Salmonella enterica es una especie de bacterias anaeróbicas facultativas que han sido empleadas con gran éxito como vector bacteriano vivo atenuado con fines vacunales. Recientemente se ha documentado que S. enterica tiene propiedades importantes para ser considerada como agente terapéutico contra el cáncer. Estudios preclínicos y clínicos han demostrado que S. enterica coloniza tumores sólidos, semisólidos y metástasis, además de que contribuye a disminuir la resistencia a los tratamientos. En esta revisión se aborda la capacidad de S. enterica atenuada para eliminar células tumorales y su empleo como vector bacteriano vivo acarreador de moléculas heterólogas contra el cáncer.
Salmonella enterica, a species of facultative anaerobic bacteria, has demonstrated success as a live-attenuated bacterial vector for vaccination. S. enterica has also demonstrated promise as a therapeutic agent against cancer. Pre-clinical and clinical trials have shown that S. enterica is localized in both solid and semi-solid tumors as well as in metastatic tumors. Moreover, S. enterica reduces resistance to treatment with other agents. In this review we present the novel therapeutic anti-cancer approaches that use S. enterica both for its ability as a delivery system for heterologous moieties against cancer and for its direct anti-cancer properties.
1. Introduction
Cancer is one of the main public health challenges worldwide, and although great progress has been made with regards to treatments, the problems related with abolishing metastasis, adverse effects and resistance to treatment have made the search for alternative therapies with better efficacy and selectivity for the altered cells or the microenvironment necessary.1-5 An alternative for the solution of these problems is the use of live attenuated bacterial vectors as antitumor agents or carriers of molecules with anti-tumor activity.6-8
1.1. Bacterial vectors in antitumor therapy
The idea of using bacteria as anti-tumor agents was documented in 1868 by Busch, noting that a patient’s sarcoma decreased when he acquired erysipelas.9 This observation was again described 30 years later by Coley10 and Fehleisen.9 The latter described that the causal agent of erysipelas was Streptococcus pyogenes.9 Coley’s observations on patient’s recovery with cancer after an erysipelas infection led him to develop a vaccine called Coley toxin, composed of S. pyo-genes and Serratia marcescens, to treat patients with sarcomas, carcinomas, lymphomas, melanomas and myelomas.9-11 This toxin lost importance due to the advances in radiation and chemotherapy.
The antitumor principle of bacteria was once again taken up by Holmgren in 1935 who reported that the attenuated strain of Mycobacterium bovis, bacillus Calmette-Guérin (BCG), had antitumor activity.12 This antitumor activity could explain the observation by Rosenthal13 on the low incidence of leukemia in neonates immunized with BCG.13 Later studies allowed that from 1976 BCG was applied intravesically as immunotherapy to reduce the recurrence and progression of superficial transitional cell bladder carcinoma.5,13,14
