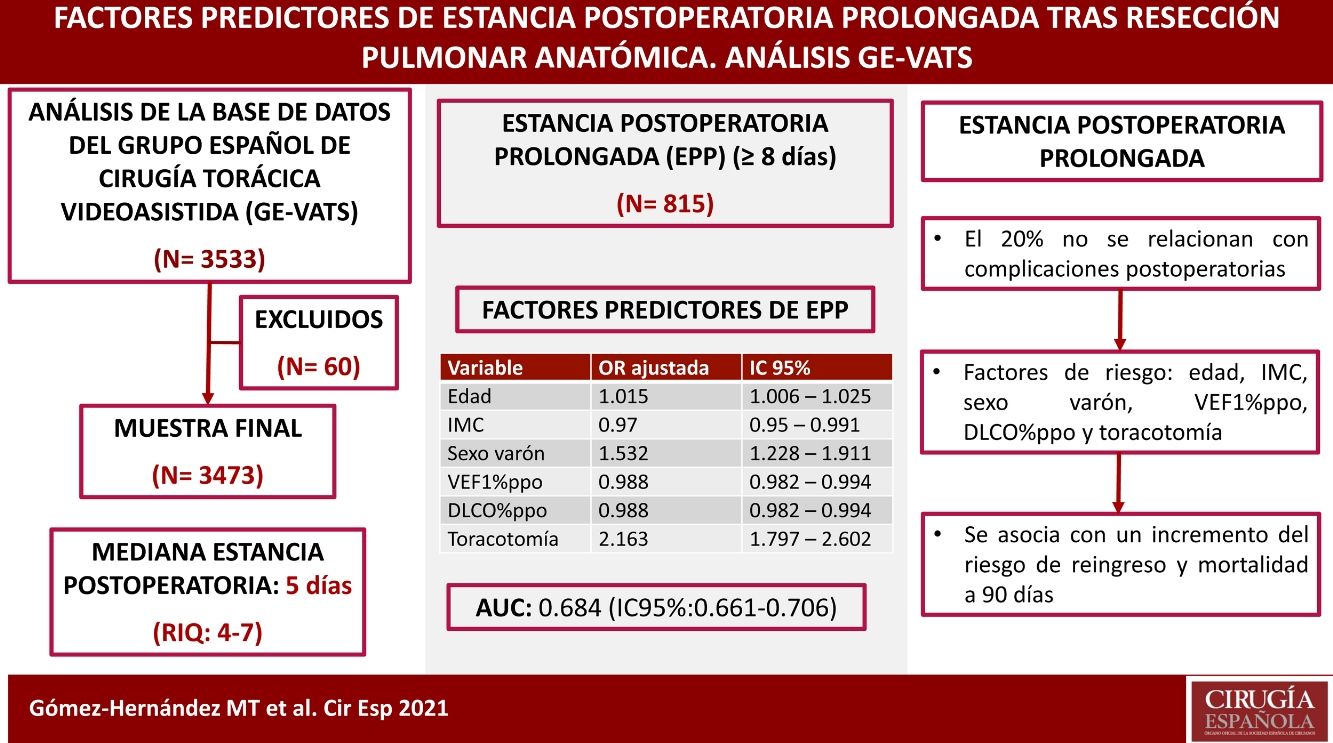
ResultadosSe incluyeron en el estudio 3473 pacientes. La mediana de estancia postoperatoria fue de 5 días (RIQ: 4–7). 815 pacientes tuvieron una EPP (≥8 días), de los que el 79.9% presentaron complicaciones postoperatorias. El modelo final incluyó como variables: edad, IMC, sexo varón, VEF1%ppo, DLCO%ppo y toracotomía; el AUC en la serie de derivación fue de 0.684 (IC95%: 0,661–0,706) y en la de validación de 0,73 (IC95%: 0,681–0,78). Se encontró una asociación significativa entre la EPP y el reingreso (p < 0,000) y la mortalidad a 90 días (p < 0,000).
ConclusionesLas variables edad, IMC, sexo varón, VEF1%ppo, DLCO%ppo y toracotomía afectan a la EPP. La EPP se asocia con un incremento del riesgo de reingreso y mortalidad a 90 días. El 20% de las EPP no se relacionan con la ocurrencia de complicaciones postoperatorias.
