Pro-inflammatory cytokines play an important role in diabetic retinopathy. There is conflicting evidence about their serum elevation in this condition and that they also may be possible serum inflammatory biomarkers of diabetic retinopathy.
ObjectiveTo evaluate the presence of serum pro-inflammatory cytokines and acute phase reactants in the serum of patients with and without diabetic retinopathy.
Material and methodsComparative case series with 36 patients divided into three groups were included: 12 patients with diabetes mellitus and diabetic retinopathy (group 1), 12 diabetic patients without diabetic retinopathy (group 2), and 12 healthy patients as a control group. Serum levels of the following pro-inflammatory cytokines were measured in all patients: TNF-α, IL-1β and IL-6. Pro-inflammatory biomarkers measurements were also performed, such as erythrocyte sedimentation rate and C-reactive protein.
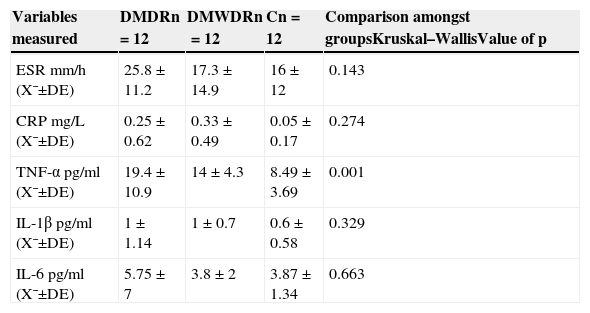
ResultsThe levels of TNF-α and IL-6 were higher in group 1 (TNF-α: 19.4±10.9pg/ml, IL-6: 5.75±7pg/ml) compared to the other two groups, although the difference was statistically significant only in the case of TNF-α (group 1: 19.4±10.9pg/ml, group 2: 14±4.3pg/ml and control: 8.49±3.69pg/ml, p=0.001). There were no differences among pro-inflammatory biomarkers such as erythrocyte sedimentation rate and C reactive protein. Among the three groups (p>0.05).
ConclusionsPro-inflammatory serum cytokine levels were higher in the diabetes mellitus with diabetic retinopathy group. Larger studies are warranted to establish the real impact of this finding.
Las citoquinas proinflamatorias desempeñan un papel importante en la retinopatía diabética; existe evidencia contradictoria sobre la elevación sérica de las mismas en esta condición y el posible rol que tienen como su marcador sérico.
ObjetivoEvaluar la presencia de citoquinas séricas proinflamatorias en pacientes con y sin retinopatía diabética.
Material y métodosSeries de casos comparativas. Se incluyeron 36 pacientes divididos en 3 grupos: 12 pacientes con diabetes mellitus con retinopatía diabética (grupo 1), 12 pacientes con diabetes mellitus sin retinopatía diabética (grupo 2) y 12 pacientes sin diabetes mellitus como grupo control. Se midieron los niveles séricos de las siguientes citoquinas proinflamatorias en todos los pacientes: TNF-α, IL-1β e IL-6. También se realizó medición de biomarcadores proinflamatorios o reactantes de fase aguda, como velocidad de sedimentación globular y proteína C reactiva.
ResultadosLos niveles de TNF-α e IL-6 resultaron mayores en el grupo 1 (TNF-α: 19.4±10.9pg/ml, IL-6: 5.75±7pg/ml) comparados con los de los otros 2 grupos, aunque la diferencia resultó estadísticamente significativa solo en el caso del TNF-α (grupo 1: 19.4±10.9pg/ml, grupo 2: 14±4.3pg/ml y grupo control: 8.49±3.69pg/ml, p=0.001). No hubo diferencias entre biomarcadores proinflamatorios como velocidad de sedimentación globular y proteína C reactiva entre los 3 grupos (p>0.05).
ConclusionesLos niveles séricos de citoquinas proinflamatorias fueron mayores en el grupo 1. Se requieren mayores estudios para establecer mejor el impacto de este hallazgo.
The aethiopathogenesis of diabetic retinopathy includes various theories, such as: the aldose reductase pathway,1,2 free radical tissue damage3,4 non-enzymatic protein glycosylation,5,6 among others.
One theory that has gained importance in recent times is related to the concept that in diabetes and its late complications, such as diabetic retinopathy, a “low grade” inflammation occurs, where pro-inflammatory cytokines play an important role.7–14 Moreover, some of these cytokines tend to present increases both at the vitreous cavity and serum levels in late complications of diabetes.15 The above could make them candidates to become serum markers of these, including diabetic retinopathy itself as part of the assessment of patients.
