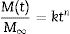Hybrid organic–inorganic aerogels are highly hydrophobic porous solids that avoid the brittleness and moisture adsorption of the standard silica aerogels. Superhydrophobic porous materials have attracted great interest because of their ability for selective absorption of organic solvents while repelling water, as excellent candidates for remediation techniques. This work shows a comparative of three drying procedures of DEDMS/TEOS (diethoxydimethylsilane/tetraethylorthosilicate) gels, namely, by supercritical CO2, by supercritical ethanol, and dried at ambient conditions. Supercritical CO2 and ambient drying produced superhydrophobic aerogels (θ>150°), while supercritical ethanol drying produces denser aerogels with smaller both porous volume and specific surface area. Regarding the absorption of organic liquids, swelling is observed in all cases. Hexane had faster diffusion that obeyed Fick's law (∝t0.5) whereas liquid polydimethylsiloxane exhibited slower non-Fickian diffusion process (∝tn, n<0.5).
Los aerogeles híbridos orgánicos-inorgánicos son sólidos porosos altamente hidrofóbicos, que mejoran la fragilidad e impiden la absorción de humedad típicas de los aerogeles de sílice. El estudio de materiales superhidrofóbicos porosos ha generado gran interés debido a la capacidad que estos poseen para absorber de forma selectiva solventes orgánicos, mientras repelen el agua, resultando ser excelentes candidatos en técnicas de remediación ambiental. En el presente trabajo se comparan tres métodos de secado de geles de dietoxidimetilsilano/tetraetilortosilicato (DEDMS/TEOS), concretamente realizados en atmósfera supercrítica de CO2, en etanol supercrítico y, por último, en condiciones ambientales. Mientras los procedimientos de secado llevados a cabo en CO2 supercrítico y en condiciones ambientales producen aerogeles superhidrofóbicos (θ>150°), el realizado en etanol supercrítico dio lugar a aerogeles de mayor densidad, con menor volumen poroso y superficie específica. En cuanto a la absorción de líquidos orgánicos, en todos los casos tiene lugar el hinchamiento de la red polimérica (swelling). El hexano presentó una difusión rápida, que obedece a la ley de Fick (∝t0,5), mientras que el polidimetilsiloxano líquido mostró un proceso de difusión más lento que no obedece la ley de Fick (∝tn, n<0,5).
Industrialization and urbanization in developed and emerging nations have had significant negative consequences for freshwater and seawater ecosystems. Industrial wastewater is one of the most important sources of contamination, which includes very diverse organic compounds and suspended solids that must be removed or reduced to limit pollution by basic or complex wastewater treatment facilities. Water-soluble organic compounds usually degrade faster than lipophilic compounds, which are particularly most persistent, resulting in the accumulation of specific industrial contaminants, many of them with associated toxic effects. Hence, one of the most urgent tasks for environmental preservation is to reduce the huge accumulation of water contaminants. To address this challenge, several remediation techniques have been developed and a large number of experiments to evaluate their efficiency have been reported [1,2]. One of the most important remedies for oil or organic solvents spills in practice is mechanical recovery by the use of sorbent materials. Among these, superhydrophobic absorbers show noteworthy benefits such as selectivity to absorb oil or organic solvents. Various examples that have been studied involve modified organophilic clays, silica, exfoliated graphite, polyurethane sponges, cellulose-based materials and polydimethylsiloxane elastomers and hybrid gels [3]. Also, materials derived from silica aerogels materials have been used for oil sorption [4–6]. This type of material is very attractive due to its high specific surface area, pore volume and low density, together with a high mechanical stability, which makes them inspiring proposals for remediation techniques.
Silica aerogels are complex structures which consist of a coherent solid 3D-network formed by the bonding and arrangement of nanometric-sized silica particles (3–4nm in diameter), to give hierarchically-ordered structures or randomly-arranged particles [7]. They are extremely porous (pore volume higher than 85%) with an average pore size between 30 and 50nm, and they have very low density (<5kg/m3) and large surface area (>500m2/g). Nevertheless, the potential applications of aerogels always appear limited by their poor mechanical properties. For example, under compression, they exhibit classical fragile fracture, after undergoing small plastic deformation due to pore collapse, which is favoured by the large pore structure and loose cluster structure [8].
Also, the extreme fragility response under stress due to high absorption of water make it difficult to maintain its physical integrity when being handled [9]. The inclusion of polymers based on Si–C bonds, such as polydimethylsiloxane (PDMS), are a solution to avoid problems derived from fragility and/or moisture absorption, as it modifies the inorganic network of the silica aerogels and creates homogenous organic–inorganic hybrid materials with physical and mechanical properties that can be modulated depending on the composition [10–12].
These type of aerogels are typically obtained by, firstly, processing of alcogel by the sol–gel method. This step can also be assisted by the use of ultrasound (sonocatalysis), which significantly affects the chemical reactions and gelation rate, improving the thermal stability and increasing the apparent density of the resulting sonogels [13,14] and secondly, removing pore residual solvent from wet gel in an autoclave, at temperatures and pressures slightly higher than the critical point of a predetermined fluid (typically CO2 or ethanol). The SiO2-PDMS sono-aerogel was first obtained by Kramer et al. [10], by removing the pore solvent under supercritical CO2 conditions. Due to its easily accessible critical conditions (31°C, 7.39MPa), CO2 is the most widely used supercritical fluid to obtain this type of material. Hence, it enables carrying out the process at mild temperatures (40–60°C). This procedure makes it possible for the microstructure of the wet hybrid sonogel in a dry form to be preserved as a monolithic sample. Following this drying method, the synthesis of a lot of new silica-based hybrid aerogels combining different organic compounds has been proposed [15]. Alternatively, hybrid SiO2-PDMS monolithic sono-aerogels with a high content in the organic phase were successfully obtained using supercritical ethanol (241°C, 6.1MPa). Thermal analysis demonstrated that the organic chains in these samples do not degrade thermally until 260°C under inert atmosphere [16].
In the case of conventional drying, a xerogel is obtained, in which, commonly, the very high porous structure has collapsed, yielding denser hybrid samples. Other procedures use chemical additives that reduce the surface tension of the liquid–vapor interphase, reducing the capillary pressure during drying, and avoiding pore collapse during conventional drying. Samples obtained through this method are known as ambigels.
These different drying processes have been researched and discussed previously. For example, the higher temperatures used during ethanol-dried supercritical drying process, in comparison to the CO2-based process, yield hydrophobic silica aerogels, while CO2-dried silica aerogels are hydrophilic [17,18].
These hybrid aerogels acquired an elastomeric type of behaviour, instead of the characteristic brittle behaviour of the pure silica aerogels, and the resulting porosity and specific surface area decreased to a great extent due to the addition of the organic phase. Beyond this simple modification in the microstructure and the mechanical properties, hybrid aerogels are also hydrophobic and superhydrophobic nanostructures [7,9,19]. In other words, these procedures allow the fabrication of highly homogeneous hybrid materials with tuneable physicochemical properties, with a wide range of compositions [20]. For example, in a previous work [5], monolithic sono-aerogels using tetraethoxysilane (TEOS) and diethoxydimethylsilane (DEDMS) as the organic component were successfully obtained by performing supercritical drying in ethanol. The results showed how increasing the organic content produced a significant decrease in the specific porous volume, from 3.31 to 0.14cm3/g, and the specific surface, from 900 to 40m2/g, for pure silica aerogel and the rubbery TEOS-DEDMS 50wt.% aerogel (1:1.25 molar ratio), respectively. Otherwise, the presence of the methyl radicals made the silica aerogels superhydrophobic and oleophilic. Thus, due to these new properties, the TEOS-DEDMS sono-aerogels were successfully used to selectively separate silicone and hydrocarbon oils from water, exhibiting a high absorption capacity, fast absorption and excellent reusability.
The first objective of the present study was to study the effects of the different drying conditions on the structural, physicochemical and mechanical properties, of different sets of hybrid silica-organic samples: aerogels dried through supercritical ethanol, through supercritical CO2, and one conventionally ambient-dried sample, that is, a xerogel. Secondly, the absorption performance of these hydrophobic TEOS-DEDMS samples has been tested for different solvents. Lastly, the mechanical properties of both the as-prepared (dry) samples and those immersed in PDMS organic solvent (wet) states have also been compared in order to discuss the reusability of the samples for their technological applications as wastewater cleaners.