Most healthcare systems struggle to contain rising costs and allocate available resources wisely. In this scenario, the role of healthcare professionals is fundamental1. The duration of postoperative hospital stay is an important component of the total cost of elective surgical procedures2–4. In addition to these economic implications, postoperative hospital stay has been analyzed as an indicator of quality after cancer-related lobectomies and has been used to establish comparisons among hospitals5,6. Although the occurrence of postoperative complications is the main determinant of the length of postoperative hospital stay, it is likely that certain preoperative demographic or clinical factors that are not controlled by the surgeon and not related to the quality of care may also affect the duration of postoperative stay4,7,8. In our opinion, the identification of predictors for prolonged hospitalization could be relevant to establish the expected hospital stay prior to surgery, which would facilitate surgical planning for hospital bed requirements and optimize the use of available resources. Likewise, the risk-adjusted hospital stay could be used as an indicator of quality when comparing results among different thoracic surgery units with the aim to improve quality of care.
Furthermore, measures aimed at reducing postoperative hospital stay after thoracic procedures can potentially and significantly reduce costs while providing other patients access to hospitalization, but these benefits must be weighed against the risk of readmission among patients discharged early9. Nevertheless, the relationship between postoperative prolonged length of stay (PLOS) and the risk of readmission or mortality after major thoracic procedures has not been well studied.
The objective of this study is to identify the predictive factors of PLOS in patients treated with anatomical lung resection using the database of the Spanish Group of Video-Assisted Thoracic Surgery (GEVATS)10, creating a predictive model, validating it in an external cohort, and evaluating the influence of PLOS on readmission and 90-day mortality in patients undergoing anatomical lung resection.
MethodsStudy populationAll patients who had been prospectively registered in the GEVATS database and had undergone anatomical lung resection were included in the study. The patients were recruited from December 20, 2016 to March 20, 2018 (15 months) by 33 Spanish thoracic surgery departments. The study was approved by the Ethics Committees of all participating hospitals, and patients signed a specific informed consent form to use their data for scientific purposes. Patients who died during postoperative hospitalization and cases with no hospital stay data were excluded.
Statistical analysisThe selected outcome variable was the PLOS, which was defined as the hospital stay in days above the 75th percentile of the stay of all patients included in the study. First, the predictive factors for PLOS were analyzed in all the patients included in the study. Baseline patient demographic, oncological, and surgical variables were evaluated to detect a possible association with PLOS. The variables were initially evaluated with a univariate analysis. Only statistically significant variables were used as independent predictor variables in the logistic regression analysis. Data for continuous quantitative variables were expressed as mean ± standard deviation. The normal distribution of the numerical variables was previously evaluated with the Kolmogorov–Smirnov normality test. Numerical variables with normal distribution were analyzed with the Student’s t test for independent data, while those without normal distribution were analyzed with the Mann–Whitney U test. Categorical variables were expressed as frequencies and percentages and were analyzed with the chi-squared test or Fisher’s exact test if the expected frequency was less than 5. Statistically significant variables in the univariate analysis were used as independent variables in the multivariate analysis. The variables that showed collinearity after the analysis of the correlation matrix among the independent variables were excluded from the model. Backward stepwise logistic regression was used, in which variables with a significance level in the Wald test P > .05 were successively eliminated from the model. Results are presented as odds ratio (OR) with 95% confidence interval (CI) and P-value. To estimate the discrimination capacity of the model, a ROC curve was constructed, and the area under the curve (AUC) and its 95% CI were calculated. Model calibration was evaluated using the Hosmer–Lemeshow goodness-of-fit test. The resulting model was tested in an external validation series using the same application criteria.
Second, the associations between PLOS and 30-day readmission and 90-day mortality were analyzed using the chi-squared test.
For all analyses, a P-value <.05 was considered statistically significant. The data analysis was performed using the SPSS version 26 statistical package (IBM Corp, Chicago, Illinois, 2019).