The objective of this study is to describe the levels of some of these cytokines and acute phase reactants, in diabetic patients with and without retinopathy.
Material and methodsThe type of study was a comparative series of cases study. 36 patients were chosen from General Hospital “Dr. Miguel Silva” in the city of Morelia, Michoacán, México, with simple random sampling, and 3 study groups of 12 patients each were formed.
The inclusion criteria were: patients with diabetes mellitus diagnosis, patients with any degree of diabetic retinopathy and healthy patients.
Based on the above, the first group was the group of patients with diabetes mellitus with diabetic retinopathy, the second group was of patients with diabetes mellitus without diabetic retinopathy and the third group was of patients in the control group.
The exclusion criteria were: patients with eye disease such as uveitis, thyroid disease, autoimmune disease, connective tissue or malignant disease, terminal nephropathy, known cardiac ischaemic disease, chronic inflammatory diseases (chronic non-specific ulcerative colitis, Crohn's disease, acute or chronic infectious processes).
Patients were subject to a full ophthalmologic examination, which included measuring corrected visual acuity, biomicroscopy with slit lamp, tonometry and ophthalmoscopy with pharmacological dilation.
Diabetic retinopathy was classified based on its presence and absence according to the criteria of the Early Treatment Diabetic Retinopathy Study (ETDRS).
The demographic characteristics of the patients studied included: age, gender, smoking, body mass index, waist circumference, systemic arterial hypertension, systolic blood pressure, diastolic blood pressure, presence of metabolic syndrome, (NCEP-ATPIII) and risk of cardiovascular event at 10 years.
This study was approved by the Ethics Committee of General Hospital “Dr. Miguel Silva”, with the informed consent of all patients pursuant to the Declaration of Helsinki.
Samples and measurement of cytokinesSerum levels of cytokines were measured in all patients of the 3 groups studied. Fresh serum samples were evaluated using ELISA R&D kits (Systems, Inc., Minneapolis, MN; and Endogen, Rockford, IL, USA.). Cytokines and acute phase reactants measured were: erythrocyte sedimentation rate (mm/h), c-reactive protein (mg/dl), TNF-α, IL-1β and IL-6 (pg/ml).
An analysis with descriptive statistic was carried out, and standard deviation means were obtained. Regarding inferential statistic, the Kruskal–Wallis test was used as a global test and the U de Mann–Whitney test as post hoc test for comparisons among specific groups.
Regarding correlation tests, Spearman correlation coefficients were calculated (Spearman's ρ).
A p<0.05 was considered statistically significant. The SPSS programme for MAC, version 20 was used to perform statistical tests.
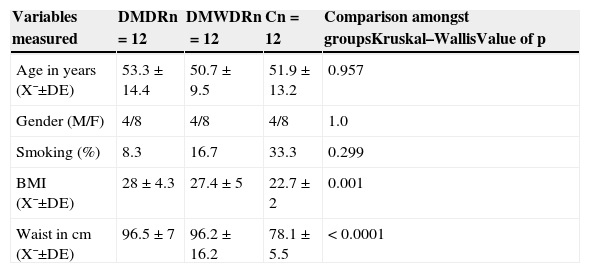
ResultsAs regards the demographic characteristics, there were no statistically significant differences among the 3 groups, and there were also no differences in smoking habits. The body mass index was normal on average in the control group (Table 1).
Demographic characteristics, history, body mass index and waist of patients studied.
| Variables measured | DMDRn=12 | DMWDRn=12 | Cn=12 | Comparison amongst groupsKruskal–WallisValue of p |
|---|---|---|---|---|
| Age in years (X¯±DE) | 53.3±14.4 | 50.7±9.5 | 51.9±13.2 | 0.957 |
| Gender (M/F) | 4/8 | 4/8 | 4/8 | 1.0 |
| Smoking (%) | 8.3 | 16.7 | 33.3 | 0.299 |
| BMI (X¯±DE) | 28±4.3 | 27.4±5 | 22.7±2 | 0.001 |
| Waist in cm (X¯±DE) | 96.5±7 | 96.2±16.2 | 78.1±5.5 | < 0.0001 |
C: control group; SD: standard deviation; DMDR: group of diabetes mellitus with diabetic retinopathy; DMWDR: group of diabetes mellitus without diabetic retinopathy; BMI: body mass index; n: number of patients per group; X¯: mean.