Three distinct groups of bacteria with anti-tumor activity have been proposed as the result of their ability to tolerate oxygen, which is found in very low concentrations in the tumor microenvironment. In group I are found bacteria that are strictly anaerobic of the genus Bifidobacterium (which produce lactic acid); in group II are found intracellular bacteria of the genus Salmonella and Listeria (which are nonfacultative); finally, in group III are found strictly anaerobic bacteria of the genus Clostridium (formed by spores).3
Among these groups of bacteria, Salmonella enterica serovar Typhi (S. Typhi), which infects humans and Salmonella enterica serovar Typhimurium (S. Typhimurium), which infects mice and humans, have called attention due to the availability of attenuated strains with low toxicity15 and because of its high specificity for tumor tissue16,17 including metastasis.18
2. Salmonella enterica and its selectivity for the tumor microenvironment
At present, S. enterica is the most used bacterial vector as a therapeutic agent in pre-clinical models of cancer. However, the mechanisms that explain the selectivity of these bacteria towards soft tissue are not very clear. It has been described that the microenvironment generated by the physiology of the tumor; characterized by hypoxia (at oxygen concentrations ≤ 10 mmHg compared normal tissue of 50-60 mmHg3), acidity (caused as a consequence of lactic acid, product of the anaerobic metabolism induced by decrease in oxygen) and necrosis (the result of death of tumor cells due to lack of nutrients and uncontrolled growth), could contribute to the bacterial proliferation in the tumor microenvironment.6
In vitro studies by Kasinskas and Forbes where the micro-environment of the tumor cells of colon carcinoma and the gradient of the metabolytes in human tumors was mimicked, have demonstrated that S. enterica migrates to the tumor tissue by the molecular attraction that would act as chemotaxic agents by uniting to their respective receptors in the bacteria, favoring tumor colonization.19 In this manner, the aspartate receptor in S. enterica begins the chemotaxis of the bacteria towards the tumor zone, the serine receptor initiates penetration and the ribose/galactose receptor directs S. enterica towards the area of tumor necrosis20. In this process, motility of the bacteria mediated by molecules such as CheA/CheY is indispensable for an effective distribution and for the recruitment of the bacteria in tumor tissue.20-22 However, these findings have been challenged recently with in vivo studies by Crull et al. in a murine model of colon cancer. These demonstrate that the invasion and colonization of the tumor by S. Typhimurium is independent of the islands of pathogenicity type I and type II, and even independent of the motility and of the chemotactic response. Their results also suggest that the colonization and anti-tumoral activity are affected by the route of administration, with the intravenous and intraperitoneal routes being the most effective.23 This study also confirmed that the strains of S. Typhimurium mutated in the metabolic pathways of the synthesis of aromatic amino acids slightly decreased the colonization of the tumor tissue compared with the bacteria of the field strain.21