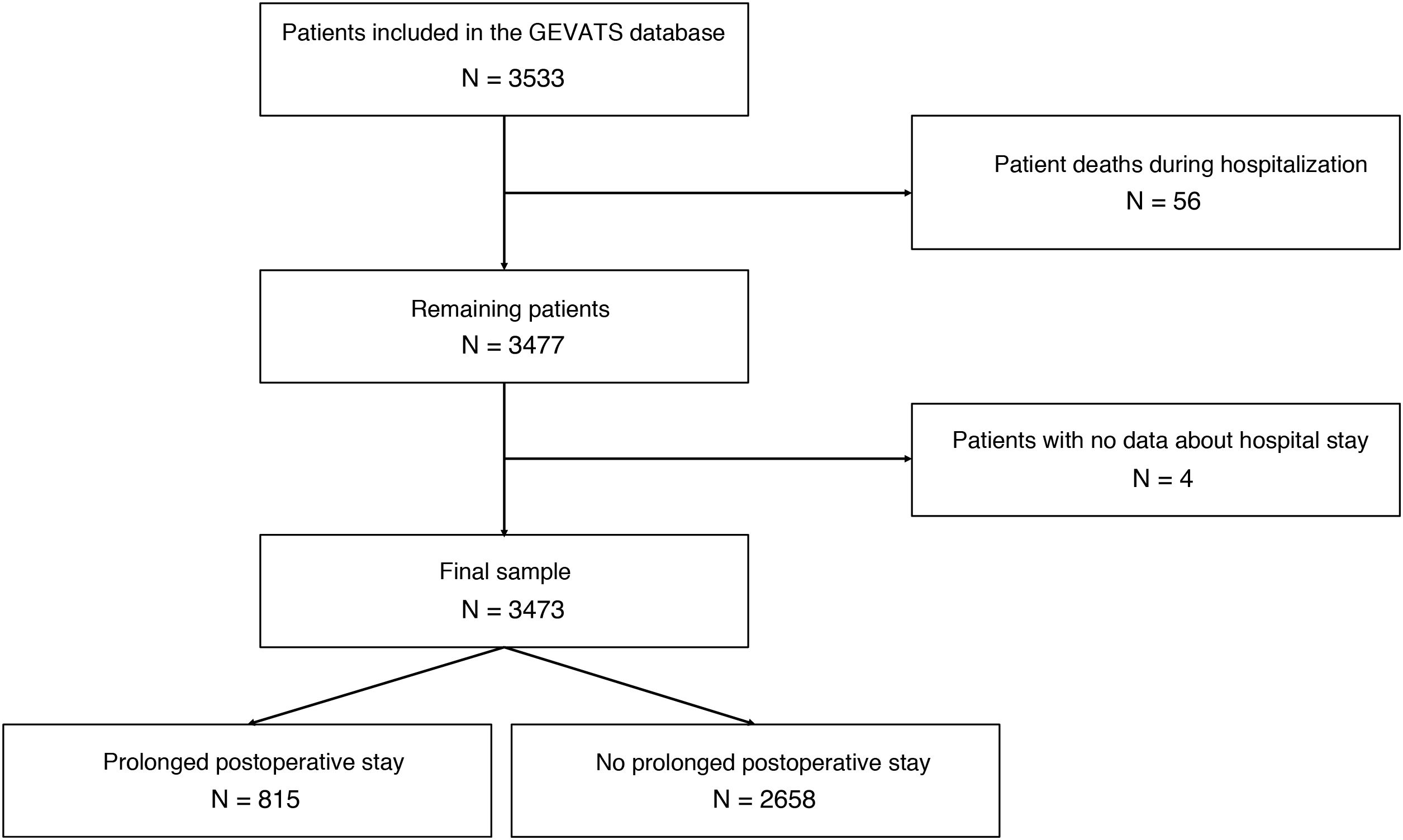
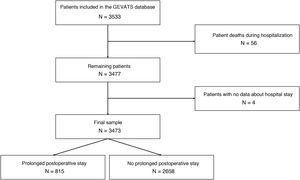
ResultsA total of 3533 patients underwent anatomic resection during the study period. Fifty-six patients died during admission after surgery (1.6%), and 4 cases were excluded due to the lack of data on the length of postoperative stay. Thus, the final sample is comprised of 3473 patients. The median postoperative hospital stay was 5 days (interquartile range: 4–7 days). A total of 815 patients had a hospital stay ≥8 days (Fig. 1), and 79.9% of the patients who had PLOS presented some type of postoperative complication compared to 14.3% of the patients with a hospital stay ≤7 days (P < .000).
Table 1 provides details about the main demographic and clinical characteristics of the patients included in each group, as well as the annual surgical volume of the study center where they were operated on.
Comparison of patient characteristics (prolonged hospital stay versus no prolonged hospital stay).
