Salmonella Gallinarum is a host-restrict pathogen that causes fowl typhoid, a severe systemic disease that is one of the major concerns to the poultry industry worldwide. When infecting the bird, SG makes use of evasion mechanisms to survive and to replicate within macrophages. In this context, phoPQ genes encode a two-component regulatory system (PhoPQ) that regulates virulence genes responsible for adaptation of Salmonella spp. to antimicrobial factors such as low pH, antimicrobial peptides and deprivation of bivalent cations. The role of the mentioned genes to SG remains to be investigated. In the present study a phoPQ-depleted SG strain (SG ΔphoPQ) was constructed and its virulence assessed in twenty-day-old laying hens susceptible to fowl typhoid. SG ΔphoPQ did cause neither clinical signs nor mortality in birds orally challenged, being non-pathogenic. Furthermore, this strain was not recovered from livers or spleens. On the other hand, chickens challenged subcutaneously with the mutant strain had discreet to moderate pathological changes and also low bacterial counts in liver and spleen tissues. These findings show that SG ΔphoPQ is attenuated to susceptible chickens and suggest that these genes are important during chicken infection by SG.
The poultry-adapted pathogen Salmonella enterica subsp. enterica serovar Gallinarum biovar Gallinarum (S. Gallinarum) causes a disease so-called fowl typhoid, which is known as a severe systemic illness that concerns the animal health authorities worldwide due to its economic significance and negative impact on the poultry industry.1 Among the domestic poultry, chickens belonging to semi-heavy and heavy lineages are usually those susceptible to fowl typhoid.2
In the field conditions S. Gallinarum reaches the host system after oral intake of bacteria but it does not evoke overt host immune response in the intestines as do some other Salmonella serovars.3 The systemic invasion begins when S. Gallinarum crosses the gut barrier at the Gut Associated Lymphoid Tissues (GALT)4 from where the pathogen is thought to meet resident macrophages that after phagocytosis will internalize single bacteria within each modified phagossome in the cytosol called Salmonella-Containing Vacuoles (SCV).5,6 The acid pH, restriction of bivalent cations and antimicrobial peptides are some of the harmful conditions faced by the bacteria in this microenvironment.5,7 Thus, S. Gallinarum must transcribe several genes located on chromosomal regions named Salmonella Pathogenicity Islands (SPIs) in order to survive and multiply in this hostile place.6
Several SPIs have been described to date but the SPI-2 and SPI-3 appear to play a pivotal role on the systemic phase of Salmonella-caused diseases.6,8,9 SPI-2 genes synthesize a Type Three Secretion System (TTSS) that delivers effector proteins from the SCV into the host cytosol whose functions encompass, among others, the disruption of bactericidal compounds including reactive oxygen and nitrogen molecules.6,8 The SPI-3 genes in turn act enhancing Mg2+ uptake from the SCV favoring bacterial replication.9
Previous studies with S. Typhimurium-infected mice demonstrated the importance of the regulatory system PhoPQ on controlling the expression of several other genes scattered throughout the Salmonella genome.9,10 This two-component system is composed by a membrane sensor protein kinase PhoQ and by the cytoplasmic regulator PhoP9,10 and is activated at low pH,11 Mg2+ and Ca2+ deprivation,12 and by antimicrobial peptides activity.13 Therefore, the intramacrophage signaling is sensed by the PhoPQ system, a critical step on the SPI-2 and SPI-3 mediated virulence.9,10,14–18
Although the PhoPQ regulatory system has proven to play a key role on the systemic phase of Salmonella-provoked diseases based on data from other serovars,14,19,20 none is known of its contribution on S. Gallinarum pathogenicity to the best of our knowledge. In order to shed light on this subject, the present study aimed at evaluating the importance of both genes (phoPQ) on the fowl typhoid pathogenesis.
Materials and methodsBacterial strainsAll strains were stored at −80°C in LB supplemented with 30% glycerol. The spontaneous nalidixic acid-resistant Salmonella Gallinarum 287/91 (SG hereafter) was isolated from a chicken involved in an outbreak of fowl typhoid in Brazil and used in this study.21 SG provided the genetic background for constructing the phoPQ-depleted mutant strain (SG ΔphoPQ) and was used to reproduce the fowl typhoid in susceptible chickens (positive control) in the in vivo experiments. Standard culture methods were used to grow bacteria. In short, they were inoculated in Lysogeny broth (LB – Becton Dickinson, Sparks, Maryland, USA) from the frozen cultures and incubated at 37°C for 18h at 150 rotations per minute (rpm). These pure LB cultures were used for oral infection of chickens. Subcutaneous infection demanded a cleaning step before inoculation that consisted of centrifugations at 4000×g for 10min at 4°C followed by discard of supernatants and bacterial pellet suspension in appropriate volume of phosphate-buffered saline (PBS) pH 7.4.22 Cleaning procedure was performed thrice before infection.