It has been proposed that once S. enterica arrives at the tumor microenvironment, the mechanisms that allow it to stay are associated with the little activity of macrophages and neutrophyls24 due to the hypoxemia inside the tumor, to the suppression of the immune response mediated by the presence of cytokines (as TGF-b) and to the difficult access of the anti-Salmonella antibodies and of the factors of complement by the irregular growth of the blood vessels inside the tumor25.
3. S. enterica and its intrisic antitumoral activity
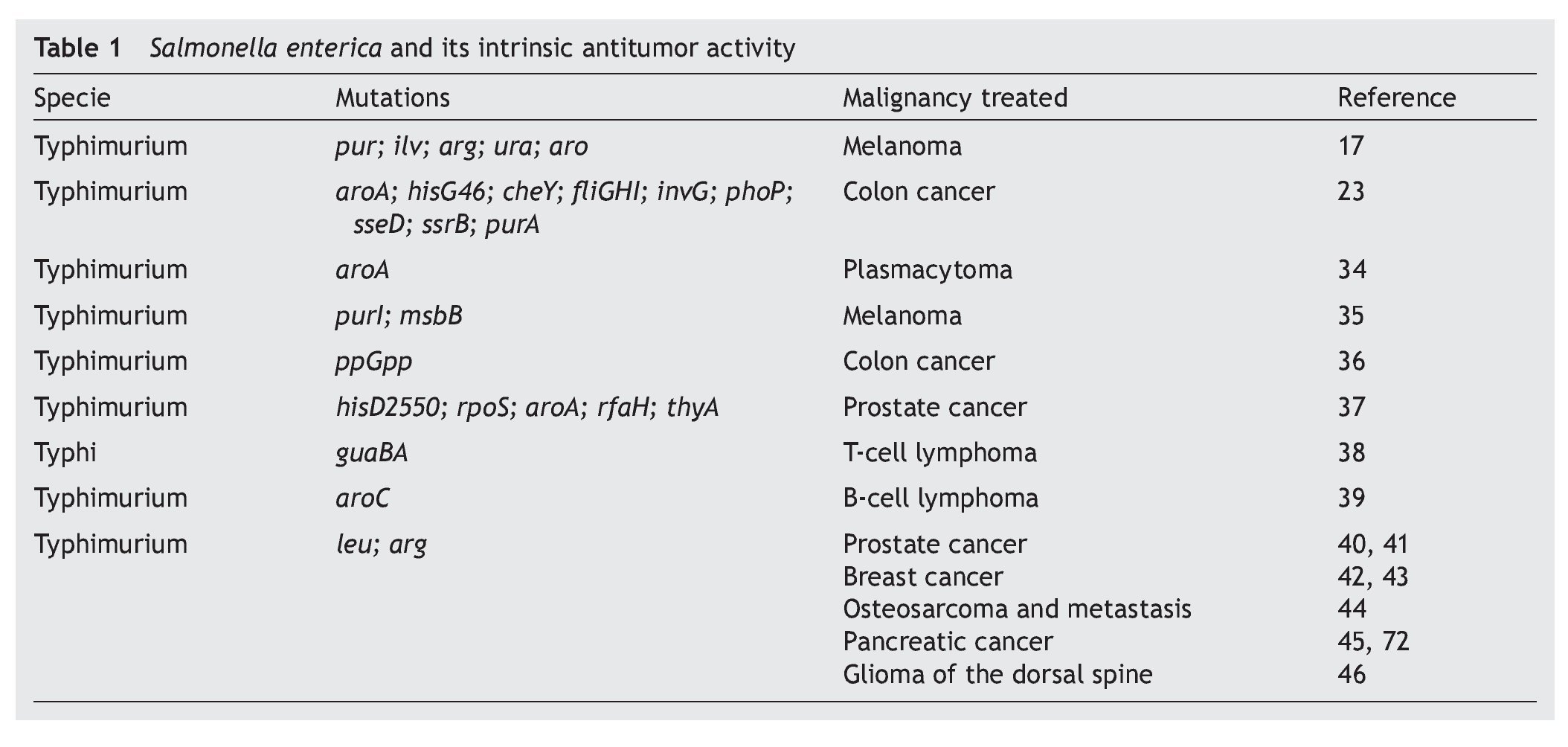
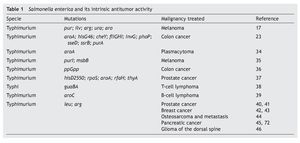
S. enterica is a facultative anaerobic bacterium that has been used with great success as live attenuated bacterial vector as a vaccine because of its affinity for antigen presenting cells.8,26,27 This characteristic is associated with mechanisms of induction or activation of the innate immune28,29 or specific response,30,31 which explain part of the antitumoral immunotherapeutic activity possessed by these bacteria. The intrinsic antitumoral activity of S. enterica is complemented by the competition between the tumor cells and the bacteria for the nutrients or by the release of anti-tumoral bacterial components due to lysis of the bacteria attached to the tumor cell.15 Diverse studies have documented the intrinsic antitumoral activity possessed by S. enterica, per se (Table 1).
Since 1982, Kurashige and Mitsuhashi documented the induction of the immune response with antitumoral activity by the use of mini cells (bacterial cells without genomic DNA) obtained from S. Typhimurium in a murine model of sarcoma32 as well as T-cell lymphoma.33 In these models, the mini cells restored the activity of the macrophages in the tumor microenvironment. Subsequently, studies by Eisenstein et al. in 1995 using mutations in metabolic pathways (aroA mutations) of S. Typhimurium SL3235 demonstrated that these attenuated strains were effective in inhibiting growth and reducing the size of the tumor in a murine model of plastocytoma.34 The ability of the attenuated strains of S. enterica to colonize and replicate within the tumors was demonstrated by Pawelek et al. in 1997 using a murine melanoma model. The results demonstrated a colonization ratio of 1000:1 with respect to normal tissue and its usefulness as carrier of therapeutic proteins towards tumor cells in vivo was confirmed.17 Since that time different reports have arisen demonstrating that the attenuated strains of S. enterica have the capacity of reducing the size of the tumor and of delaying the development of metastasis and extending survival in different models of murine cancer such as the case of lung carcinoma,35 colon carcinoma,23,36 prostate cancer,37 T-cell metastatic lymphoma38 and B-cell lymphoma,39 among others.