| Variable | Prolonged hospital stay | No prolonged hospital stay | P value |
|---|---|---|---|
| Age, mean ± SD, years | 65.67 ± 10.01 | 64.6 ± 10.1 | 0.003 |
| BMI, mean ± SD | 26.47 ± 4.74 | 26.99 ± 4.53 | 0.005 |
| Sex, male, n (%) | 635 (77.9) | 1784 (67.1) | < 0.000 |
| Ischemic cardiopathy, n (%) | 76 (9.3) | 230 (8.7) | 0.554 |
| Creatinine >2 mg/dL, n (%) | 19 (2.3) | 71 (2.7) | 0.591 |
| CVA, n (%) | 47 (5.8) | 129 (4.9) | 0.295 |
| Diabetes mellitus, n (%) | 160 (19.6) | 482 (18.1) | 0.337 |
| FEV1%ppo, mean ± SD | 64.53 ± 17.12 | 71.82 ± 18.25 | < 0.000 |
| DLCO%ppo, mean ± SD | 60.37 ± 17.87 | 67.18 ± 18.2 | < 0.000 |
| Induction treatment, n (%) | 66 (9.1) | 193 (8.4) | 0.546 |
| Tumor size >3 cm, n (%) | 320 (44.5) | 831 (36.2) | < 0.000 |
| Lung cancer diagnosis, n (%) | 726 (89.1) | 2305 (86.7) | 0.077 |
| Pneumonectomy, n (%) | 81 (9.9) | 143 (5.4) | < 0.000 |
| Extended resection, n (%) | 78 (9.6) | 97 (3.6) | < 0.000 |
| Approach, n (%) | |||
| Thoracotomy | 508 (62.3) | 1065 (40.1) | < 0.000 |
| Minimal invasion | 307 (37.7) | 1593 (59.9) | |
| Surgical volume, n (%)* | |||
| < 100 cases | 237 (29.1) | 745 (28) | 0.528 |
| 100–150 cases | 281 (34.5) | 886 (33.3) | |
| > 150 cases | 297 (36.4) | 1027 (38.6) | |
CVA: cerebrovascular accident; DLCO%ppo: predicted postoperative diffusing capacity for carbon monoxide; SD: standard deviation; BMI: body mass index; FEV1%ppo: predicted postoperative forced expiratory volume in the first second.
Table 2 describes the postoperative complications presented by patients with PLOS. Those who presented major Clavien–Dindo complications11 (III–IV) had longer mean hospital stays (20.65 days) than those who had minor complications (Clavien–Dindo I–II) (12.37 days).
Description of the main postoperative complications of the patients with prolonged postoperative hospital stay.
| Complications | N (%) |
|---|---|
| Major complications (CD III–IV) | 198 (30.4) |
| Respiratory complications | 538 (66) |
| Pneumonia | 111 (17.1) |
| Empyema | 31 (4.8) |
| ARDS | 21 (3.2) |
| Bronchial fistula | 10 (1.5) |
| PE | 6 (0.9) |
| CD I–II | 365 (67.8) |
| CD III–IV | 173 (32.2) |
| Cardiovascular complications | 122 (15) |
| Arrythmia | 87 (13.4) |
| Heart failure | 15 (2.3) |
| CVA | 2 (0.3) |
| AMI | 2 (0.3) |
| CD I–II | 96 (78.7) |
| CD III–IV | 26 (21.3) |
| Other complications | 135 (16.6) |
| Digestive | 31 (4.8) |
| Urological | 47 (7.2) |
| Psychiatric | 14 (2.2) |
| Hematologic | 10 (1.5) |
| Wound infection | 32 (4.9) |
| CD I–II | 114 (84.4) |
| CD III–IV | 21 (15.6) |
CVA: cerebrovascular accident; CD: Clavien–Dindo classification; AMI: acute myocardial infarction; ARDS: adult respiratory distress syndrome; PE: pulmonary embolism.
The predictive variables associated with PLOS in the final logistic regression model were age, male sex, BMI, predicted postoperative percentage of forced expiratory volume in the first second (FEV1%ppo), predicted postoperative percentage of diffusion capacity of carbon monoxide (DLCO%ppo), and the thoracotomy approach. Results are shown in Table 3.
Results of the logistic regression.
| Variable | Adjusted OR | 95% CI | P value |
|---|---|---|---|
| Age | 1.015 | 1.006–1.025 | 0.002 |
| BMI | 0.97 | 0.95–0.991 | 0.005 |
| Male sex | 1.532 | 1.228–1.911 | < 0.000 |
| FEV1%ppo | 0.988 | 0.982–0.994 | < 0.000 |
| DLCO%ppo | 0.988 | 0.982–0.994 | < 0.000 |
| Thoracotomy | 2.163 | 1.797–2.602 | < 0.000 |
DLCO%ppo: predicted postoperative diffusing capacity for carbon monoxide; BMI: body mass index; FEV1%ppo: predicted postoperative forced expiratory volume in the first second.
The coefficients that made up the final formula for calculating the risk of PLOS (logit) were obtained from the regression study: −0.46 + 0.015 × age – 0.03 × BMI + 0.427 × sex (1 for male and 0 for female) – 0.012 × FEV1ppo% – 0.012 × DLCOppo% + 0.771 × approach (1 for thoracotomy and 0 for minimally invasive).
