The antimicrobial properties of a silver ion (Ag+)-releasing polyurethane foam were evaluated using different microorganisms. The diffusion of Ag+ from the medium, as well as any possible cytotoxicity on human cells, was also studied.
Material and methodsSilver release from V.A.C. GranuFoam Silver® was assessed by using inductively coupled plasma mass spectrometry (ICP-MS). An in vitro experimental study was designed to evaluate the bactericide capacity using lethal dose curves on Acinetobacter baumannii, Pseudomonas aeruginosa, Stenotrophomonas maltophilia, K. pneumoniae, Escherichia coli, Proteus mirabilis, methicillin resistant Staphylococcus aureus, Enterococcus faecium, Streptococcus pyogenes and Corynebacterium minutissimum. A cytotoxicity study was also performed on human fibroblasts.
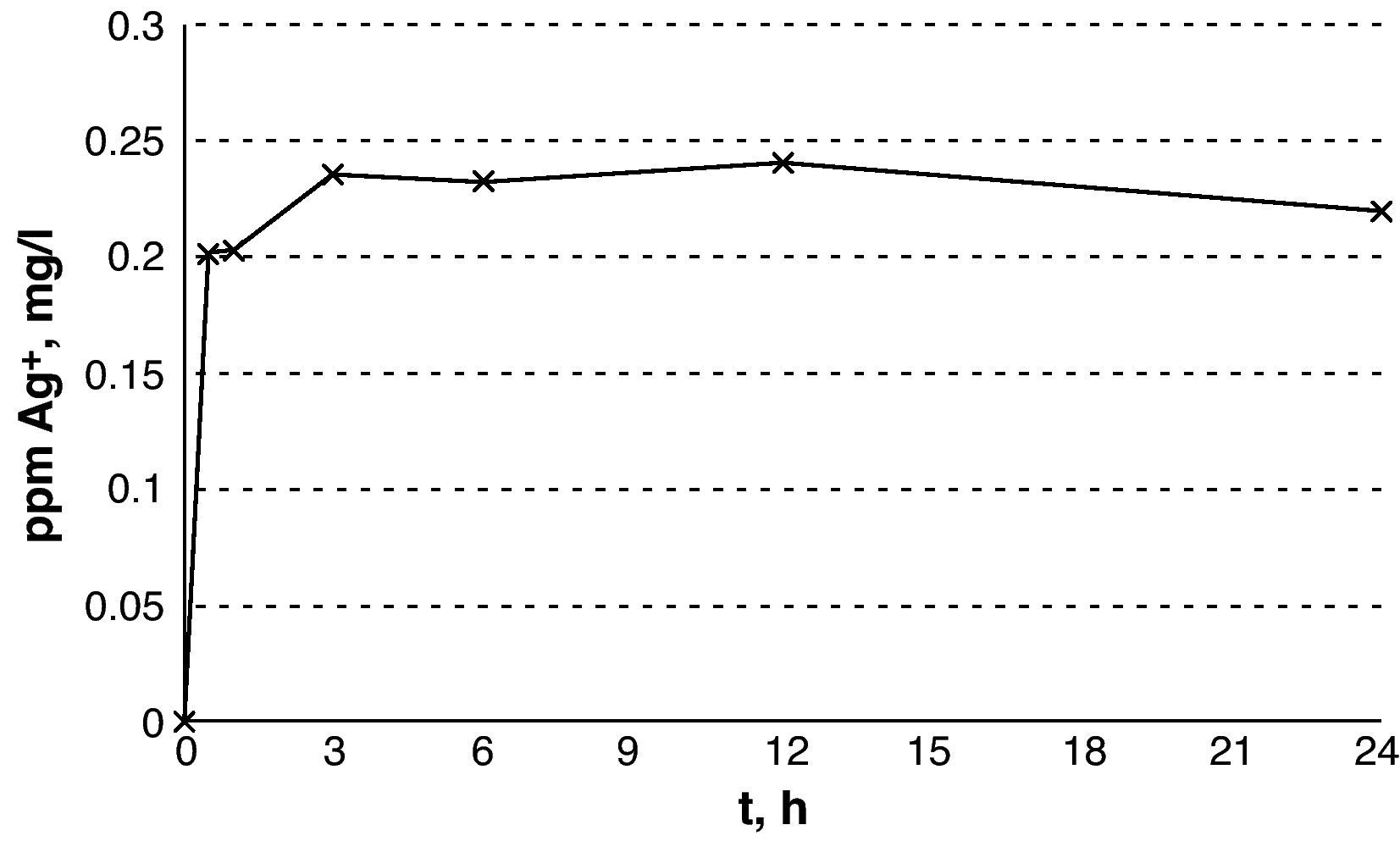
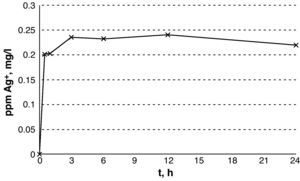
ResultsThe silver release showed an exponential curve with a stable meseta phase after 3h, with levels of 0.22–0.24mg/l. A reduction of 99.9% of all the Gram-negatives was achieved at 3h. The reduction was greater than 99% at 2h in S. pyogenes and C. minutissimum, at 6h in S. aureus and at 14h in E. faecium. In an in vivo simulation model, these reductions were achieved in 6h in the Gram negatives and 24h in the Gram positives. The silver concentrations were no cytotoxic to human fibroblasts, with no differences being observed between the cells exposed to Ag+ and the controls (P=.7).