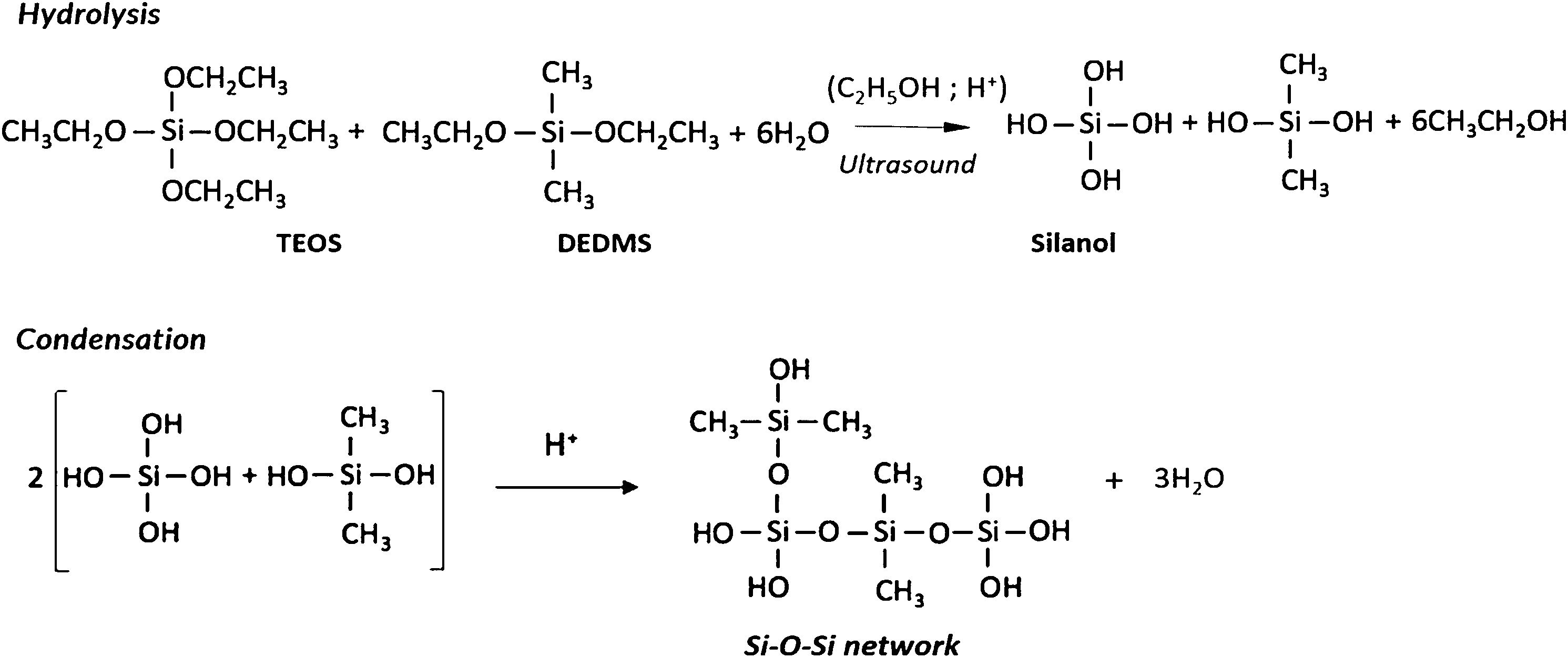
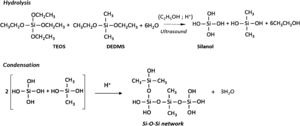
Materials and methodsSynthesis of hybrid samplesThe synthesis of the hydrophobic samples in this study involved two major steps: the preparation of the alcogels and the drying procedure. The chemical reactions responsible for the formation of three-dimensional gel network are outlined in Scheme 1.
Initially, tetraethoxysilane (TEOS) was mixed with diethoxydimethylsilane (DEDMS) in absolute ethanol (EtOH) solvent and were totally hydrolyzed with water under acidic conditions with nitric acid (0.1M). The DEDMS/TEOS molar ratio was fixed to 1.25, corresponding to 50wt.% of the organic phase with regard to the total silica content of the sample. The first step is the condensation of these hydrolyzed species by a one-step acid-catalyzed sol–gel process in ethanol solvent to get the alcogels, followed by their ageing soaked in ethanol for four weeks at 50°C and then being washed in new ethanol for three days at ambient temperature before being subjected to drying. All chemical reactions were carried out under the catalytic effects of ultrasound, by supplying 0.6kJ/cm3 of high power ultrasound, in a flask immersed in a cooling bath at 0°C.
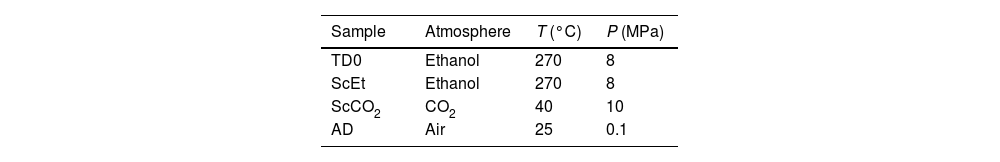
The drying proceduresThe second step for the synthesis of the samples is the drying of the gels to remove the solvent. This second step was performed by three different procedures: in supercritical ethanol, in supercritical carbon dioxide, and by ambient evaporation at atmospheric pressure. By means of these procedures, cylindrical monolithic samples were obtained, with heights of 15–20mm and diameters of 8–10mm. The samples were labelled as “ScEt”, “ScCO2” and “AD”, for drying performed under supercritical ethanol, supercritical CO2 and ambient drying conditions, respectively. In addition, pure silica aerogels were also synthesized from TEOS by sol–gel, followed by supercritical drying in ethanol, (referred to as “TD0”) as a reference, in order to make pertinent comparisons with the hybrid aerogel samples. All the samples were kept at 50°C in an oven for stability. In summary, 45 TEOS-DEDMS samples were prepared, 15 for each drying procedure, plus 5 pure silica aerogels. The critical temperatures and pressures of the solvents, conditions of their synthesis (temperature and pressure), and the designations of the samples are summarized in Table 1.
Characterization of the samplesThe density of the samples was obtained by measuring the mass and the volume of the cylindrical samples with a microbalance (precision±0.1mg) and a calliper (precision±1/20mm), respectively. The nanostructural characteristics of the hybrid aerogels (texture) were investigated by means of nitrogen physisorption experiments (Micromeritics ASAP2010, working at 77K and equipped with pressure transducer resolution of 10−4mmHg). The specific surface area, specific pore volume and pore diameter were determined, using standard models for the analysis (BET and BJH, respectively). Prior to these experiments, the samples were milled in an agate mortar and degasified at 150°C for 2h at 100Pa. Thermogravimetric analysis (TGA) was performed with a TGA Q50, TA Instruments, thermogravimetric analyzer at a heating ramp of 10°C/min under N2 atmosphere. The initial degradation temperature and the residual weight were determined. The microstructural surface morphology was studied by scanning electron microscopy (SEM) with a thermoionic filament emission (FEI Quanta; resolution: 3.5nm) using the low-vacuum capability in the pressure range 10–130Pa, which enables the analysis of wet specimens without previous preparation. The hydrophobicity of the samples was studied by measuring the contact angle θ using the sessile drop method: a drop of 5μL is deposited on the flat surface of the aerogel with a micro syringe of 50μL DS 500/GT. The values of the static contact angle were determined using a commercial video-based software-controlled contact angle analyzer (SCA22, model OCA 15plus, from Dataphysics Instruments). The chemical structure was studied by Fourier transform infrared spectroscopy (FTIR). All the infrared spectra were collected at room temperature by a Fourier transform spectrometer (Bruker Tensor 37) with a resolution of 4cm−1 and 32 scans in the region 4000–400cm−1. The samples were stored overnight in a stove at 60°C, then ground and mixed with KBr and pressed into a self-supporting wafer. The wafer was put on a sample holder for the spectrum collection.
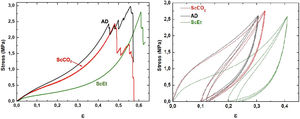
Liquid absorptionFor the liquid absorption experiments, the cylindrical specimens of the hybrid samples were immersed in two different organic solvents up to the point of saturation at room temperature (20±2°C). A silicone oil (silanol-terminated PDMS) fluid of medium viscosity (450g/mol in average molar mass, density=0.965g/cm3, viscosity=20–32cSt at 15°C) was used as the reference oily fluid; and n-hexane (density=0.692g/cm3) as a reference for hydrocarbon liquid. The samples were removed periodically from the liquid bath to measure their weight in a sensitive microbalance. Before each measurement was made, the excess surface liquid was wiped off of the specimen. After the weight was registered, the sample was immediately returned to the liquid bath. This process was repeated until no weight change was observed for several hours, at which time the saturation of the absorption test is attained. The liquid absorption process was studied by measuring the relative weight gain M(t) defined in terms of the mass of the absorbed fluid per mass of the dry sample, that is, the actual mass for a given time, m(t), minus the mass of the dry sample, m(0), divided by the mass of the dry sample (Eq. (1)):
The time-dependence of the normalized weight gain, M(t)/M∞, was registered for the kinetic studies, where M∞ is the weight of the sample after saturation (namely, for t→∞). Finally, the absorption capacity was also assessed as the ratio of the volume of absorbed liquid compared to the original porous volume of the sample, revealing the presence of swelling if this ratio exceeds unity.
Mechanical testsThe mechanical tests were performed by uniaxial compression on the as-prepared samples (dry), and also on the samples immersed in liquid silicone oil after saturation in the absorption experiments. Uniaxial compression tests were performed in a Shimadzu universal testing machine, AG-I Autograph, equipped with a load cell of 5kN (within ±1% of the indicated test force at a load cell rating of 1/250). For the dry samples, a sample deformation rate of 0.5mm/min was considered; for the wet samples, this rate was 0.01mm/min. Compression was performed until the breakage of the sample was achieved. The mechanical parameters were obtained by unconfined compression from cylindrical specimens of 15–20mm in height and 8–10mm in diameter, in accordance with the ASTM D7012 standard. The compressive strength was obtained from the maximum stress before sample failure, and the elastic modulus was estimated from the slope of the stress–strain curve. All the dry experiments were performed in triplicate to get statistical significance. The wet experiments were performed once.