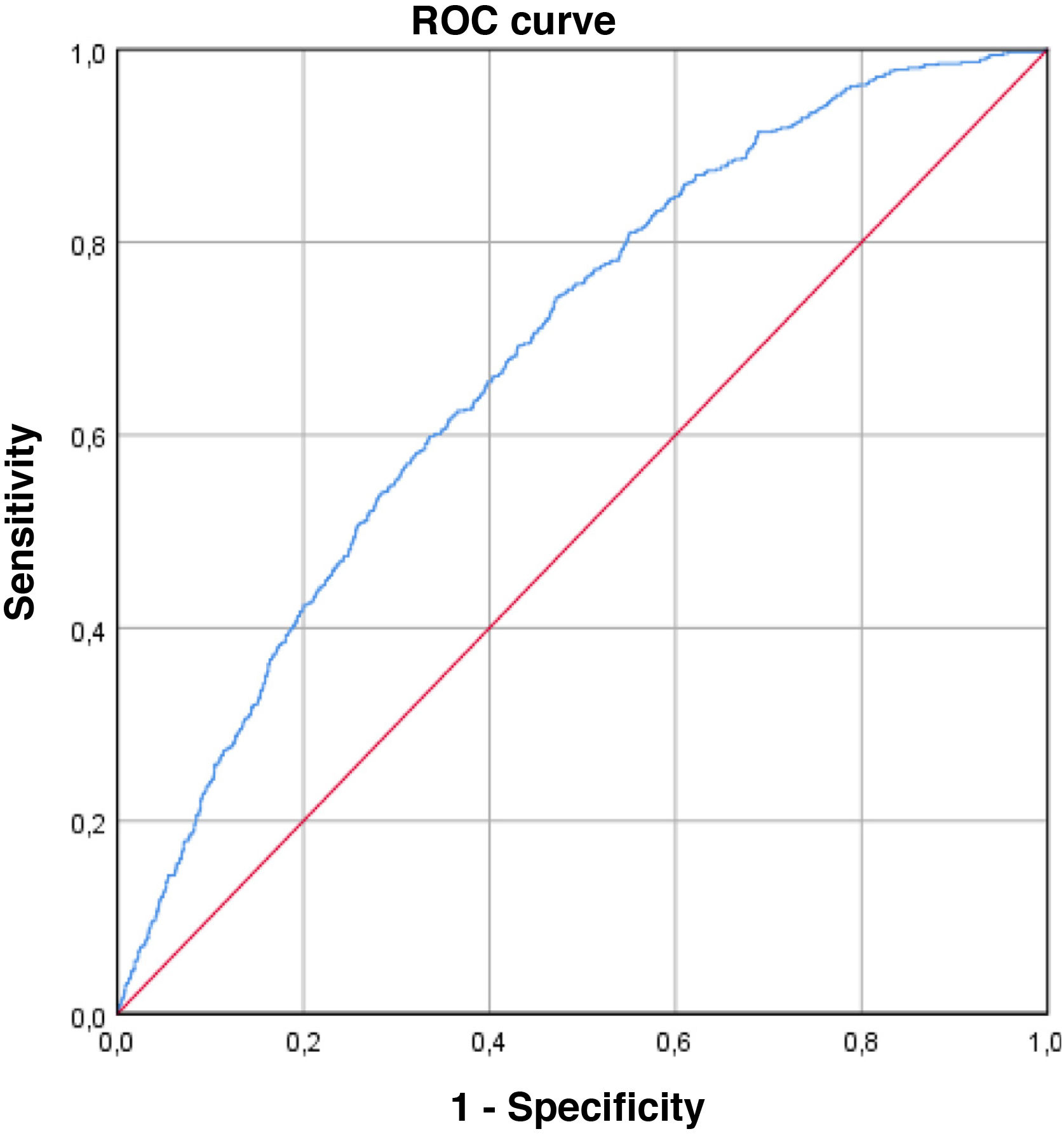
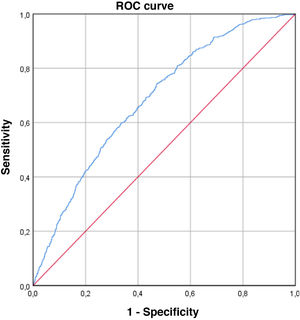
The ROC curve obtained, which estimates the predictive capacity of the model, can be seen in Fig. 2. The AUC was 0.684 (95% CI: 0.661–0.706), indicating a moderate discriminative capacity. The Hosmer–Lemeshow goodness-of-fit test showed a P = .079, which indicates a good goodness-of-fit for the model.