ConclusionV.A.C. Granufoam Silver® releases bactericide concentrations of Ag+ that did not damage human fibroblasts. It appears to be a good alternative for the control and prevention of local infections.
Evaluar las propiedades antimicrobianas de una espuma de poliuretano que libera iones de plata sobre diversos microorganismos. Se estudia la difusión al medio de Ag+, así como la posible citotoxicidad sobre células humanas.
Material y métodosEstudio de liberación de plata de V.A.C. GranuFoam Silver® mediante espectrometría de masas (Inductively Coupled Plasma Mass). Estudio experimental in vitro para evaluar la capacidad bactericida mediante curvas de letalidad sobre A. baumannii, P. aeruginosa, S. maltophilia, K. pneumoniae, E. coli, P. mirabilis, S. aureus resistente a meticilina, E. faecium, S. pyogenes y C. minutissimum. Estudio de citotoxicidad sobre fibroblastos humanos.
ResultadosLa liberación de Ag+ muestra una curva exponencial con una fase estable de meseta a partir de las 3h, con niveles de 0,22-0,24mg/l. En 3h se logró una reducción superior al 99,9% en todos los gramnegativos excepto en E. coli que fue del 92,5%. La reducción fue superior al 99% a las 2h en S. pyogenes y C. minutissimum, a las 6h en S. aureus y a las 14h en E. faecium. En simulación in vivo estas reducciones se alcanzaron en 6h en los gramnegativos y en 24h en los grampositivos. Las concentraciones de Ag+ no fueron citotóxicas sobre fibroblastos humanos, sin observar diferencias entre las células expuestas a Ag+ y los controles (p = 0,7).
ConclusiónV.A.C. Granufoam Silver® liberó concentraciones bactericidas de Ag+ que no fueron perjudiciales para los fibroblastos humanos. Se presenta como una buena alternativa para el control y prevención local de las infecciones.
Silver (Ag+) has been used for antimicrobial purposes for centuries. Herodotus described how Cyrus's troops stored water in silver jugs to stop it from becoming stagnant whilst on military campaigns.1 The Romans would also place silver coins in glasses of water to act as a disinfectant.2
At present, due to the growing number of multidrug-resistant microorganisms, there is a need for alternatives to normal antimicrobial treatments, especially in topical treatment and infection prophylaxis. The use of material containing silver could be one of the solutions. However, despite the appearance of silver-releasing dressings and catheters, the studies that have been performed confuse medical professionals.2–5 These new materials have the potential to benefit a patient's health when it comes to preventing infections that complicate their symptoms. They could also have a great impact on the control of antimicrobial resistance. As a result, the excessive use of antimicrobial agents would become unnecessary when preventing infections caused by microorganisms, which pose a therapeutical challenge in some units because of multidrug resistance.
The aim of this study is to evaluate the antimicrobial properties of new silver ion-releasing polyurethane foam using different multidrug-resistant microorganisms or microorganisms that can pose a risk when present in a wound. The diffusion of silver from the medium, as well as any possible cytotoxicity on human cells, was studied.
Material and MethodAn in vitro experimental study designed to evaluate the bactericidal capacity of V.A.C. GranuFoam Silver®.
MicroorganismsThe microorganisms used in this study came from clinical samples and included the following for the analysis with Phosphate Buffered Saline (PBS): colistin-sensitive Acinetobacter baumannii (A. baumannii), colistin-sensitive Pseudomonas aeruginosa (P. aeruginosa), cotrimoxazole-sensitive Stenotrophomonas maltophilia (S. maltophilia), Friedlander's bacillus (K. pneumoniae) producing carbapenems (metallo-beta-lactamase), Escherichia coli (E. coli) producing inhibitor resistant TEM (IRT) beta-lactamase, methicillin-resistant Proteus mirabilis (P. mirabilis), Staphylococcus aureus (S. aureus), Enterococcus faecium (E. faecium), Streptococcus pyogenes (S. pyogenes) and Corynebacterium minutissimum (C. pyogenes).
For the in vivo simulation study, microorganisms of the same species, except for S. pyogenes and C. minutissimum, were used.