Mutant constructionThe Lambda-Red-mediated recombination was performed to construct SG ΔphoPQ in accord to Datsenko and Wanner.23 A chloramphenicol resistance cassette (Cmr) replaced the phoPQ genes so that the suggestive positive colonies were selected on Lysogeny agar (LA; Difco™ LB Agar Lennox, 240110, Sparks, Nevada, US) containing 20μg/mL chloramphenicol. The phoPQ mutation was confirmed by Polymerase Chain Reaction (PCR). The mutated gene was transduced to a clean background using P22 bacteriophage and prior to animal experimentation the chloramphenicol-resistance gene was removed from the chromosomes through the FLP system.23 Primers used in the present study were designed using the Primer-BLAST software24 and their sequences are available in Table 1.
Primer sequences used to construct the SG ΔphoPQ mutant strain.
| Primer | Sequence (5′→3′) | Reference |
|---|---|---|
| C1 | TTATACGCAAGGCGACAAGG | 23 |
| C2 | GATCTTCCGTCACAGGTAGG | 23 |
| phoP50F | CACCATAATCAACGCTAGACTGTTCTTATTGTTAACACAAGGGAGAAGAGGTGTAGGCTGGAGCTGCTTC | This study |
| phoQ50R | CGATTATAACGGATGCTTAACGAGATGCGTGGAAGAACGCACAGAAGTGTCATATGAATATCCTCCTTAG | This study |
| phoPQ_ctrF | GCAAGCTGGAAGTAAACCGC | This study |
| phoPQ_ctrR | TCAAGAAAGTCGGGCCAGTT | This study |
Long primers were used to amplify antibiotic cassettes and short primers (20-bp long) were used to verify cassette insertion.
One hundred and sixty brown egg-layer chicks susceptible to fowl typhoid were acquired for this research. One-day-old animals were obtained from a commercial hatchery where they were vaccinated against Marek's disease. On arrival chicks were housed in metal cages in temperature controlled rooms in the School of Agricultural and Veterinary Sciences (FCAV/Unesp). Transport cardboard boxes were examined in order to exclude infection by Salmonella.25 Birds received clean water and antibiotic-free balanced feed ad libitum.
In vivo experiments were performed in accordance with the Ethical Principles on Animal Experimentation developed by the Brazilian College of Animal Experimentation and approved by the internal Ethical Committee on the Use of Animals (Process 23049/15; approved on 04 December 2015).
Experiment 1 – evaluation of SG ΔphoPQ pathogenicityForty animals were equally and randomly distributed into two groups (A and B) when they were 20 days old. One mL of SG ΔphoPQ and SG overnight cultures containing a roughly 5×108 colony forming units (CFU)/mL were orally administered into the crops of chickens from groups A and B, respectively. Mortality and clinical signs were daily recorded throughout the four-week experiment.
Experiment 2 – evaluation of systemic infectionOne hundred and twenty chickens were randomly distributed into four groups when they were 20 days old as follows: Groups C and D were comprised of 30 animals each while groups E and F were composed of 48 and 12, respectively. A larger quantity of animals within group E was necessary to compensate for the high mortality usually caused by fowl typhoid in susceptible chickens.
Group C chickens were orally administered with an overnight culture containing 5×108 CFU/mL of SG ΔphoPQ whereas group D animals received subcutaneously, in the nape of their necks, 0.2mL containing 1×109 CFU/mL of SG ΔphoPQ. Group E chickens were orally inoculated with 1mL of overnight culture containing 5×108 CFU of SG strain and group F animals were spared in a separate room as the negative control of infection.