The external validation series was made up of a total of 619 patients who underwent anatomical lung resection consecutively between March 21, 2018 and December 31, 2020 at the Hospital Universitario de Salamanca. The median postoperative hospital stay in this series was 4 days (IQR: 3–5). A total of 133 patients presented PLOS, in this case ≥6 days. Table 4 shows the characteristics of the series and compares them with the referral series. Statistical analysis showed similar magnitudes in the variables included in the model except for FEV1ppo% and the minimally invasive approach, which were significantly higher in the validation series.
Comparison of patient characteristics (referral series versus validation series).
| Variable | Referral series | Validation series | P value |
|---|---|---|---|
| Age, mean ± SD, years | 64.86 ± 10.09 | 65.39 ± 10.24 | 0.135 |
| BMI, mean ± SD | 26.87 ± 4.58 | 26.74 ± 4.67 | 0.277 |
| Sex, male, n (%) | 2419 (69.7) | 429 (69.3) | 0.855 |
| Ischemic cardiopathy, n (%) | 306 (8.8) | 47 (7.6) | 0.320 |
| Creatinine >2 mg/dL, n (%) | 90 (2.6) | 27 (4.4) | 0.015 |
| CVA, n (%) | 176 (5.1) | 0 (1.3) | < 0.000 |
| Diabetes mellitus, n (%) | 642 (18.5) | 117 (18.9) | 0.809 |
| FEV1%ppo, mean ± SD | 70.13 ± 18.26 | 76.56 ± 20.27 | < 0.000 |
| DLCO%ppo, mean ± SD | 65.58 ± 18.35 | 67.04 ± 18.45 | 0.120 |
| Induction treatment, n (%) | 259 (7.5) | 25 (4) | 0.002 |
| Tumor size >3 cm, n (%) | 1151 (38.2) | 242 (39.1) | 0.673 |
| Lung cancer diagnosis, n (%) | 3031 (87.3) | 488 (78.8) | < 0.000 |
| Pneumonectomy, n (%) | 224 (6.4) | 23 (3.7) | 0.009 |
| Extended resection, n (%) | 175 (5) | 52 (8.4) | 0.001 |
| Approach, n (%) | |||
| Thoracotomy | 1573 (45.3) | 135 (21.8) | < 0.000 |
| Minimum invasion | 1900 (54.7) | 484 (78.2) | |
DLCO%ppo: predicted postoperative diffusing capacity for carbon monoxide; BMI: body mass index; FEV1%ppo: predicted postoperative forced expiratory volume in the first second.
The predictive model was tested in the validation series, obtaining an AUC of 0.73 (95% CI: 0.681–0.78), and the Hosmer–Lemeshow goodness-of-fit test showed a value of P = .313.
Finally, a statistically significant association was found between PLOS and readmission (10.9% vs 5.8%, P < .000) and 90-day mortality (2.8% vs 0.9%, P < .000). The analysis of the subgroup of patients who did not present postoperative complications showed similar frequencies of readmission in patients with PLOS versus hospital stays ≤7 days (3.8% vs 4.2%, P = .797), although 90-day mortality was significantly higher in patients with PLOS (3.7% vs 0.7%, P < .000).
DiscussionThe median postoperative hospital stay registered in the GEVATS series is 5 days, which is lower than published reports from other national multicenter series12 and equal to the Giambrone et al. article13 in a series of more than 13 000 patients undergoing pulmonary lobectomy who did not present postoperative complications.
Although various strategies have been described to reduce postoperative stay after lung resection surgery, such as enhanced recovery protocols14, a significant number of patients have prolonged postoperative hospital stays. There are several possible explanations that may justify a PLOS. First, the occurrence of postoperative complications; in our series, almost 80% of the patients with PLOS presented some type of postoperative complication, compared to 14% of the patients with hospital stays equal to or less than 7 days. The prevention of complications and the optimization of their management could improve the overall efficiency of hospitalization for the surgical process. Second, it is possible that the PLOS and the variability in postoperative stays between the different hospitals are due to different practice styles of the healthcare professionals involved in patient care, such as the dissimilar adoption of minimally invasive approaches or enhanced recovery protocols15, different hospital policies regarding discharges during weekends or holidays, or the geographical dispersion of the population treated. Third, certain non-modifiable patient demographic and clinical characteristics can also influence the length of postoperative stay. Unlike the published surgical risk models16,17 focused on postoperative morbidity and mortality, the model obtained aims to identify patients who will require prolonged hospital stays associated or not with postoperative morbidity. In our study, 20% of the patients with PLOS did not present any postoperative complications. If we assume similar styles of care practice in the different hospitals, the excess postoperative stay in these cases could be explained by the patients’ preoperative characteristics (previous comorbidity or frailty), intraoperative variables (extended resections and approaches), and postoperative events not related to adverse effects (analgesic control) requiring greater surveillance.