These studies have been consistent with the observations made in murine models of xenotransplants with human cell lines including breast and prostate cancers40-42 where attenuated strains of S. Typhimurium have been used that induce lower toxicity in the host and maintains its anti-tumor activity. An example of this are the works described with auxotrophic strains A1 (deficient in leucine and arginine synthesis) and A1-R (deficient in leucine and arginine synthesis, with greater capacity of eliminating tumor cells), which has been used in murine models of xenotransplants of human prostate cancer40 and its metastasis41 as well as human breast cancer.42 The results of the latter model demonstrate that the strains colonize the tumor and that in ~40% of the animals treated the tumor was completely eradicated and remained apparently healthy for the 20 weeks the experiment lasted.42 Recent studies show that the A1-R strain inhibits bone metastasis caused by breast cancer.43 Other works have documented the anti-tumor and anti-metastatic activity of S. Typhimurium A1-R in osteosarcoma,44 pancreatic cancer45 and in gliomas of the dorsal spine.46
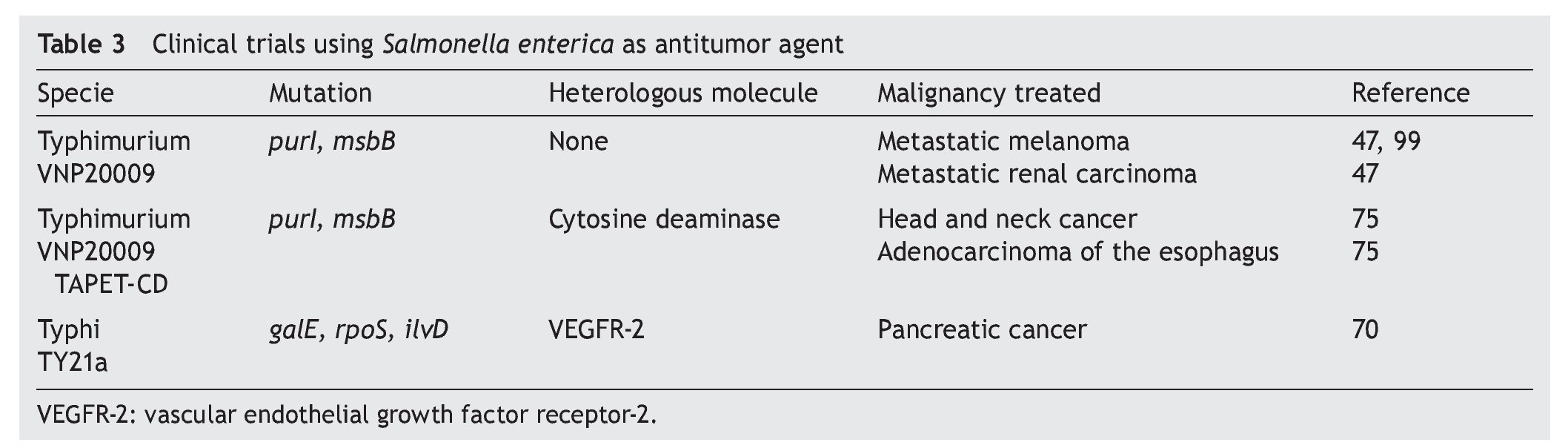
Various efforts have been made to obtain attenuated strains of S. enterica that reduce side effects in the host. For this purpose the VNP20009 strain of S. Typhimurium with mutations in genes msbB was developed (affecting the formation of lipid A, reducing the toxicity associated with lipopolysaccharide) and purI (making it dependent on an