Colonies were incubated for 18h at 36°C on blood agar.
Lethality StudyLethality curves of V.A.C. Granufoam Silver® were created introducing a product sample of 3.2cm×2cm×0.5cm (0.100g, 95% CI, 0.09–0.11) into 25ml of PBS with a trough level for the microorganism being studied of 105 colony-forming units (CFU)/ml, and with Minimum Essential Medium Eagle with 10% foetal bovine serum (MEM-10) and a microorganism concentration of approximately 107 CFU. They were incubated under agitation at 36°C and, in order to count the number of surviving microorganisms, aliquots of 100μl were taken every hour in the case of the Gram-negatives, every 2h in the study of the Gram-positives in PBS, and every 6h in the study of the Gram-positives in MEM. The aliquots were cultured on blood agar plates and the colonies were counted 48h after incubation at 36°C.
Twenty-five milliliters of PBS or MEM10 of identical microorganism concentration were used as a growth control and the surviving microorganisms were counted.
The study was repeated three times on different days under the same conditions and the limit of detection of the surviving microorganisms was ≥100CFU/ml.
In Vivo Simulation StudyIsolates of the same species as those of the lethality study with MEM10 under the same conditions and concentrations of inoculum and incubation. Likewise, aliquots of 100μl were taken every hour in the case of the Gram-negatives, and every 5h in the case of the Gram-positives. Four 25μl drops were cultured on Müller-Hinton agar plates. After an incubation period of 24h at 36°C, a semiquantitive count was carried out and growth was noted and classified as either abundant, moderate, scarce or none.