To evaluate the systemic infection, five animals belonging to groups C, D and E were euthanized at 2, 4, 7, 10 and 14 days post-infection (dpi). Overall post-mortem examinations were performed at necropsy and gross alterations on the chicken organs were recorded. Two group F chickens were euthanized at each sampling day in order to provide parameters for comparison. Liver and spleen fragments were collected to determinate bacterial counts as hereafter described. Samples were macerated and homogenized in PBS at a 1:10 proportion (w/v), followed by their serial dilution in PBS (1:10, v/v). From each dilution 0.1mL was dropped onto Brilliant-Green agar (BGA; Brilhante Green Agar, Oxoid, CM0263, US) containing 100μg/mL of nalidixic acid (BGA Nal), which was incubated at 37°C for 24h. Two-fold concentrated Selenite broth (Selenite Broth Base, CM0395, Oxoid, US) containing 0.04% novobiocin were added to the diluted tissue samples at equal volume. After incubation, the CFU per gram (CFU/g) of organ were recovered. In the absence of bacterial growth, the incubated-enriched samples were streaked on BGA Nal. If then positive, a value of 102 CFU/g was assigned for the downstream analyses.
Statistical analysisMortality rates were compared by Chi-square test and the means of bacterial counts in livers and spleens were firstly converted to Log10 and then submitted to ANOVA followed by Tukey's test. Differences were considered significant where p<0.05. Statistical analysis was carried out using the GraphPad Prism software version 6.02 for Windows (GraphPad Software, Inc.).
ResultsExperiment 1 – evaluation of SG ΔphoPQ pathogenicityNeither clinical signs nor mortality (n=0/20) were observed among SG ΔphoPQ-infected chickens (group A) whereas SG-infected animals (group B) showed clinical signs since 3 dpi when two birds presented depression, anorexia and discrete greenish-yellow diarrhea. On the next day all group B animals showed depression, somnolence, dropped wings, ruffled feathers, greenish-yellow diarrhea and reduction in feed and water intake. The onset of mortality was at 5 dpi and lasted until 9 dpi when 90% of group B animals died (n=18/20 infected). Differences between the mortality rates in groups A and B were statically significant (p<0.05; Table 2). The remaining group B animals (n=2/20 infected) showed progressive clinical recovery from 13 dpi onward.
Cumulative mortality in groups with 20 birds orally inoculated at 20 days old with mutant (group A) and wild-type (group B) strains.
| Bacteria | Days post-infection (dpi) | Total | %A | |||||
|---|---|---|---|---|---|---|---|---|
| 5 | 6 | 7 | 8 | 9 | 28 | |||