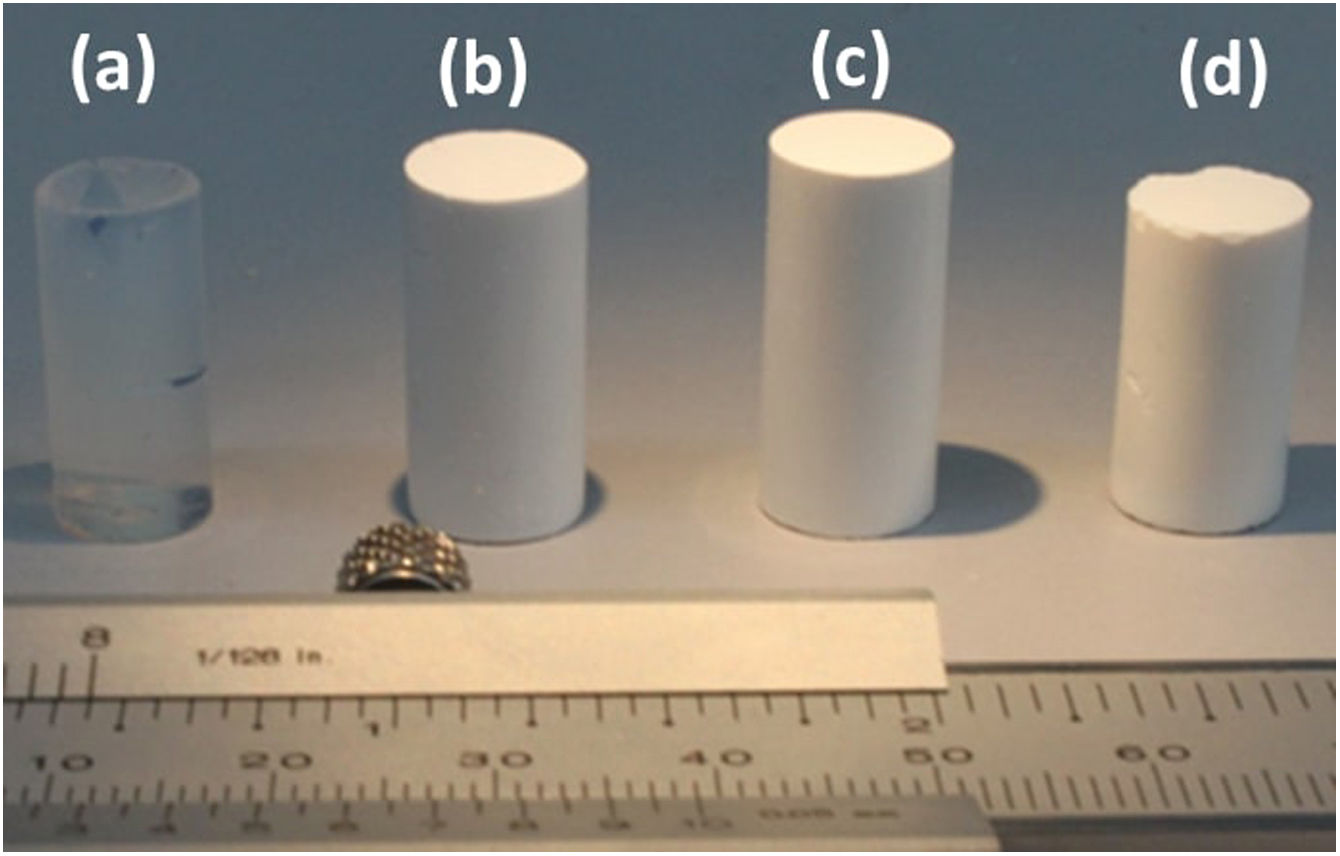
Results and discussionDensity, contact angle, microstructure and chemical propertiesThe TEOS-DEDMS samples were obtained as monolithic cylinders (Fig. 1). Sample TD0 was a typical silica aerogel, produced through an acid-catalyzed sol–gel process, transparent and brittle, with a density of ρ=0.32±0.04g/cm3. In contrast, hybrid specimens were opaque, with average bulk densities ranging from ρ=0.38 to 0.41g/cm3 (see Table 2). Although these densities seem higher than those observed in the literature for classical aerogels [s10853.-014-8480-0], they are typical values obtained when ultrasound is used in the sol–gel process [5,21].
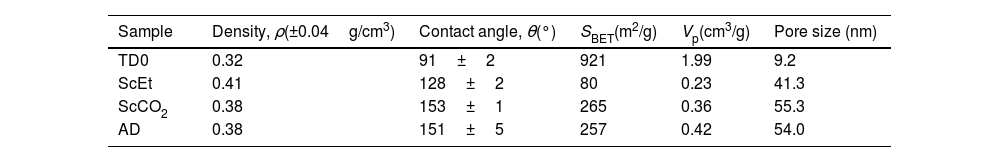
Bulk density, averaged water contact angle, specific surface area, specific pore volume and mean pore size obtained from N2 physisorption, for pure silica TD0 aerogel, and hybrid aerogels. Specific surface area SBET correlation coefficient in BET fitting was higher than 0.999 in all cases. Characteristic pore sizes are estimated from the position of the peak of the pore size distribution.
| Sample | Density, ρ(±0.04g/cm3) | Contact angle, θ(°) | SBET(m2/g) | Vp(cm3/g) | Pore size (nm) |
|---|---|---|---|---|---|
| TD0 | 0.32 | 91±2 | 921 | 1.99 | 9.2 |
| ScEt | 0.41 | 128±2 | 80 | 0.23 | 41.3 |
| ScCO2 | 0.38 | 153±1 | 265 | 0.36 | 55.3 |
| AD | 0.38 | 151±5 | 257 | 0.42 | 54.0 |
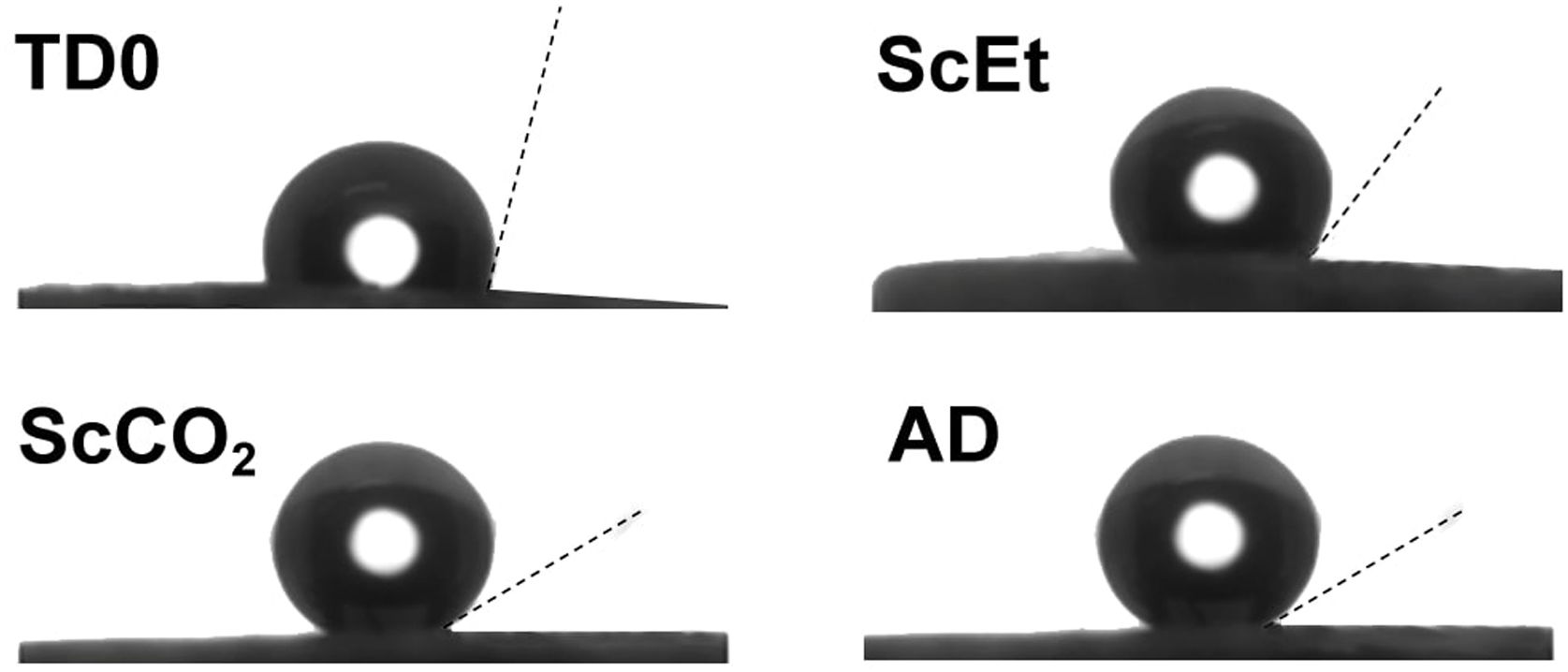
The measurement of the hydrophobicity of the samples revealed that the copolymerization with DEDMS made the samples hydrophobic, as can be clearly seen in Fig. 2, in which the composition and the influence of the drying procedure are compared. The obtained values of the contact angles are listed in Table 2. Samples dried in ethanol were both hydrophobic, with contact angles θ=91°±2° and θ=128°±2°, for samples TD0 and ScEt, respectively. The surfaces of the ScCO2 and AD samples showed a contact angle of θ>150°, similar to confirming their superhydrophobicity. These results are in agreement with those of previous studies of polysiloxane aerogels obtained in a similar manner [22,23].
This hydrophobic surface behaviour is the combined result of the copolymerized DEDMS/TEOS, which contributes hydrophobic methyl radicals, and the surface roughness of the mesoporous aerogels [24]. Therefore, the addition of the organic chains of DEDMS confers significantly higher hydrophobicity to the nanoporous silica structure. Surprisingly, despite the high temperatures employed in the ethanol-based drying procedure, which involves the hydrophobization of the silica [25], superhydrophobic samples were only obtained by the CO2-based and ambient drying procedures. In previous work, contact angles very close to 0° for common motor oil in TD0 and ScEt samples were obtained, thus confirming the good oleophilicity and, in addition, the expected ability of these samples for oil recovery from wastewaters [5].