Study of Subpopulations Below the Limit of DetectionOnce the results had been obtained, the experiment in PBS was repeated two times. The only difference was that aliquots were 1ml instead of 100μl. They were taken every hour for 24h, the time from when growth could not be detected in the previous study. The purpose was to observe surviving microorganism concentrations between 1 and 100CFU/ml.
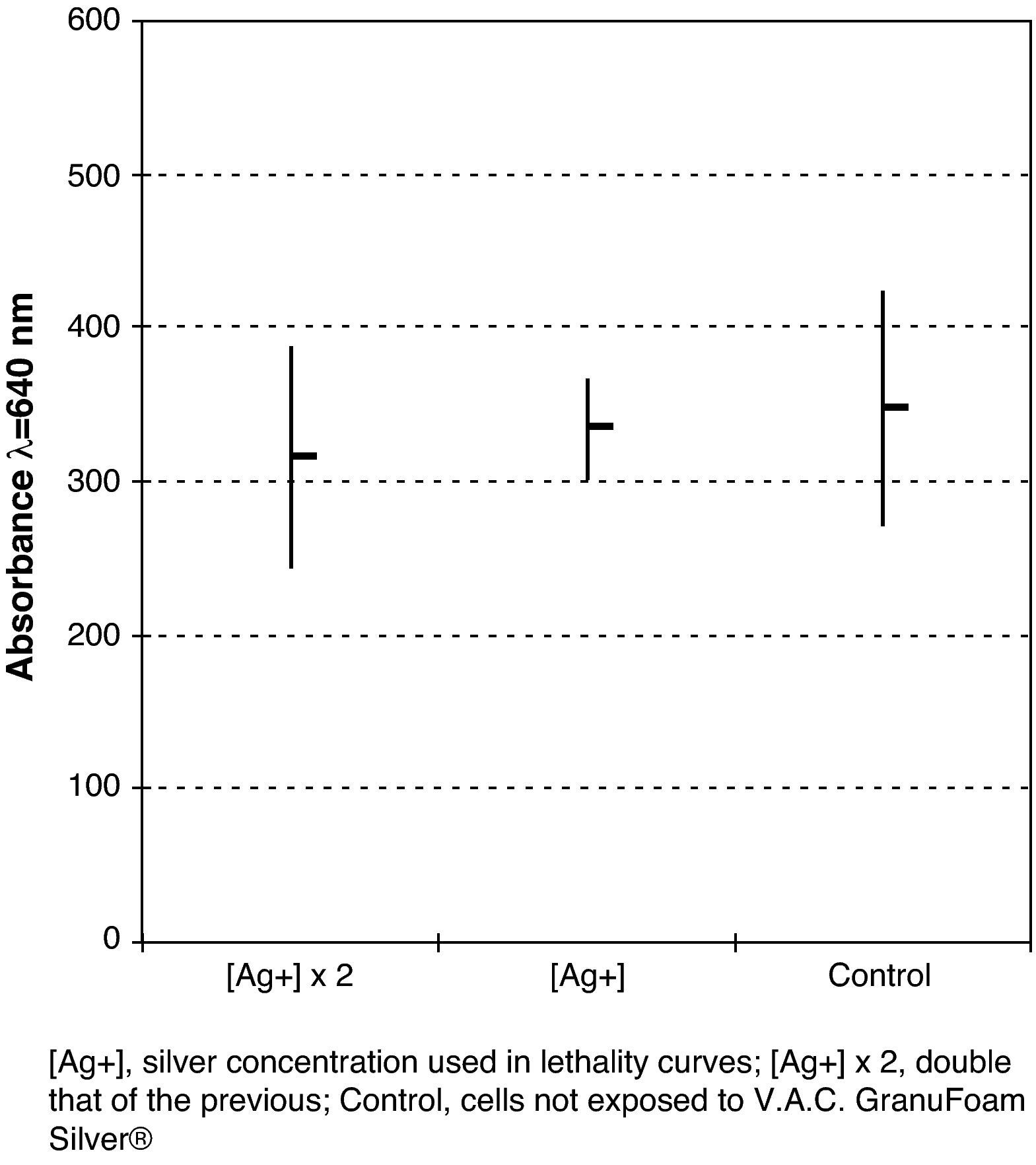
Cytotoxicity StudyThe cytotoxicity study was performed with the MRC-5 cell-line which consists of foetal human diploid lung fibroblasts. These cells were cultured in quintuplicate at a concentration of 100000 cells/ml in plates of 96 flat bottom wells with MEM10 derived from 25ml incubated for 24h with a 3.2cm×2cm×0.5cm sample of V.A.C. Granufoam Silver®, and MEM10 incubated in the same way but with double the amount of cells. MEM10 was used as a growth control. After an incubation period of 24h at 36°C and with an atmosphere of 5% CO2, the surviving cells were stained with crystal violet solution, the methanol dye was extracted and the absorbance of the dye retained by the cells was measured in a spectrophotometer at a wavelength of 640nm.