| SG ΔphoPQ | – | – | – | – | – | – | 0/20 | 0a |
| SG | 4 | 8 | 13 | 17 | 18 | 18 | 18/20 | 90b |
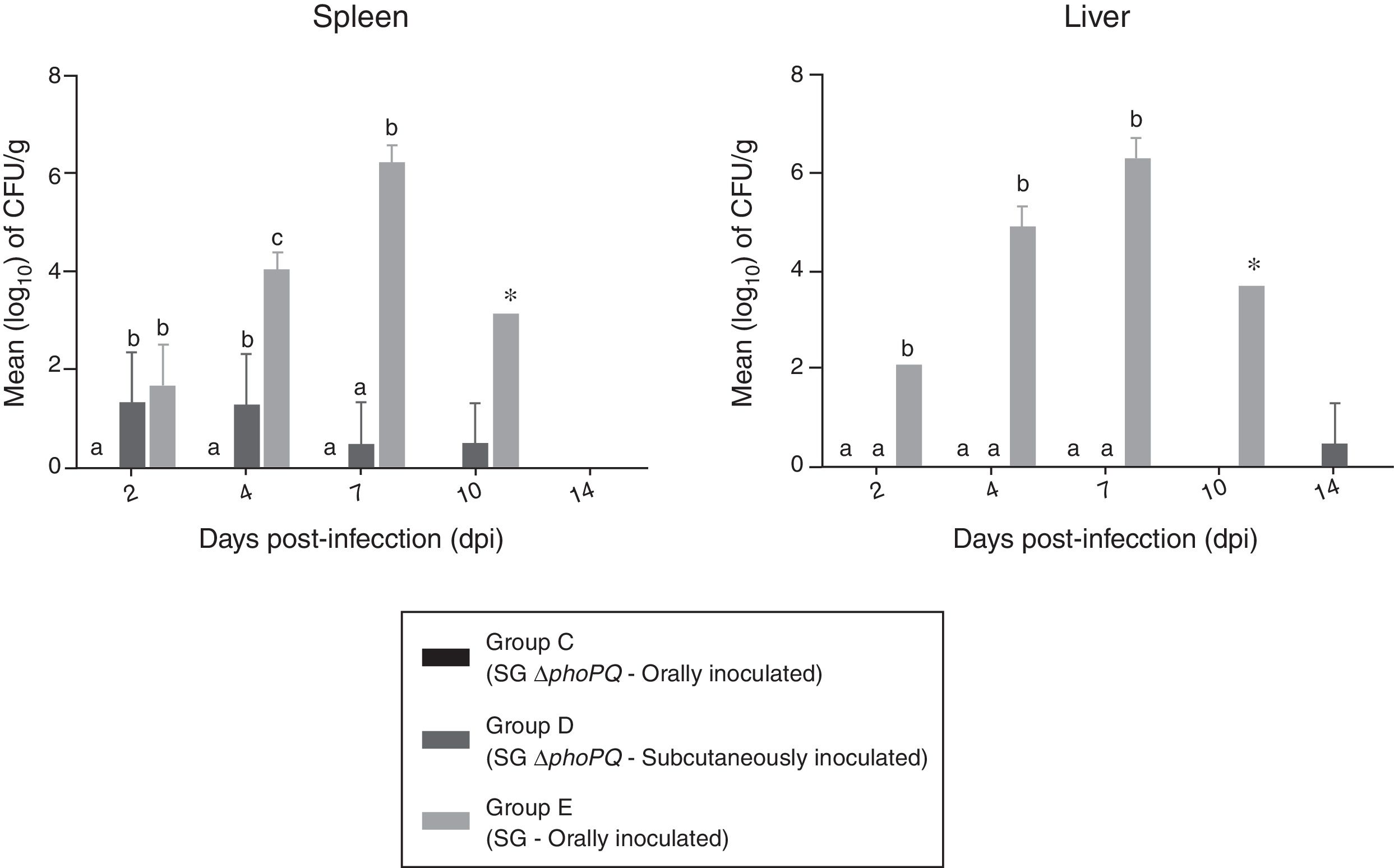
Data on bacterial enumeration in liver and spleen of challenged birds are shown in Fig. 1. Oral administration of SG ΔphoPQ (group C) resulted in no bacterial recovery from spleen or liver tissues throughout the experiment. Subcutaneous inoculation of SG ΔphoPQ (group D) led to low bacteria recovery from spleens until 10 dpi, but only after sample enrichment, while livers were mostly negative saved for a single sample collected at 14 dpi which was positive also after enrichment. Comparatively, SG (group E) was retrieved in higher quantities than SG ΔphoPQ until 10 dpi. SG quantity progressively increased and bacterial recovery peaked at 7 dpi in liver and spleen tissues. No statistical significance was found at 10 dpi because there was only a single SG-infected animal in group E to be examined. SG ΔphoPQ was not retrieved in high amounts regardless the inoculation route analyzed. Despite that, the number of SG ΔphoPQ positive spleens upon subcutaneous inoculation was higher and statistically significant at 2 and 4 dpi when compared to oral infection. There was not surviving birds in group E at 14 dpi, therefore bacterial enumeration was not compared at this time point.
Bacterial counts in livers and spleens collected from chickens inoculated with SG ΔphoPQ and SG by different routes. *At 10 dpi, only a single remaining SG-infected bird was examined (group E). Values are expressed as means±standard deviation of bacterial counts (log10CFU/g). Different letters on the plots indicates statistical differences by Tukey's test (p<0.05).
No gross alterations were observed on the organs of SG ΔphoPQ-infected chickens (group C) although those subcutaneously inoculated (group D) presented discrete to moderate alterations such as hepatomegaly, congested and friable liver.
SG-challenged chickens (group E), in turn, presented the most severe alterations over the experiment. They showed discrete congestion and enlargement on the liver and spleen at 2 dpi. Two days later, moderate to severe alterations could be observed, such as hepatosplenomegaly and severely congested livers that were friable and presented white foci and diffuse bronze discoloration. Moreover, kidneys and cecal tonsils were moderate enlarged with the latter showing red hemorrhagic spots. Besides the alterations already mentioned SG also induced atrophy of the bursa of Fabricius at 7 dpi. At 10 dpi the single remaining bird had the same severe pathological changes described above. Chickens from negative control (Group F) did not show any gross alteration.