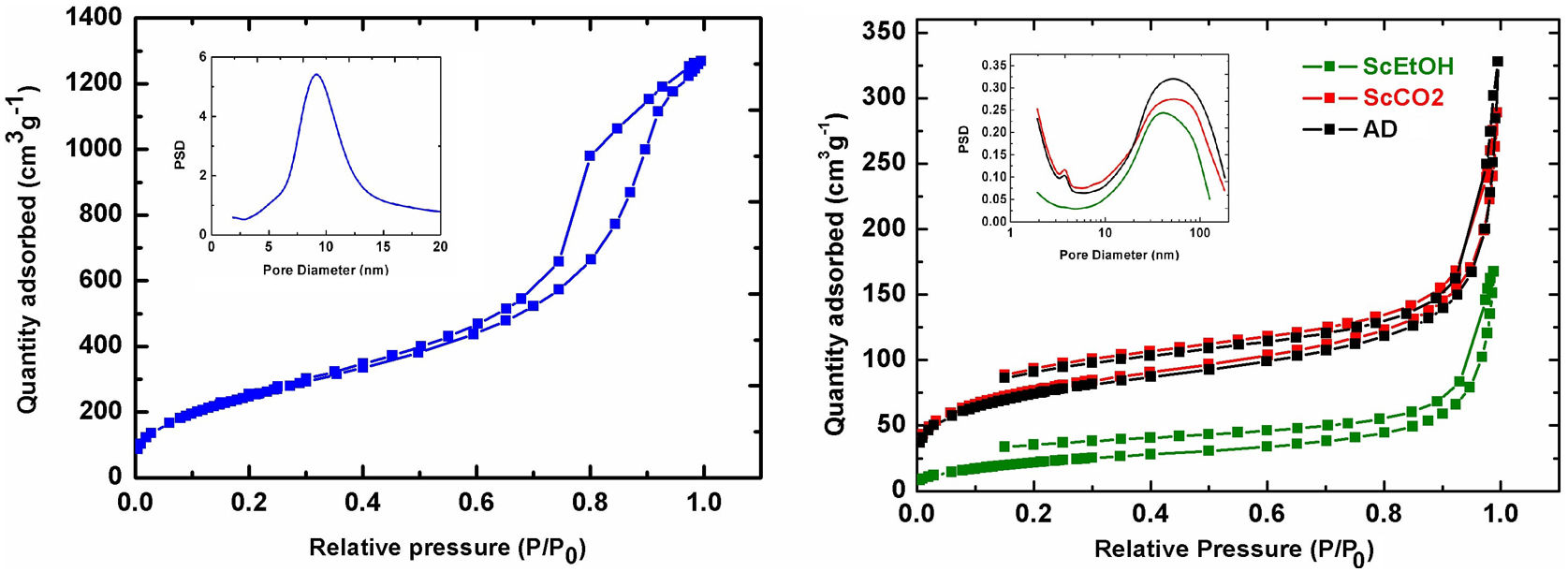
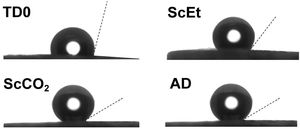
Nitrogen physisorptionThe characteristic microstructural features of these samples were revealed by physisorption experiments. The corresponding adsorption/desorption isotherms are shown in Fig. 3. The TD0 isotherm is type IV, based on the IUPAC classification, and is associated with capillary condensation through multilayer adsorption taking place in interconnected mesopores, leading to a type H1 hysteresis loop. The TD0 sample presented typical high specific surface area (921m2/g) and pore volume (1.99cm3/g), with mean pore size 9.2nm and a monomodal pore size distribution, as expected for ethanol-dried pure silica aerogel, as shown in Table 2.
The other adsorption curves, for the hybrid samples (Fig. 3), can be classified as type II, which behave like porous media containing both macropores and mesopores. Unclosed hysteresis loops belong to type H3, which is often attributed to narrow slit-shaped micropores in which the release of trapped nitrogen is difficult owing to a slow diffusion rate, which hinders reaching the equilibrium during desorption and prevents the closure of the hysteresis loop. This phenomenon may be intensified by the presence of ink-bottle pores [26] and perseveres, despite increasing the data sampling of the isotherms or doubling the equilibrium time during desorption. These qualitative structural changes can be explained in terms of the composition of the sample, given that the hybrid aerogels are made with a 1:1.25 DEDMS:SiO2 molar ratio (50wt.% organic:inorganic mass ratio) so the percolation level of the organic phase (40wt.%) was already exceeded [12]. For these reasons, the hybrid aerogels studied in this work can be considered as an organic matrix where the silica clusters are embedded, instead of a porous silica matrix with embedded polydimethylsiloxane oligomers.
The presence of DEDMS in the hybrid samples drastically decreases the specific surface area to above 260m2/g in the case of the scCO2 and AD samples, and to 80m2/g for the scEt aerogel. This decrease is similar to what has been reported previously [5] and the differences from the AD are also expected due to the collapse of the structure and densification. However, the different supercritical drying procedures produced significant differences in the submicrostructure of the aerogels. Thus, completely similar alcogels resulted in aerogels with more open structures when dried with supercritical CO2 instead of ethanol, with lower density, higher specific surface area and pore volume, and a finer pore size. In addition, the pore size distributions of all the hybrid samples showed similar characteristic pore sizes in the range of 41.3–54.0nm (Table 2), that is in the limit between the meso- and macropore domains. This fact is corroborated by the closed hysteresis loop for the TD0 sample without micropores, compared to the open loops for the other samples.
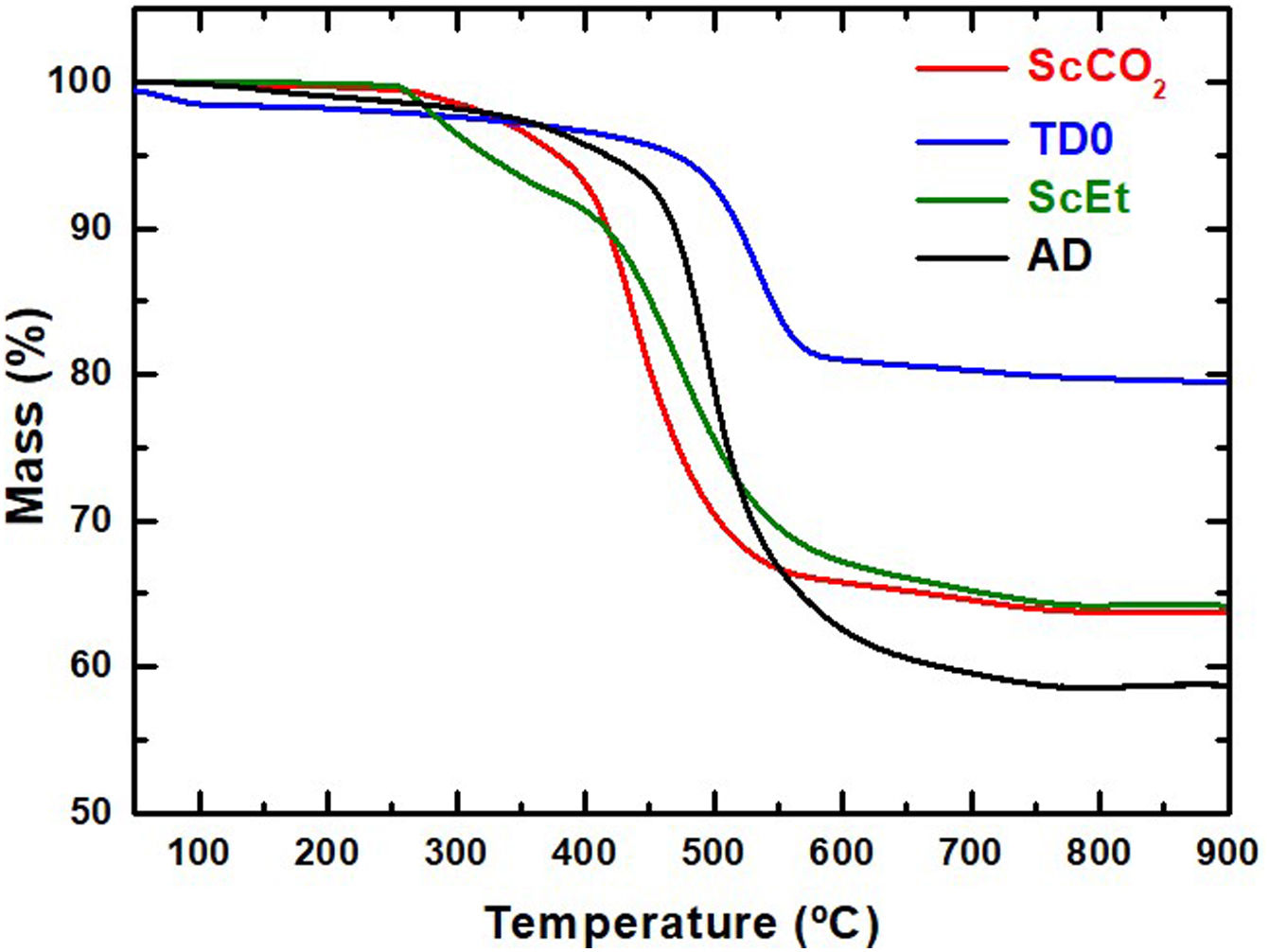
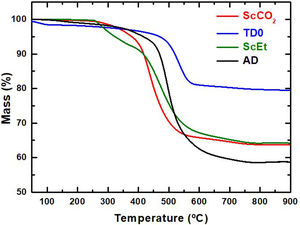
Thermal stabilityThe initial degradation atmosphere and thermal stability was studied by thermogravimetric analyses under inert atmosphere (Fig. 4). The weight loss of silica aerogel (TD0) initiates below 250°C with the extraction of adsorbed water and increases continuously up to 700°C. The total weight loss corresponds to a higher condensation of surface silanol groups (dehydroxilation) than expected for fully dense silica particles with 921m2/g and exhibiting the typical surface OH density of ∼4–5OH per nm2[17]. Thus, some loss of organic components (such as ethanol or ethoxy groups) and of structural water can be concluded. Moreover, it can also be confirmed that the thermal degradability is greatly affected by the choice of the drying method. For example, the decomposition of the aerogel ScEt started at a lower temperature than that of the samples ScCO2 and AD, and was similar to TD0. It exhibited two differentiated processes: the first one is due to the condensation of silanols of the surface and the degradation of the ethoxy radicals originating during the ethanol-based drying process [27], and the second one due to the degradation of the organic phase, mainly attributed to the decomposition of methyl groups [28].