Silver Release StudyUnder the same conditions as the lethality study (0min, 30min, 1h, 3h, 6h, 12h, and 24h), samples of PBS that had been in contact with V.A.C. Granufoam Silver® were taken, and the amount of silver released through the Inductively Coupled Plasma Mass (ICP-MS) technique was determined. In brief: the liquid sample is inserted using a nebuliser and argon gas spray in plasma which is generated simultaneously using argon current. This current reaches temperatures of up to 8000°C. At such temperature a high percentage of atoms are ionised. A beam of ions is generated and is driven through an interface at decreasing pressure until it reaches a vacuum in the mass analyser, which is capable of selecting the mass/load ratio for the metal in question. This means that any irrelevant matter is eliminated so that the metal being studied reaches the detector. The greater the amount of relevant metal that reaches the detector, the greater the signal that is generated which, in turn, means that the metal can be quantified by comparing this signal with that of a calibration line.6
The samples had been previously diluted at 1/10 in 0.5% nitric acid due to the salt levels within the PBS, analysed using the equipment and quantified with rhodium as an internal standard.
Statistical AnalysisThe lethality periods for the different microorganisms were contrasted using the Mann–Whitney U-test, whereas the results of the cytotoxicity study were contrasted using the ANOVA test. A P<.05 value was considered to define statistical significance.
ResultsThe results from the release of silver show an exponential curve with a rapid increase in the concentration of silver within the first 30min and a plateau after 3h that remains stable for up to 24h (Fig. 1) with silver levels between 0.22 and 0.24mg/l.