DiscussionSalmonella spp. harbor virulence genes clustered on chromosomal regions named as Salmonella Pathogenicity Islands (SPIs) or virulence plasmids. This wide genetic repertoire enables bacteria to cross the gastrointestinal environment, to invade the intestine epithelial cells and to survive and multiply within macrophages during systemic infection.26 In order to overcome the host physical-chemical and immunological barriers,27Salmonella must perform a fine-tuning regulation of its virulence genes and the two-component regulatory PhoPQ codified by phoPQ genes are key in this regulation process.11
Previous studies involving inoculation of phoPQ-defective S. Typhi and S. Typhimurium in humans and BALB/c mice, respectively, showed that both genes are required for systemic infection, since attenuation was observed for these mutant strains.15,16 Moreover, a phoP-depleted S. Enteritidis was also attenuated to chickens once it did not provoke any intestinal inflammation.28 However, it is still unknown the importance of phoPQ genes to the SG pathogenicity.
The data generated on the present study demonstrated that SG ΔphoPQ is attenuated for susceptible chickens, since neither mortality nor clinical signs were observed during SG ΔphoPQ infection. Such results are in agreement with previous researches in which the function of PhoPQ proteins on Salmonella pathogenicity was exploited15,16,28 and highlight that an undamaged PhoPQ regulatory system is required for a full expression of SG pathogenicity.
In the present study SG ΔphoPQ was not recovered from liver and spleen tissues collected from the orally infected chickens at any time point analyzed suggesting that bacterium was not able to reach these sites. A previous study showed that Salmonella sensitivity to acid pH increases in the lack of a functional PhoPQ system.11 Thus, the supposition is that SG ΔphoPQ viability largely decreased after it passed through the proventriculus so that just a small fraction of the initial inoculum may have reached the intestines. The infection caused by the remaining bacteria neither resulted in an efficient invasion of the host system nor in disease development.
Avoiding the physical–chemical barriers through subcutaneous inoculation led to SG ΔphoPQ recovery from the liver and spleen tissues and to the appearance of mild gross alterations in organs although this bacterium was not as pathogenic as was SG wild-type for susceptible chickens. It has been shown that phoPQ-defective Salmonella is unable to survive/replicate within macrophages in which micro-organism faces several antimicrobial mechanisms, such as acidification, antimicrobial peptides and bivalent cations deprivation.7,13,17 Data from the oral and subcutaneous inoculations put together indicate that SG ΔphoPQ is attenuated for susceptible chickens because the microorganism is unable to overcome the physical-chemical barriers imposed by the gastrointestinal tract but also due to its inability to survive/replicate within the macrophages.
Salmonella survival within phagocytic cells is dependent on the expression of genes related to the Type Three Secretion System codified by the SPI-2 (TTSS-2), which in accord to Bijlsma and Groisman14 rely on functional PhoPQ proteins. In a previous study, Jones et al.8 disrupted the coding sequence of the ssaU gene of SG str. 9 so that this bacterium was unable to express the TTSS-2 and did not cause fowl typhoid in susceptible chickens.8 Moreover, this mutant strain was highly attenuated when inoculated by both oral and intravenous routes, a phenotype that SG ΔphoPQ resembled in the present study. Therefore, it is feasible that SG ΔphoPQ was attenuated for susceptible chickens by subcutaneous inoculation due to its inability to properly express/regulate the TTSS-2 genes after undergoing phagocytosis.
In addition to SPI-2 genes, the SPI-3 mgtCBR genes transcription is also under the PhoPQ control.9 The Mg2+-transporter protein MgtB enhances the uptake of extracellular magnesium29 whereas the MgtC protein inhibits adenosine triphosphate (ATP) production by the bacteria within the SCV.30 On the bacterial cytosol ATP binds to Mg2+ ions making it unavailable for use; thus, the MgtC-dependent inhibitory activity assures the availability of free cytosolic Mg2+ for ribosome assembly and protein translation.29 In the present study SG ΔphoPQ showed reduced replication skills throughout the experiment by subcutaneous injection suggesting that a non-regulated expression of SPI-3 genes may also have taken place contributing to SG ΔphoPQ phenotype in vivo.
Defective-PhoPQ Salmonella strains are not able to evade the antimicrobial mechanisms imposed by the host, such as the physical-chemical barriers, and the hostile macrophage-within environment, in which bacteria hardly replicates.6,7,11,12 The data herein presented indicate that the phoPQ genes play a fundamental role on the Salmonella Gallinarum pathogenicity since their inactivation was enough to attenuate SG ΔphoPQ to susceptible chickens.
Conflicts of interestThe authors declare no conflicts of interest.
This work was supported by the São Paulo Research Foundation (FAPESP) [Grant Number 2016/10369-0 (Angelo Berchieri Junior)], the National Council of Technological and Scientific Development (CNPq) and the Coordination of Improvement of Higher Education Personnel (CAPES), Brazil.