The samples TD0, AD and ScCO2 showed a one-step degradation process and more stable organic phases (degradation at higher temperatures). These results revealed that the thermal stability of the organic phase in this type of sample can be better achieved through mild drying temperatures, between 40 and 50°C, instead of the 270°C used in the case of the ScEt sample. The total weight loss of the AD sample is higher than those of the ScEt and scCO2 samples. Assuming the full degradation of the organic phase (the same in all hybrid samples), this is due to the lower temperature of the drying process when working in ambient drying than with supercritical ethanol, which leads to a higher hydroxyl surface density.
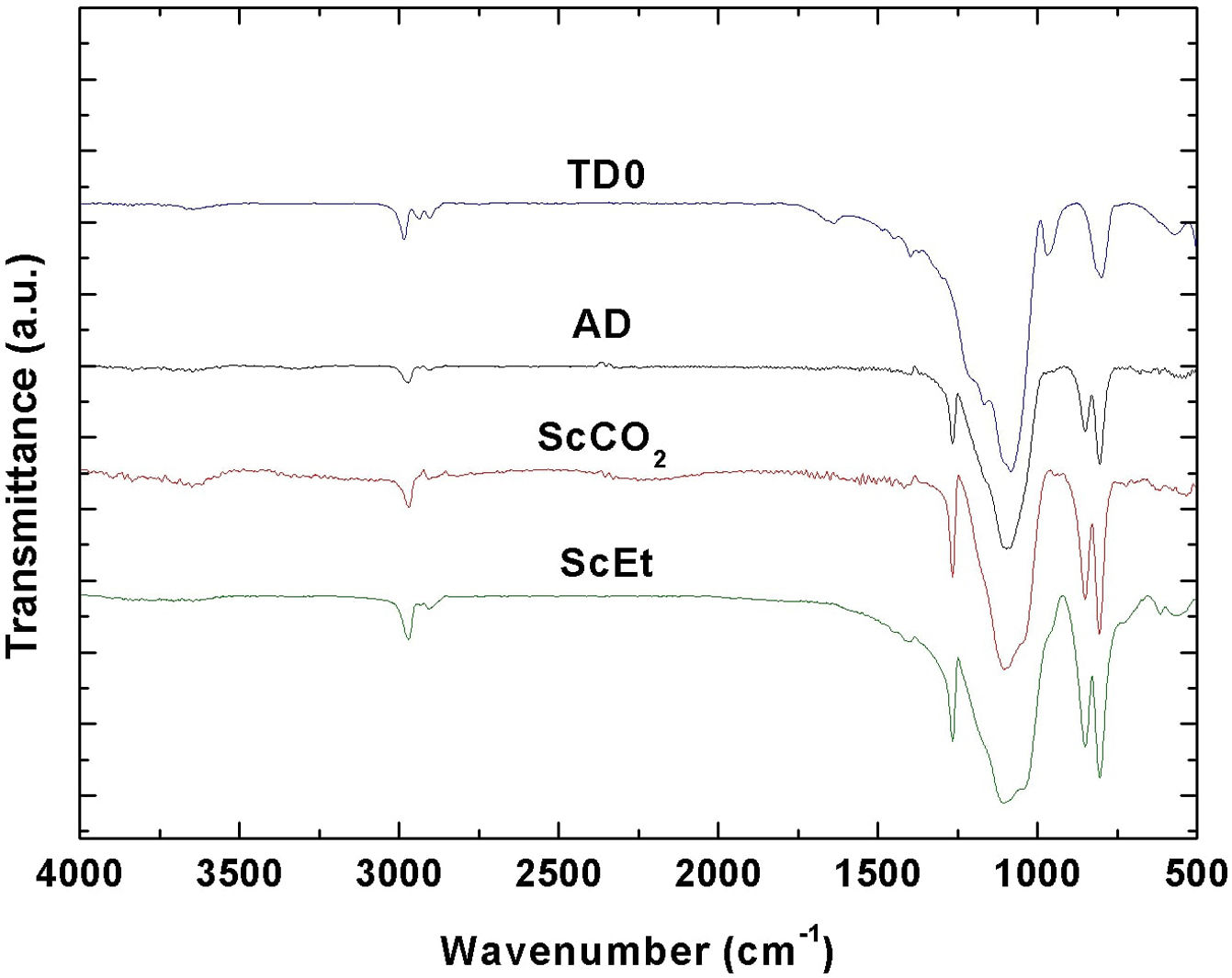
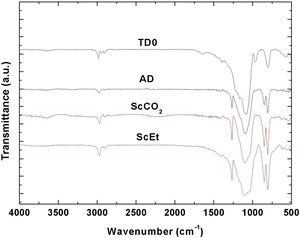
FTIRThe typical FTIR spectra obtained for the samples are shown in Fig. 5. The observed absorption bands agree with a predominant inorganic silica network composed of Si–O–Si bonds, with two methyl groups per silicon and hydroxyl groups at the network ends [29]. Thus, three bands centred at 800, 1040, and 1100cm−1 associated with the Si–O–Si asymmetric and symmetric stretching vibration modes are found in the four samples [30]. Absorption bands corresponding to stretching vibrations of Si–C bonds at 850cm−1 and symmetric and asymmetric flexural vibrations of Si–CH3 group at 1265 and C–H bending at 1400cm−1 confirm the existence of methyl groups in the hybrid structures [31,32]. Also, symmetric and asymmetric stretching vibrations of C–H bonds between 2800 and 3000cm−1 accounts for residual ethoxy radicals in TD0 and ScEt [33] in coherence with the degradation processes observed in TGA (Fig. 3). This band is clearer in the ScCO2 sample and demonstrates the great extent of the condensation reactions favoured by its drying procedure. Two additional bands accounting for surface Si–OH and H–O–H bonds are found at 961 and 1640cm−1 respectively, in the TD0 sample [31]. Also narrow peaks at 1100 and 1200cm−1 probably indicate that the TEOS molecule is not fully hydrolyzed. Therefore, the band at 961cm−1 in TD0 could also account for ethoxy non-hydrolyzed groups. Finally, it is worth noting the almost complete absence of any absorption peak in the region of 3500cm−1, associated with O–H vibrations, therefore indicating the deficiency of hydroxyl groups on the surfaces of the aerogels and the strong hydrophobic character of all the samples [28].
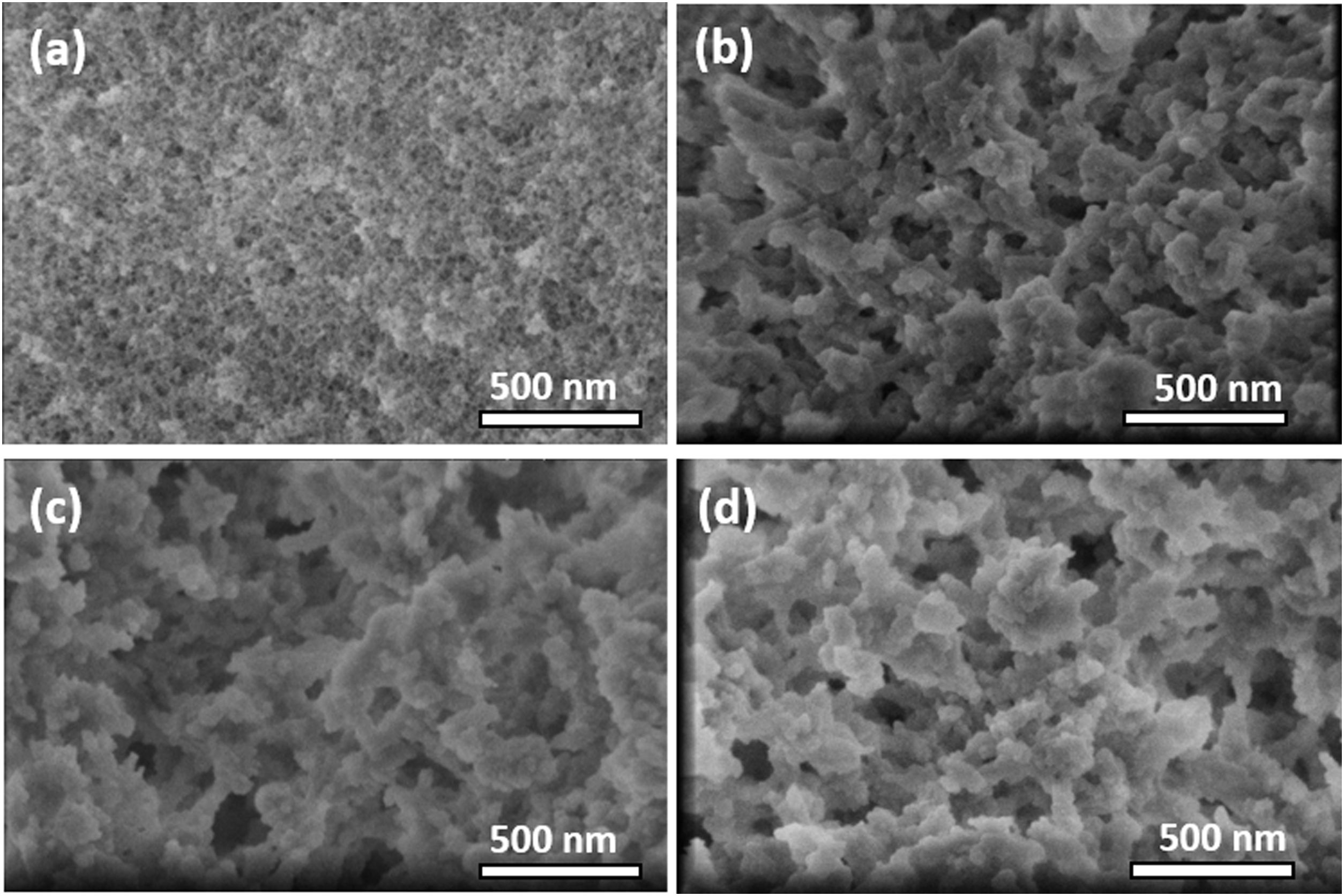
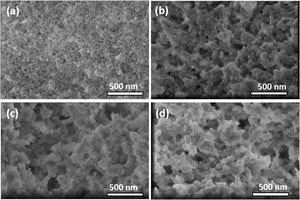
Scanning electron microscopyA comparison of the scanning electron micrographs of the samples was also performed. Fig. 6a shows the regular surface aspect of the inorganic silica aerogel TD0, with extremely fine structural features, far below the 500nm length scale. In contrast, some differences are clearly seen with Fig. 6b, c and d, from ScEt, scCO2, and AD, respectively. These hybrid samples presented a broader particle size distribution with larger particles (∼200nm in all hybrid samples), abundant large pores (100nm), and very similar submicrostructural characteristics with an associated macroporous distribution, showing a well-branched and highly nanostructured porous network, typical of polysiloxane hydrophobic aerogels [23,33], which is in good agreement with the reported results from N2 physisorption experiments (Table 2 and Fig. 3), making it difficult to discern the differences between them.