The final predictive model included age, male sex, BMI, FEV1%ppo, DLCO%ppo and open surgical approach as predictors of PLOS, all of which were included in surgical risk models published previously16,17. The main predictive factor was the open approach (OR: 2.2). The association between reduced hospital stay and minimally invasive approaches has been previously described in large series of patients from Europe and the USA18,19.
The resulting predictive model has a moderate predictive capacity (AUC: 0.684), although its validation in the external series showed a higher predictive capacity (AUC: 0.73). This circumstance could be explained by the lower complexity of the validation series due to the homogeneity in the perioperative management of patients at a single center. It is likely that the postoperative stay in the GEVATS series is also influenced by other variables not recorded in the database, which could differ significantly among the participating hospitals, such as the application or not of postoperative intensive physiotherapy protocols, whose identification would increase the precision and complexity of the model. Furthermore, the model shows good predictive capacity regardless of the variability in individual care practice styles, since it uses the 75th percentile of the hospital stay as the cut-off point to define PLOS in the referral series (7 days) and the validation series (5 days). Therefore, we believe that the model could be useful as an indicator of the quality of medical care to compare the results of different thoracic surgery units.
One of the most relevant findings of our study is the relationship between PLOS and readmission and 90-day mortality. In our series, patients with PLOS have a higher risk of readmission and 90-day mortality compared to patients with non-prolonged postoperative stays. These findings are consistent with what was published by Quero-Valenzuela et al.20, who found that patients requiring readmission had longer postoperative stays and that readmission significantly increased the risk of 90-day mortality. Since the occurrence of postoperative complications is the main risk factor for readmission21,22 and 90-day mortality23, we analyzed the subgroup of uncomplicated patients. Our results indicate that PLOS is associated with an increased risk of 90-day mortality, while the risk of readmission is not altered by length of stay in uncomplicated patients.
LimitationsThis study presents certain limitations:
-
This is a study based on a voluntary registry, which may associate patient selection bias. However, it should be noted that patients from centers with low recruitment rates were excluded, and an internal audit was carried out for data quality (98% agreement)10.
-
There is a potential influence of variability in the perioperative management of patients among the different participating study centers. The variability in hospital stay after pulmonary lobectomy has been associated with surgical volume; hospitals with low surgical volumes are 1.46–2.36 times more likely to exceed the median postoperative stay than hospitals with high volume13. However, in the present study, no significant association was found between surgical volume and PLOS.
-
There are patient-specific factors (performance status, frailty or adequate analgesic control) that were not considered in the database records and may affect the hospital stay duration24, which could explain PLOS in uncomplicated patients.
In conclusion, the calculated predictive model using age, BMI, male sex, FEV1%ppo, DLCO%ppo, and thoracotomy demonstrates acceptable performance for predicting PLOS. PLOS is associated with an increased risk of readmission and 90-day mortality. However, 20% of PLOS are not associated with the existence of postoperative complications, so other variables that influence the quality of the hospital care process must be analyzed.
FinancingThe costs related to the development and maintenance of the GEVATS database have been covered by Ethicon, Johnson & Johnson. The authors have had absolute freedom and control in all aspects related to the design, methodology, analysis and composition of the study. GEVATS received a research grant from the Spanish Society of Thoracic Surgery in 2015.
Conflict of interestsThe authors have no conflict of interests to declare.