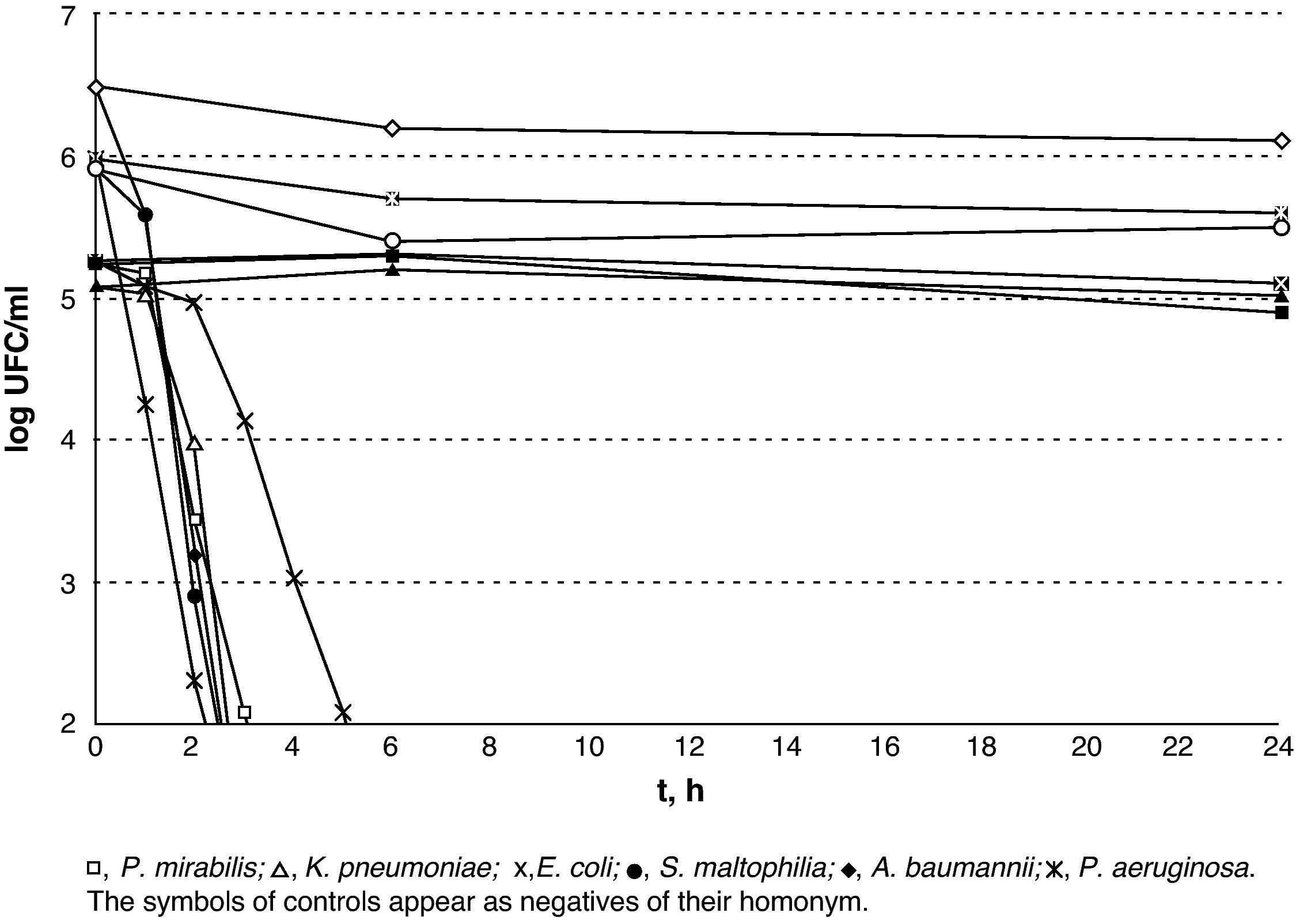
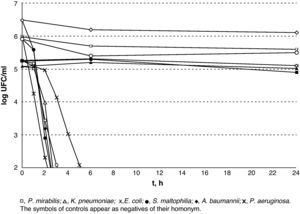
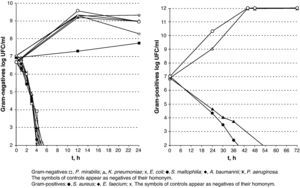
The results of lethality in PBS for Gram-negatives are shown in Fig. 2. The non-fermenting Gram-negative bacilli did not display a rapid decrease upon contact with silver, whereas the enterobacteria displayed an initial plateau followed by rapid decrease. E. coli was the most resistant type of Gram-negative and counts were lower than 100CFU/ml after 6h in incubation. A subpopulation of S. maltophilia lower than 100CFU/ml was detectable for up to 8h and a subpopulation of K. pneumoniae was detectable for up to 5h.
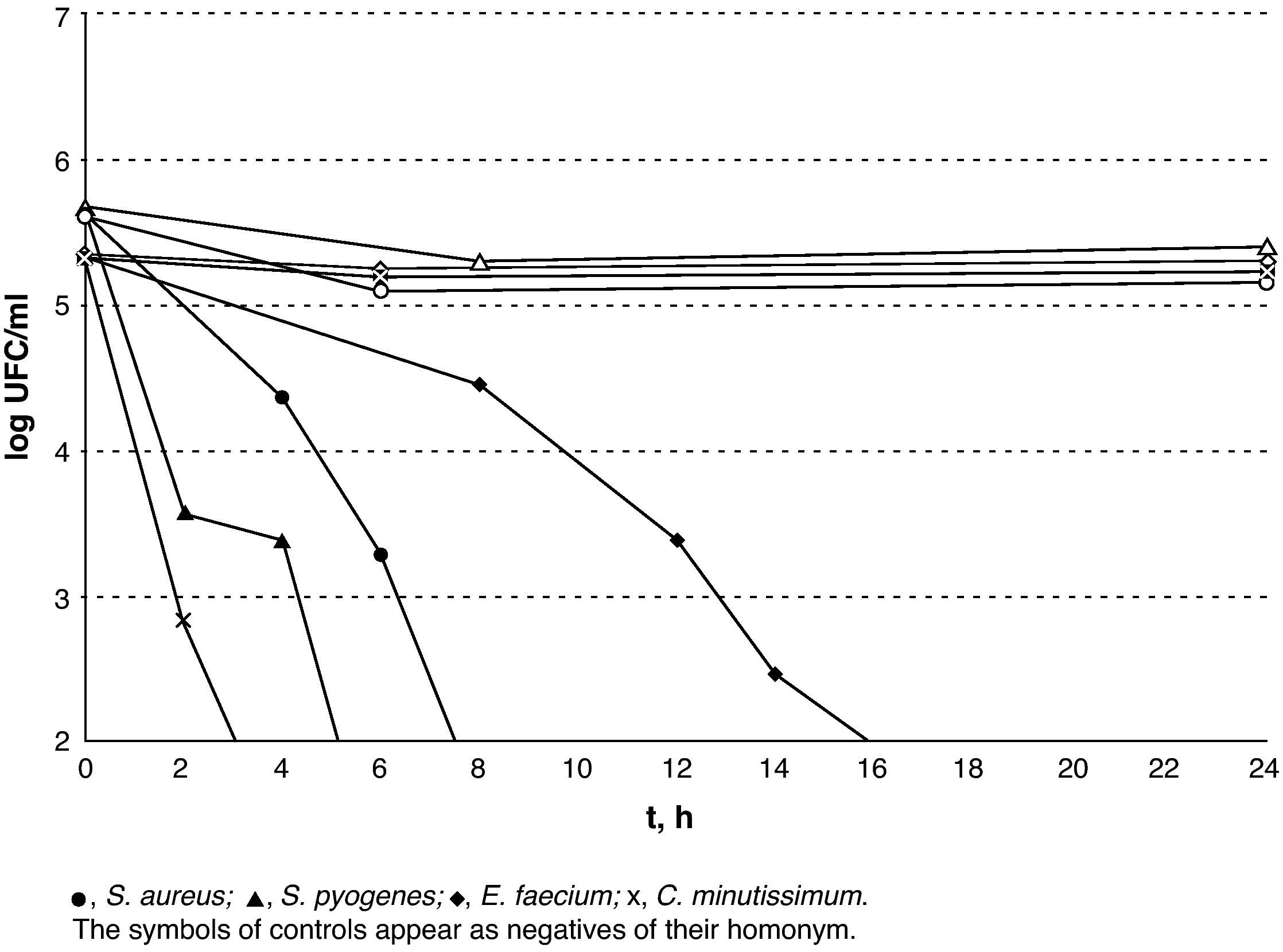
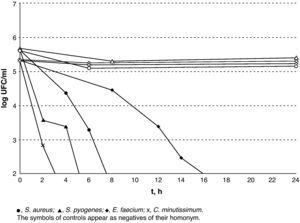
The results of lethality in PBS for the Gram-negatives are shown in Fig. 3. The Gram-positives were the most resistant microorganisms (P=.02; Mann–Whitney U-test) and E. faecium was the most resistant of all those that were tested with V.A.C. GranuFoam Silver® as counts were lower than 100CFU/ml after 16h but with a subpopulation that could be detected for up to 24h.
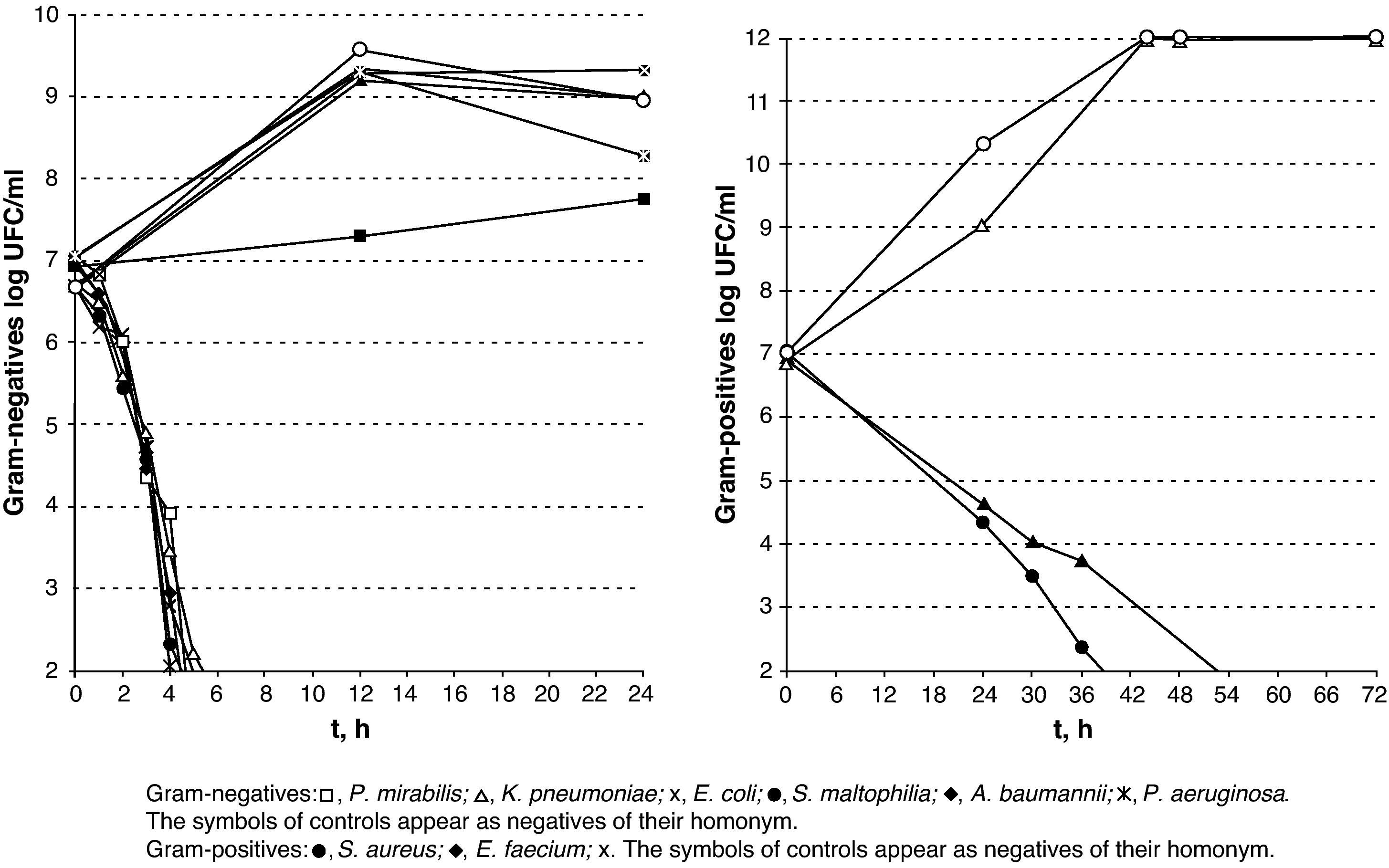
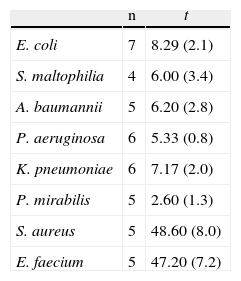
The results of lethality in MEM10 are shown in Table 1 and Fig. 4. They were similar to those obtained with PBS apart from the tailing of CFU/ml decreasing times and survival times, mainly in the Gram-positives and particularly in S. aureus.