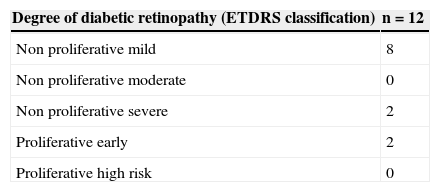
In the stratification of retinopathy pursuant to the ETDRS severity scale, the absence of cases of high-risk proliferative diabetic retinopathy stands out (Table 2).
Degree of diabetic retinopathy in patients of the group with diabetes mellitus and diabetic retinopathy.
| Degree of diabetic retinopathy (ETDRS classification) | n=12 |
|---|---|
| Non proliferative mild | 8 |
| Non proliferative moderate | 0 |
| Non proliferative severe | 2 |
| Proliferative early | 2 |
| Proliferative high risk | 0 |
ETDRS: Early Treatment Diabetic Retinopathy Study; n: number of patients.
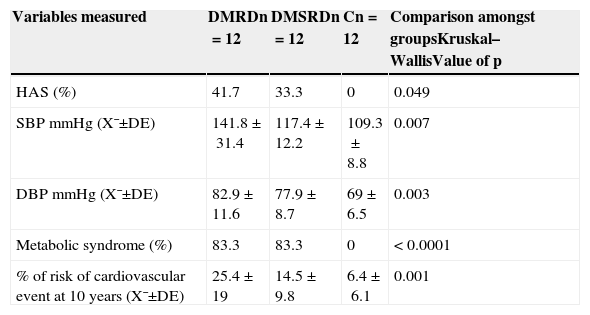
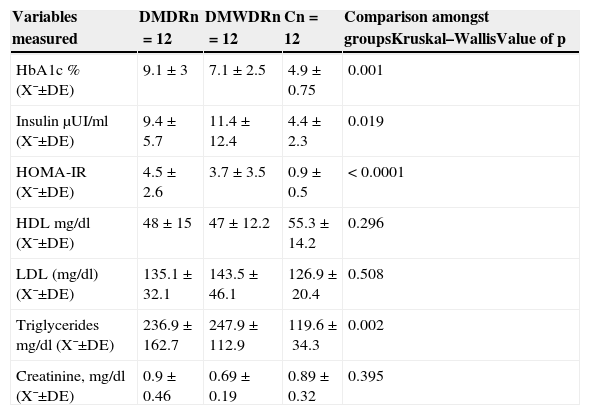
The levels for blood pressure, metabolic syndrome, glucose serum levels, insulin and lipid levels showed significant differences among the 3 groups of patients studied (Tables 3 and 4).
Clinicometabolic and cardiovascular risk variables in the patients studied.
| Variables measured | DMRDn=12 | DMSRDn=12 | Cn=12 | Comparison amongst groupsKruskal–WallisValue of p |
|---|---|---|---|---|
| HAS (%) | 41.7 | 33.3 | 0 | 0.049 |
| SBP mmHg (X¯±DE) | 141.8±31.4 | 117.4±12.2 | 109.3±8.8 | 0.007 |
| DBP mmHg (X¯±DE) | 82.9±11.6 | 77.9±8.7 | 69±6.5 | 0.003 |
| Metabolic syndrome (%) | 83.3 | 83.3 | 0 | < 0.0001 |
| % of risk of cardiovascular event at 10 years (X¯±DE) | 25.4±19 | 14.5±9.8 | 6.4±6.1 | 0.001 |
C: control group; SD: standard deviation; DMDR: group of diabetes mellitus with diabetic retinopathy; DMWDR: group of diabetes mellitus without diabetic retinopathy; HAS: hypertension; n: number of patients per group; DBP: diastolic blood pressure; SBP: systolic blood pressure; X¯: mean.
Laboratory variables of the patients studied. Glycosylated haemoglobin, insulin levels and lipids profile.
| Variables measured | DMDRn=12 | DMWDRn=12 | Cn=12 | Comparison amongst groupsKruskal–WallisValue of p |
|---|---|---|---|---|
| HbA1c % (X¯±DE) | 9.1±3 | 7.1±2.5 | 4.9±0.75 | 0.001 |
| Insulin μUI/ml (X¯±DE) | 9.4±5.7 | 11.4±12.4 | 4.4±2.3 | 0.019 |
| HOMA-IR (X¯±DE) | 4.5±2.6 | 3.7±3.5 | 0.9±0.5 | < 0.0001 |
| HDL mg/dl (X¯±DE) | 48±15 | 47±12.2 | 55.3±14.2 | 0.296 |
| LDL (mg/dl) (X¯±DE) | 135.1±32.1 | 143.5±46.1 | 126.9±20.4 | 0.508 |
| Triglycerides mg/dl (X¯±DE) | 236.9±162.7 | 247.9±112.9 | 119.6±34.3 | 0.002 |
| Creatinine, mg/dl (X¯±DE) | 0.9±0.46 | 0.69±0.19 | 0.89±0.32 | 0.395 |
C: control group; SD: standard deviation; DMDR: group de diabetes mellitus with diabetic retinopathy; DMWDR: group de diabetes mellitus without diabetic retinopathy; HbA1c: glycosylated haemoglobin; HDL: high-density lipoproteins; HOMA-IR: homeostatic model assessment: insulin resistance; LDL: low-density lipoproteins; n: number of patients per group; X¯: mean.