Raúl Embuna, Iñigo Royo-Crespoa, José Luis Recuero Díaza, Sergio Boluferb, Sergi Callc, Miguel Congregadod, David Gómez-de Antonioe, Marcelo F. Jimenezf, Nicolás Moreno-Matag, Borja Aguinagaldeh, Sergio Amor-Alonsoi, Miguel Jesús Arrarásj, Ana Isabel Blanco Orozcok, Marc Boadal, Alberto Cabañero Sánchezg, Isabel Cal Vázquezm, Ángel Cilleruelo Ramosn, Silvana Crowley Carrascoe, Elena Fernández-Martínn, Santiago García-Barajaso, María Dolores García-Jiménezp, José María García-Primq, José Alberto García-Salcedor, Juan José Gelbenzu-Zazpes, Carlos Fernando Giraldo-Ospinat, María Teresa Gómez Hernándezf, Jorge Hernándezu, Jennifer D. Illana Wolfv, Alberto Jauregui Abularachw, Unai Jiménezx, Iker López Sanzh, Néstor J. Martínez-Hernándezy, Elisabeth Martínez-Téllezz, Lucía Milla Colladoaa, Roberto Mongil Pocet, Francisco Javier Moradiellos-Díezi, Ramón Moreno-Balsalobrem, Sergio B. Moreno Merinod, Carme Obiolsc, Florencio Quero-Valenzuelaab, María Elena Ramírez-Gils, Ricard Ramos-Izquierdoac, Eduardo Rivoq, Alberto Rodríguez-Fusterad,ae, Rafael Rojo-Marcosx, David Sánchez-Lorentel, Laura Sánchez Morenoaf, Carlos Simónag, Juan Carlos Trujillo-Reyesz, Florentino Hernando Tranchon.
a) Servicio de Cirugía Torácica, Hospital Universitario Miguel Servet y Hospital Clínico Universitario Lozano Blesa, IIS Aragón, Zaragoza
b) Servicio de Cirugía Torácica, Hospital General Universitario de Alicante, Alicante
c) Servicio de Cirugía Torácica, Hospital Universitari Mútua Terrasa, Universidad de Barcelona, Terrasa, Barcelona
d) Servicio de Cirugía Torácica, Hospital Universitario Virgen Macarena, Sevilla
e) Servicio de Cirugía Torácica, Hospital Universitario Puerta de Hierro Majadahonda, Madrid
f) Servicio de Cirugía Torácica, Hospital Universitario de Salamanca, Universidad de Salamanca, IBSAL, Salamanca
g) Servicio de Cirugía Torácica, Hospital Universitario Ramón y Cajal, Madrid
h) Servicio de Cirugía Torácica, Hospital Universitario de Donostia, San Sebastián-Donostia
i) Servicio de Cirugía Torácica, Hospital Universitario Quironsalud Madrid, Madrid
j) Servicio de Cirugía Torácica, Fundación Instituto Valenciano de Oncología, Valencia
k) Servicio de Cirugía Torácica, Hospital Universitario Virgen del Rocío, Sevilla
l) Servicio de Cirugía Torácica, Hospital Clinic de Barcelona, Instituto Respiratorio, Universidad de Barcelona, Barcelona
m) Servicio de Cirugía Torácica, Hospital Universitario La Princesa, Madrid
n) Servicio de Cirugía Torácica, Hospital Clínico Universitario, Valladolid
ñ) Servicio de Cirugía Torácica, Hospital Clínico San Carlos, Madrid
o) Servicio de Cirugía Torácica, Hospital Universitario de Badajoz, Badajoz
p) Servicio de Cirugía Torácica, Hospital Universitario de Albacete, Albacete
q) Servicio de Cirugía Torácica, Hospital Universitario Santiago de Compostela, Santiago de Compostela
r) Servicio de Cirugía Torácica, Hospital Universitario 12 de Octubre, Madrid
s) Servicio de Cirugía Torácica, Complejo Hospitalario de Navarra, Pamplona
t) Servicio de Cirugía Torácica, Hospital Regional Universitario, Málaga
u) Servicio de Cirugía Torácica, Hospital Universitario Sagrat Cor, Barcelona
v) Servicio de Cirugía Torácica, Hospital Puerta del Mar, Cádiz
w) Servicio de Cirugía Torácica, Hospital Universitario Vall d’Hebron, Barcelona
x) Servicio de Cirugía Torácica, Hospital Universitario Cruces, Bilbao
y) Servicio de Cirugía Torácica, Hospital Universitario La Ribera, Alcira, Valencia
z) Servicio de Cirugía Torácica, Hospital Santa Creu y Sant Pau, Universidad Autónoma de Barcelona, Barcelona
aa) Servicio de Cirugía Torácica, Hospital Arnau de Vilanova, Lleida
ab) Servicio de Cirugía Torácica, Hospital Virgen de las Nieves, Granada
ac) Servicio de Cirugía Torácica, Hospital Universitario de Bellvitge, Hospitalet de Llobregat, Barcelona
ad) Servicio de Cirugía Torácica, Hospital del Mar, Barcelona
ae) IMIM (Instituto de Investigación Médica Hospital del Mar), Barcelona
af) Servicio de Cirugía Torácica, Hospital Universitario Marqués de Valdecilla, Santander
ag) Servicio de Cirugía Torácica, Hospital Universitario Gregorio Marañón, Madrid