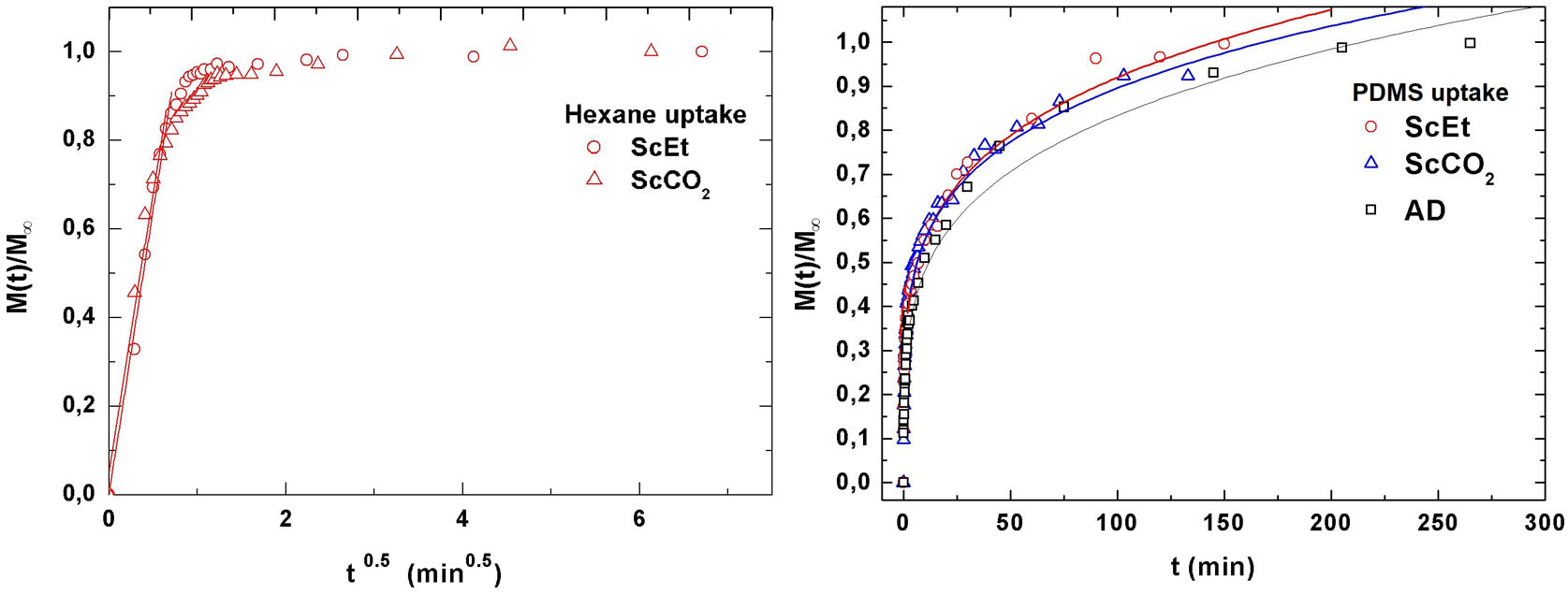
Liquid absorption experimentsThe influence of the drying procedure and type of solvent on the absorption kinetics of the hybrid samples was evaluated by the study of the normalized weight gain, namely, the ratio M(t)/M∞ (see Fig. 7) for two different organic solvents at room temperature, hexane and silanol-terminated PDMS fluid. At first glance, Fig. 7, left, shows that the type of drying procedure does not influence the liquid absorption behaviour. Thus, the nanostructures that they exhibit are similar regarding the absorption kinetics. However, well differentiated absorption regimes for each type of solvent are observed. Due to its lower viscosity, hexane is a liquid that diffuses faster than liquid PDMS. Thus, in the case of hexane, the diffusion process happens almost instantaneously, and the saturation level M∞ is achieved in a matter of minutes, following the normalized weight gain M(t)/M∞ vs. t0.5 as Fick's law of diffusion fits the linear trend perfectly (R2>0.99) before reaching saturation (M(t)/M∞≈0.8) [34]. In Table 3, the information about the corresponding linear curve fitting data is shown.
Left: normalized weight gain for hexane uptake in ScEt and ScCO2 samples versus square-root of time. Lines corresponding to linear fittings performed up to ∼ 80% of the process in hexane. Correlation values R2>0.99 in all cases. Right: plots of normalize weight gain values versus time for PDMS uptake. Lines correspond to non-linear fittings according to semi-empirical power law equation for swelling of polymers [22]. Fitting parameters are shown in Table 3.
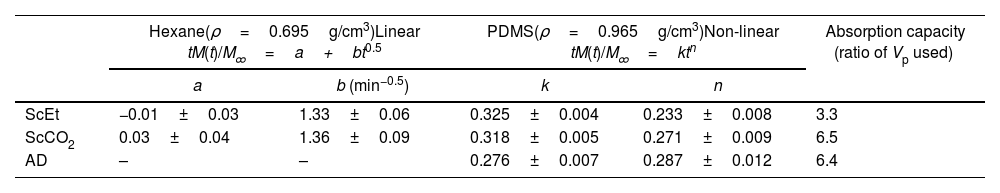
Fitting parameters of the different mathematical models considered for normalized weight gain of hexane and liquid PDMS in hybrid aerogels. Linear fittings were performed up to 80% of the process, with correlation values R2>0.99.
| Hexane(ρ=0.695g/cm3)Linear tM(t)/M∞=a+bt0.5 | PDMS(ρ=0.965g/cm3)Non-linear tM(t)/M∞=ktn | Absorption capacity (ratio of Vp used) | |||
|---|---|---|---|---|---|
| a | b (min−0.5) | k | n | ||
| ScEt | −0.01±0.03 | 1.33±0.06 | 0.325±0.004 | 0.233±0.008 | 3.3 |
| ScCO2 | 0.03±0.04 | 1.36±0.09 | 0.318±0.005 | 0.271±0.009 | 6.5 |
| AD | – | – | 0.276±0.007 | 0.287±0.012 | 6.4 |
Non-linear absorption kinetics is observed for the liquid PDMS diffusion (Fig. 7, right), which includes swelling. In this case, the following simple and semi-empirical power law equation is commonly used to determine the mechanism of diffusion in this type of polymeric network:
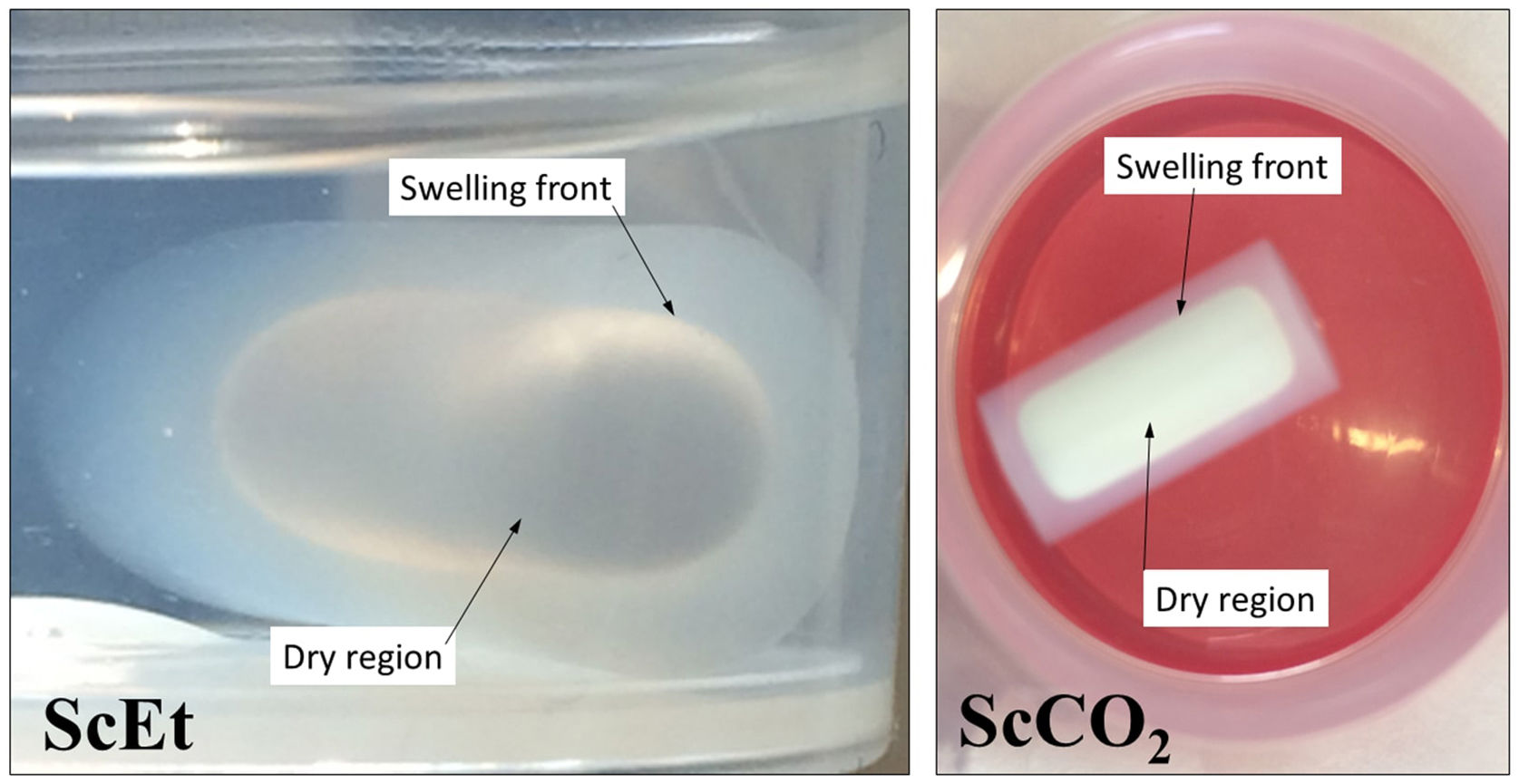
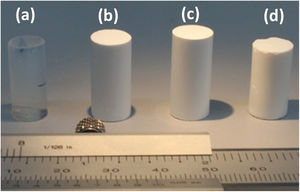
where the constants k and t, are characteristics of the solvent–polymer system and the diffusional exponent n are characteristics of the solvent–polymer system depends on the geometry of the polymeric network, as well as on the physical mechanism of the solvent uptake. The non-linear fitting curves to Eq. (2) are plotted in Fig. 7, right, for the three hybrid samples immersed in PDMS, and Table 3 summarizes the fitting parameters for each sample and solvent. The case of n<0.5 refers to a slower non-fickian situation, as has resulted for the hybrid samples and liquid PDMS solvent system under