Statistically significant differences were only found in TNF-α levels among the 3 groups, with the highest levels in the group of patients with diabetes mellitus with diabetic retinopathy (19.4±10.9pg) in relation to the other 2 groups (those in the group of patients with diabetes mellitus without diabetic retinopathy [14±4.3pg] and the control group [8.49±3.69pg] [p=0.001]) (Table 5).
Comparison among the 3 groups of serum levels of pro-inflammatory cytokines and acute phase reactants.
| Variables measured | DMDRn=12 | DMWDRn=12 | Cn=12 | Comparison amongst groupsKruskal–WallisValue of p |
|---|---|---|---|---|
| ESR mm/h (X¯±DE) | 25.8±11.2 | 17.3±14.9 | 16±12 | 0.143 |
| CRP mg/L (X¯±DE) | 0.25±0.62 | 0.33±0.49 | 0.05±0.17 | 0.274 |
| TNF-α pg/ml (X¯±DE) | 19.4±10.9 | 14±4.3 | 8.49±3.69 | 0.001 |
| IL-1β pg/ml (X¯±DE) | 1±1.14 | 1±0.7 | 0.6±0.58 | 0.329 |
| IL-6pg/ml (X¯±DE) | 5.75±7 | 3.8±2 | 3.87±1.34 | 0.663 |
C: control group; SD: standard deviation; DMDR: group of diabetes mellitus with diabetic retinopathy; DMWDR: group of diabetes mellitus without diabetic retinopathy; IL: interleukin; n: number de patients per group; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; TNF-α: tumour necrosis factor alpha; X¯: mean.
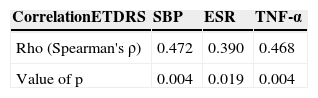
There were some statistically significant correlations: the first one, among the degree of diabetic retinopathy and TNF-α, as well as between the degree of retinopathy and the erythrocyte sedimentation rate (Table 6).
Statistically significant correlations among the degree of severity of diabetic retinopathy and SBP, TNF-α, ESR.
| CorrelationETDRS | SBP | ESR | TNF-α |
|---|---|---|---|
| Rho (Spearman's ρ) | 0.472 | 0.390 | 0.468 |
| Value of p | 0.004 | 0.019 | 0.004 |
ETDRS: Early Treatment Diabetic Retinopathy Study; SBP: systolic blood pressure; TNF-α: tumour necrosis factor alpha; ESR: erythrocyte sedimentation rate.
The inflammatory theory, which is considered as part of the pathogenesis of diabetic retinopathy, has been an intense subject of worldwide debate in recent years.9
According to this theory, pro-inflammatory cytokines play an essential role in that pathogenesis. Furthermore, high levels of those cytokines have been found both in the vitreous cavity and blood in patients suffering from this late complication of diabetes.15
As regards other studies on this subject, Meleth et al.15 found increased levels of some cytokines proportional to the severity of the retinopathy, although in their study, unlike in ours, there was no control group with healthy subjects.
Furthermore, Chen et al.16 found increased levels of IL-22 in diabetic patients with retinopathy, but also unlike our study, they found no statistically significant differences among the group of patients with retinopathy and the control group in TNF-α levels. In line with our findings, no differences among groups were found in the levels of IL-6 and IL-1β. It is also worth noting that they only report 2 groups, one of diabetic patients but without staging according to the diabetic retinopathy like our study, and a control group. On the contrary, the study by Mirza et al.17 on Mexican population like ours, is in line with our study in the sense that they found higher serum levels of TNF-α. However, one difference with our findings was that IL-6 levels they found had statistically significant differences between the group of patients with diabetic retinopathy and the control group.