Survival Mean Time of the Microorganisms Studied With MEM10.
| n | t | |
| E. coli | 7 | 8.29 (2.1) |
| S. maltophilia | 4 | 6.00 (3.4) |
| A. baumannii | 5 | 6.20 (2.8) |
| P. aeruginosa | 6 | 5.33 (0.8) |
| K. pneumoniae | 6 | 7.17 (2.0) |
| P. mirabilis | 5 | 2.60 (1.3) |
| S. aureus | 5 | 48.60 (8.0) |
| E. faecium | 5 | 47.20 (7.2) |
n: number of strains; t: meant time in hours (standard deviation).
After contacting with silver for 1h in the case of the Gram-negatives and 2h in the case of the Gram-positives, the viable microorganism counts showed two types of colonies: normal morphology ones and a variable quantity (10%–50%) of microcolonies depending on the microorganism.
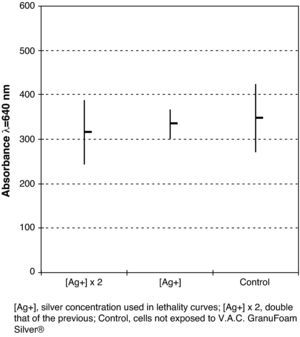
The cytotoxicity study did not show any significant difference (P=.7; ANOVA) between cell growth control and the cells that were incubated with V.A.C. Granufoam Silver® at different concentrations (Fig. 5). No cytopathic effect was observed.
DiscussionThe most relevant findings of this study are: (1) V.A.C. Granufoam Silver® quickly reached and maintained levels of silver that turned out to be bactericidal against all the microorganisms upon which it was tested; (2) within 3h, a reduction in the initial inoculum was achieved and was higher than 99.9% in all of the Gram-negatives, except for E. coli (92.5%); (3) reduction was higher than 99% after 2h in S. pyogenes and C. minutissimum, after 6h in S. aureus and after 14h in E. faecium; (4) the silver concentrations used were not cytotoxic to human fibroblasts.