study. Hence, the normalized weight gain of the absorption kinetics of liquid PDMS corresponds to non-fickian behaviour [35]. These results show that the absorption kinetics in this type of sample seems not to depend on the method of drying. On the contrary, regarding the total amount of liquid absorbed, it was observed that aerogel ScEt exhibited almost one-half of the absorption capacities of the other two hybrid samples (Table 3). Thus, while ScEt absorbed a total volume of liquid that was more than three times its own porous volume, ScCO2 and AD absorbed more than six times their respective porous volumes and up to 2.6 times of the dry weight. However, the range of absorption capacities of our aerogels was lower than that of similar superhydrophobic materials prepared using methyltriethoxysilane (MTES) [36], vinyl-terminated PDMS [23] and gelatin–silica based aerogels [37], although they were in agreement with previous studies on DEDMS-based silica aerogels [5,33].The absorption phenomenon of liquid PDMS could be directly observed thanks to the sharp well-defined boundary between the swollen and un-swollen regions of hybrid aerogels, namely, the “swelling front” indicated by the arrows in Fig. 8, therefore confirming features of non-fickian diffusion behaviour [35]. Whereas the dry hybrid samples are white and completely opaque with air inside of the pores, the uptake of the liquid PDMS reduces the opacity of the samples, allowing the clear observation of the swelling front due to the increase of the refraction index.
Mechanical propertiesThe porous interconnected volume, the rough surfaces observed in SEM as well as the abundant methyl content are responsible not only for the hydrophobic behaviour observed in the obtained aerogels, but also for their mechanical responses [23,36]. As a general description, the monolithic samples exhibited high compressibility and extraordinary flexibility, indicating that the hybrid networks were covalently bonded and provided high thermal and mechanical stabilities, as described below.
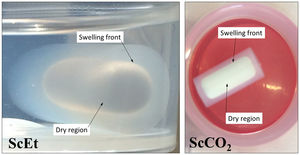
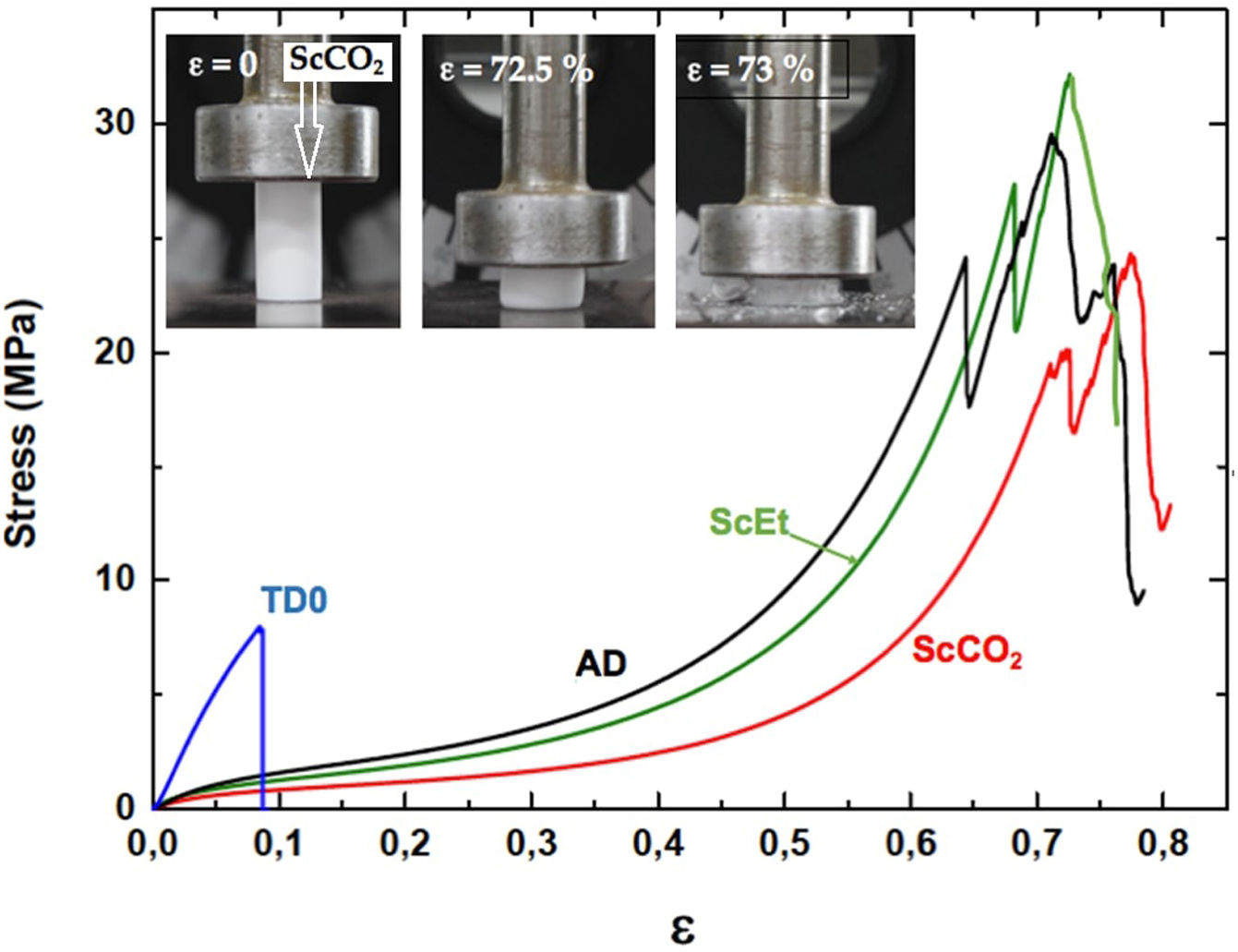
Uniaxial compression tests for as-prepared aerogelsIn Fig. 9, the corresponding stress–strain curves of the TD0, ScEt, ScCO2, and AD samples in the dry state are shown, providing sample-specific information about the typical mechanical behaviour, namely, the Young's modulus, compressive strength and rupture strain. All the curves of the hybrid samples exhibit a very similar initial response, much less stiff than the pure silica aerogel, followed by strain hardening up to the ultimate compressive strength, when the materials failed completely. This description corresponds to an elastomeric behaviour which is known to be rate-dependent and to exhibit hysteresis upon cyclic loading. The results are in accordance with the general behaviour previously reported for similar samples reinforced with organic contents [5,38], and in contrast to other inorganic reinforcing strategies [39]. In addition, a very slight influence of the drying method on the mechanical behaviour of the hybrid samples can be seen, and only small variations in the mechanical parameters were observed (Table 4).