Another study with similar results was carried out by Koleva-Georgieva et al.18, who found statistically significant differences in IL-1β and IL-6 levels in the group of patients with retinopathy. In contrast, our results show that there were no differences in the levels of these cytokines. In this study, there was staging of retinopathy according to severity, and statistically significant differences were found in IL-1β and TNF-α levels among the different subgroups of patients. Our results showed that there was only one significant correlation in the case of TNF-α.
Doganay et al., in their study carried out also on diabetic patients and healthy controls, found a positive correlation among some cytokines as sIL-2R, IL-8, TNF-α (like in our study) and the degree of diabetic retinopathy.19
Another study proposing TNF-α as a serum marker for proliferative diabetic retinopathy is the one carried out by Gustavsson et al. where a statistically significant correlation was also found among this degree of retinopathy and this cytokine in patients with type 1 diabetes mellitus.20 We also found this correlation, as well as a positive correlation between the erythrocyte sedimentation rate and the degree of retinopathy. This is not the case with the other pro-inflammatory cytokines evaluated herein.
Summing up, we found the following when comparing our results with what was reported in the literature9,10,16–20; in addition to finding a statistically significant difference among TNF-α levels of the different groups of patients studied, it is the first one to find a significant correlation among the erythrocyte sedimentation rate and the degree of diabetic retinopathy.
On another note, one of the limitations of our study is that there was no calculation of the sample, which can make it prone to systematic type II and type I errors; that is to say, that in the case of type I errors, statistically significant differences can be found when there are actually none, and vice versa in the case of type II systematic errors. Also, the correlations carried out through Spearman's ρ may be subject to this type of bias, not compensated with this non-parametric correlation test.
An important aspect of the results worth noting is that the 3 groups were balanced regarding its demographical characteristics as well as history such as smoking, which is why we can rule them out as confusion factors in the statistical analysis.
As it was previously mentioned, TNF-α was the only pro-inflammatory cytokine with a statistically significant difference; this is probably not specific for diabetic retinopathy, since the difference among the groups of patients with diabetes mellitus with diabetic retinopathy and patients with diabetes mellitus without diabetic retinopathy was not significant in post hoc tests. However, there was a statistically significant correlation among the degree of diabetic retinopathy in accordance with the ETDRS classification and TNF-α serum levels (Spearman's ρ=0.468, p=0.004).
The above may be explained by the fact that there are other conditions associated in diabetic patients, which may increase the levels of pro-inflammatory cytokines such as dyslipidaemia or hypertension, regardless of the presence of late complications such as diabetic retinopathy.
Some questions pending in relation to the previous statement are, first, if there would be some statistically significant differences in cytokines serum levels among the group of patients with diabetes mellitus and diabetic retinopathy and the group of patients with diabetes mellitus without diabetic retinopathy if the sample size increases.
Second, there is also the question on whether there would be any difference in the levels of pro-inflammatory cytokines and acute phase reactants among the different degrees of diabetic retinopathy, which remains unanswered also due to the fact that the sample cannot detect it.
Third, the TNF-α family is a family of cytokines with a broad range of isoforms. It would be interesting, in view of the above and since the levels of this cytokine were those with statistically significant differences in the global test, to see if some of these isoforms have a higher sensibility and specificity in diabetic retinopathy in relation with the different degrees of severity.
Lastly, it would be worth investigating the presence of higher levels of some cytokines according to different ethnic groups as proposed in the study of Mirza et al.17
All of the above opens a very wide field regarding the diagnosis of diabetic retinopathy, with very interesting implications: there would be a possibility of determining through the increase of some of these pro-inflammatory cytokines and/or acute phase reactants what patients may have a higher probability of suffering from diabetic retinopathy in the future, as some authors have proposed.21
ConclusionIn this study we found statistically significant differences among the 3 groups of patients regarding TNF-α levels, although the difference among the groups with diabetes mellitus without diabetic retinopathy and those with diabetes mellitus with diabetic retinopathy in post hoc tests were not conclusive.
The differences among the levels of other pro-inflammatory cytokines were not significant among the 3 groups.
Studies with more subjects, randomised, are necessary to determine more precisely the association among the different pro-inflammatory cytokines and diabetic retinopathy.
Conflict of interestThe authors declare that there are no conflicts of interest.
The authors wish to thank the academic staff of the postgraduate programme at the School of Medicine of Universidad Michoacana de San Nicolás de Hidalgo for their support during the preparation of this work, especially in the biostatistical analysis.
Please cite this article as: Hernández-Da Mota SE, Soto-Bahena JJ, Viveros-Sandoval ME, Cardiel-Ríos M. Citoquinas séricas proinflamatorias en retinopatía diabética. Cir Cir. 2015; 83: 100–106.