In this study, V.A.C. Granufoam Silver® has proved to hold a good bactericidal capacity against all the microorganisms for which it was tested. Furthermore, the results obtained show that the sensitivity of silver varies amongst the different pathogens. On the one hand, non-fermenting Gram-negative bacilli are a very sensitive group. Upon contacting with silver, they showed a sharp decrease in the viable cell-count, reaching 99.9% after 2h, and 98.1% in the first hour in the case of P. aeruginosa. However, S. maltophilia showed a very small subpopulation that was able to survive for up to 8h. On the other hand, the enterobacteria displayed greater initial tolerance. This can probably be attributed to the silver concentration, which only reached its peak after 3h. With regard to the Gram-positive C. minutissimum and S. pyogenes, they displayed elimination times similar to those of the enterobacteria. Methicillin-resistant S. aureus survived for longer than any other Gram-negative but 99.5% of its population had been eradicated within 6h. The case of E. faecium stands as a separate matter as it was viable after 24h of exposure. The mechanisms through which this isolate is able to survive for such a long time are unknown to us. However, it is interesting that while the resistance mechanisms to silver described to date in several species correspond to plasmid-encoded efflux pumps,7,8 a member of the Enterococcus species, E. hirae, has a chromosomally encoded copper efflux pump (CopB) that is capable of eliminating silver from the cell cytoplasm.9
Our data concur with previous studies that found a greater sensitivity to silver in Gram-negatives compared to Gram-positives.10–15 This can partly be explained by the thicker peptidoglycan layer, which acts as a protector.10 Furthermore, the microcolonies found after exposure to silver could be described as less viable microorganisms due to the damage caused to their cell membrane,10 enzymes, proteins or ribosomes,16 and they would correspond to the previous state of active but not cultivable bacteria, according to Jung et al.10
With regards to silver release, V.A.C. Granufoam Silver® quickly reached concentrations that turned out to be bactericidal. The numerous published studies on this subject could be deceiving and confusing given that the information that can be brought together is ambiguous.2,4,7 There is no standard methodology between studies, the material used for lethality studies differs from that used to study the concentration of silver and we found that a wide range for the silver concentration required to achieve a bactericidal effect (between 0.1mg/l and 80mg/l).2,4 Furthermore, although all speak of silver concentrations, some speak of AgNO3, others of silver nanoparticles and others of silver ions.2,12,13 We designed our study according to the situation that V.A.C. Granufoam Silver® would face under real conditions, namely, a wound with isotonic exudate. Our results were comparable to those described by Jung et al.10 in terms of bactericidal concentrations of silver in PBS. In our study, however, we observed the desired answer at 0.20–0.24ppm whereas they report 0.2ppm.
Another very important factor that must be borne in mind when comparing results is the level of halogens, such as the Cl− present in the medium in which the test was carried out, because at low levels they tend to form slightly soluble precipitates, reducing bioavailability. Also, at high concentrations, anionic complexes such as AgCl2− and AgCl32− are formed which penetrate the cell membrane more easily and increase the amount of bioavailable silver to the point where they make plasmid-containing bacteria sensitive despite the fact that they normally offer resistance to silver by using efflux pumps or porin deficiency.17 Our experiment was performed in an isotonic medium with a chlorine concentration that was ¼ higher than that expected in a wound exudate, i.e. 139mM/l in PBS vs 106mM/l in plasma, and in MEM10 of very similar characteristics to those of human exudate.
Conducting the lethality study using MEM10 and very high microorganism inocula has allowed us to approximate an in vivo situation and obtain very positive results. The Gram-negatives were found to be the most sensitive to silver, whereas S. aureus and E. faecium displayed a greater resistance despite being completely eradicated between 48 and 72h.
Finally, all of the above mentioned would serve no purpose at all if the area that is going to be in contact with the polyurethane mesh was damaged or if the scarring process delayed. Our data indicates that the silver quantities used were safe, given that they did not affect the human fibroblasts that were exposed.
To conclude, V.A.C. Granufoam Silver® released bactericide concentrations of silver that did not damage human fibroblasts. Despite the appearance of some more resistant subpopulations along with the varying time periods in which total eradication was achieved depending on the microorganism, all of the isolates decreased by more than 99% after 6h of exposure in PBS, except for E. faecium, which took 14h. The results obtained with MEM10 deserve to be studied in much greater detail but it must be pointed out that an elimination higher than 99% was achieved among the Gram-negatives alter 6h, and among the Gram-positives after 24h. The specifications of use for V.A.C. Granufoam Silver® recommend that the dressing is changed every 48h. Therefore, it appears to be a good alternative for the control and prevention of wound infections.
Conflict of InterestThe authors have no conflicts of interest to declare.
We would like to thank KCi Spain for providing us with the V.A.C. GranuFoam Silver® silver polyurethane foam samples.
Please, cite this article as: Sahuquillo Arce JM, et al. Estudio in vitro de las propiedades antimicrobianas de una espuma de poliuretano que libera iones de plata. Cir Esp. 2011; 89:532–8.