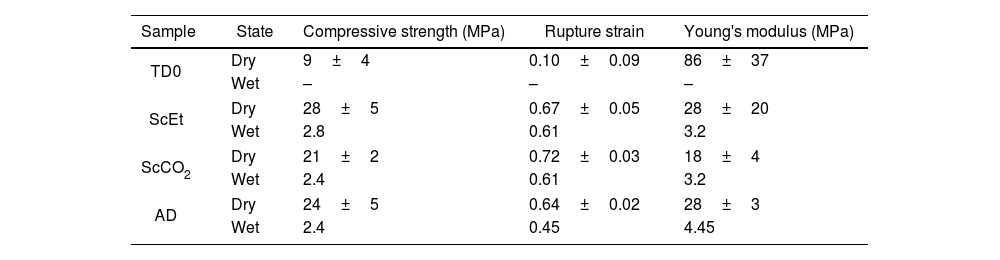
Mechanical properties of dry and wet samples (immersed in liquid PDMS) obtained from the uniaxial compression tests.
| Sample | State | Compressive strength (MPa) | Rupture strain | Young's modulus (MPa) |
|---|---|---|---|---|
| TD0 | Dry | 9±4 | 0.10±0.09 | 86±37 |
| Wet | – | – | – | |
| ScEt | Dry | 28±5 | 0.67±0.05 | 28±20 |
| Wet | 2.8 | 0.61 | 3.2 | |
| ScCO2 | Dry | 21±2 | 0.72±0.03 | 18±4 |
| Wet | 2.4 | 0.61 | 3.2 | |
| AD | Dry | 24±5 | 0.64±0.02 | 28±3 |
| Wet | 2.4 | 0.45 | 4.45 | |
TD0 showed the classical perfect elastic and brittle behaviour and fractured in a catastrophic brittle manner by rapid crack propagation with low energy release, exhibiting a relatively high Young's modulus (85.7MPa) and low compressive strength (8.8MPa) and rupture strain (9%) (data included in Table 4). In contrast, the compressive strength of the hybrid samples ranged from 20.5 to 27.5MPa and Young's modulus ranged from 17.6 to 28.1MPa, while the rupture strain varied from 64% to 72% (see Table 4). The images in the inset of Fig. 9 show the different strain stages for the ScCO2 aerogel, from initial loading to the starting point of the rupture, being representative of the rest of the hybrid samples. Although similar large deformations (up to 80%) have been previously reported [36], both the compressive strength and elastic modulus of our hybrid aerogels were two to three orders of magnitude higher than those reported in most studies on this subject [33,36,40–42].
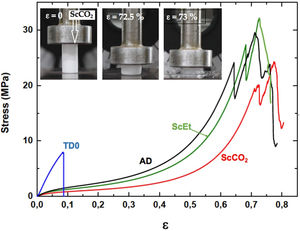
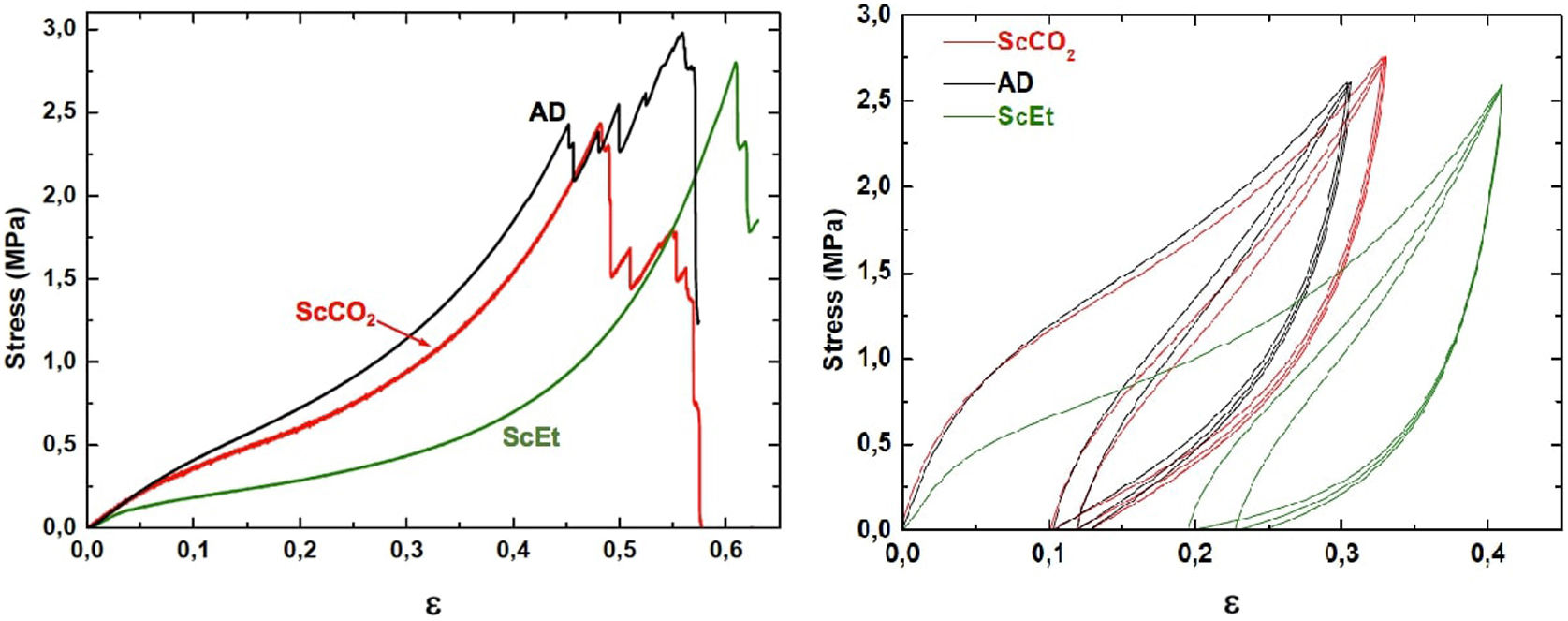
Uniaxial compression test for wet aerogelsFig. 10 shows a series of stress–strain curves from the uniaxial compression test on the hybrid samples immersed in liquid PDMS. It was observed that these samples exhibited high swelling and associated strain softening, but retained good mechanical performance when reaching their fully swollen state. As a result, they become more compliant and the estimated corresponding mechanical parameters were significantly smaller than those of the dry samples (see Table 4). At the same time, the mechanical properties of the swollen hybrid samples allowed their handling, in contrast to the TD0 silica aerogel, which led to immediate failure at a very low strength and strain after the absorption of the liquid (data not shown). In addition, as in the dry state, no significant differences were found between the different drying procedures: similar curves and similar mechanical parameters were observed for the three hybrid samples. Nevertheless, among the set of hybrid samples, the ethanol-dried sample ScEt showed the highest strain-at-break, suggesting it has the best performance in absorbing and expelling liquids, from the mechanical point of view.
To verify the usefulness of the samples for solvent recovery, cyclic loading tests were performed by uniaxial compression inside the PDMS liquid. The hybrid samples were tested in three consecutive cycles between 60% and 80% of the maximum load (Fig. 10), without any significant change in the mechanical response, except for the Mullins effect for cyclic stress, a stress-softening viscoelastic effect that takes place in rubber-like materials [43]. All the samples recovered their shapes and sizes inside the liquid bath, minutes after the last unloading. These results revealed the existence of a viscoporoelastic effect, that is, the PDMS liquid was expelled from the inner core of the cylindrical samples exhibiting a high diffusivity due to the high crosslink density of the hybrid network. This was confirmed by the good mechanical behaviour and the corresponding elastic parameters of the wet samples (see Table 4). As a result, the wet samples presented physical properties that were substantially better than other conventional absorbent gels [33,44].
Specifically, when compared to the ScEt and AD samples, the ScCO2 aerogel was highly resistant to fracture and was stretchable (with elongations at break above 70%), and highly inert to chemical attack and degradation by hydrocarbon liquids such as hexane. These properties make this type of sample potentially attractive for new environmental applications as they enable selectively absorbing a wide range of organic solvents and oils from water mixtures.
ConclusionsIn conclusion, monolithic, flexible, and superhydrophobic silica-DEDMS (50wt.%) hybrid samples were synthesized by the sol–gel process assisted by sonocatalysis, followed by drying under three different conditions: two aerogels, one obtained by supercritical drying in (i) ethanol and (ii) the other by supercritical drying in CO2, and (iii) a xerogel obtained by ambient drying. Their structure and pore size distributions were found to be dependent on the drying procedure and on the DEDMS content. It was observed that the ScEt samples were those that presented the lowest values of specific surface (80m2/g) and specific pore volume (0.32m3/g), while the ScCO2 and AD samples shared very similar textural and microstructural characteristics. TGA, FTIR and water contact angle analysis confirmed that there was a different behaviour for ScEt, as it was the first to decompose thermally (270°C) and exhibited hydrophobic behaviour (128°±2°) instead of superhydrophobicity, as occurred with ScCO2 (153°±1°) and with the ambient dried sample (151°±5°). From liquid absorption experiments and a characterization of their mechanical properties, it was observed that the ScCO2 and AD samples displayed the best performance in terms of their structural stabilization, mechanical properties, and absorption capacity. In all cases, the diffusion of the liquid uptake follow a fickian behaviour. Consequently, they are suggested as the best option for environmental applications for oil spill and organic solvent remediation.
This work has been co-financed by the 2014–2020 ERDF Operational Programme and by the Department of Economy, Knowledge, Business and University of the Regional Government of Andalusia. Project reference: FEDERUCA18-106598. V- M.-F. thanks the grant from V Plan Propio de Investigación de la Universidad de Sevilla. Funding help for the Ayuda 2018 VI-PPTIUS and the SGI functional characterization from the CITIUS-Central services of the University of Seville are also acknowledged. Mr. Adrián Pavón Duarte is deeply acknowledged for his help during simple drying.





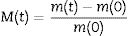
![Left: normalized weight gain for hexane uptake in ScEt and ScCO2 samples versus square-root of time. Lines corresponding to linear fittings performed up to ∼ 80% of the process in hexane. Correlation values R2>0.99 in all cases. Right: plots of normalize weight gain values versus time for PDMS uptake. Lines correspond to non-linear fittings according to semi-empirical power law equation for swelling of polymers [22]. Fitting parameters are shown in Table 3. Left: normalized weight gain for hexane uptake in ScEt and ScCO2 samples versus square-root of time. Lines corresponding to linear fittings performed up to ∼ 80% of the process in hexane. Correlation values R2>0.99 in all cases. Right: plots of normalize weight gain values versus time for PDMS uptake. Lines correspond to non-linear fittings according to semi-empirical power law equation for swelling of polymers [22]. Fitting parameters are shown in Table 3.](https://static.elsevier.es/multimedia/03663175/0000006300000001/v1_202402270536/S0366317523000171/v1_202402270536/en/main.assets/thumbnail/gr7.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)