external source of adenine). This strain has been used in phase I clinical studies in 24 patients with meta-static melanoma and in a patient with metastatic renal cancer. The patients who received intravenous dosages of 3 x 108 CFU/m2 of the strain VNP20009 did not have severe adverse effects. However, colonization of the tumor tissue was moderate and its anti-tumor effect was not significant.47
4. Salmonella enterica as carrier of heterologous antitumor molecules
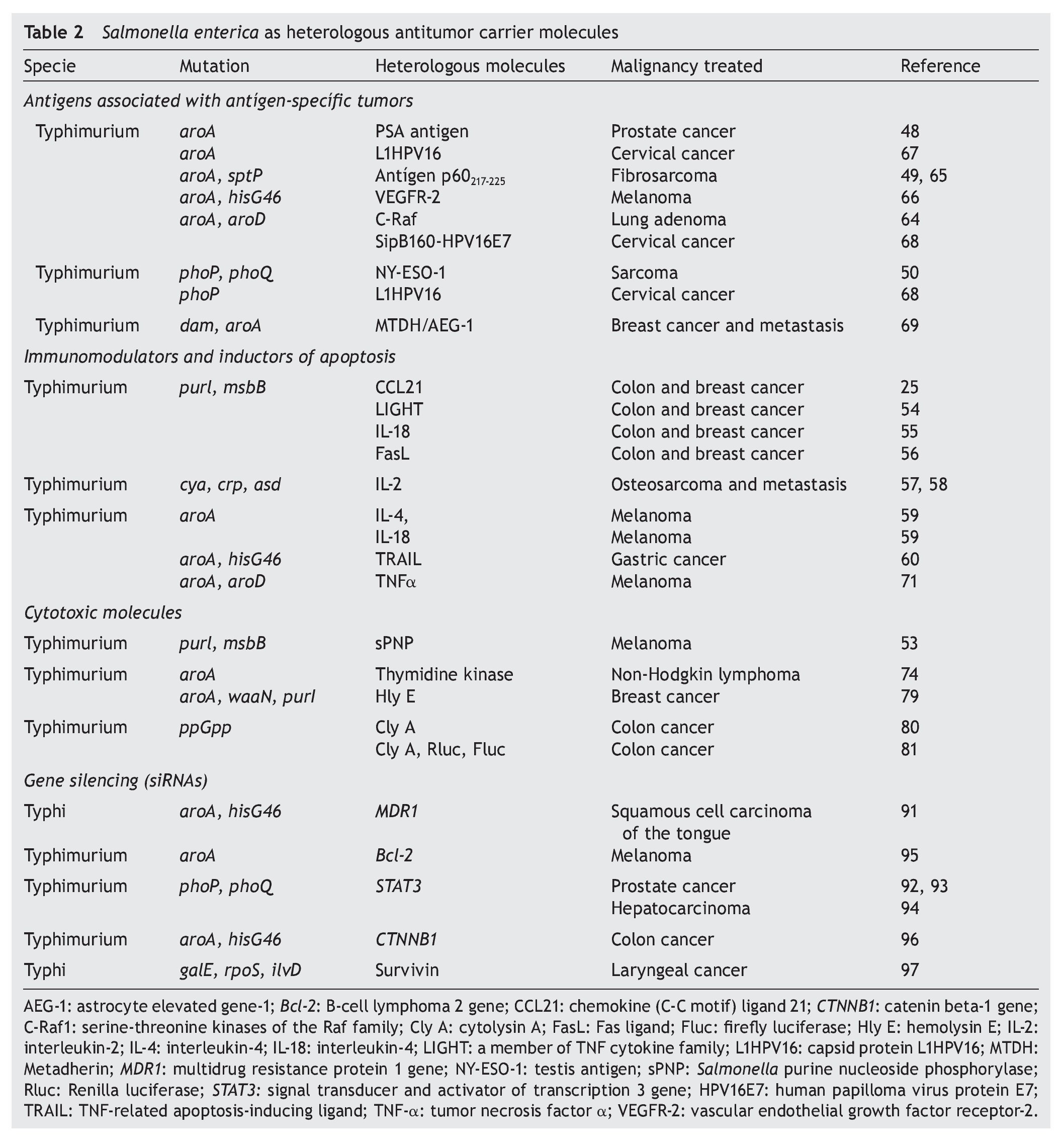
The modest anti-tumor activity induced by S. enterica in clinical studies47 makes clear the need for alternative mechanisms for potentiating and assuring reversion of the tumor. In the case of immunogenic tumors, S. enterica antigens have been expressed associated with tumors and tumor-specific antigens that are overexpressed in the tumor cell in order to induce or potentiate the specific immune response against the tumor.48-50 In the case of non-immunogenic tumors, in S. enterica there are proteins expressed that activate the cytotoxic molecules51-53 or immunomodulators that potentiate or induce the tumor cell to die by apoptosis.25,54-60
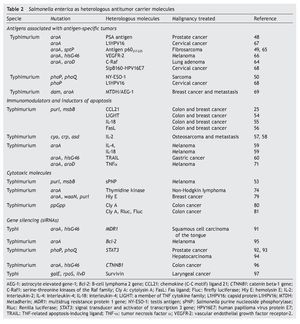
This strategy has also been useful in immunogenic tumors (Table 2).
5. Salmonella enterica and antigens associated with or specific to tumors
The presence and overexpression of proteins that promote the transformation and tumorigenesis in tumor cells, currently classified as “tumor associated antigens” (TAA) or “tumor specific antigens” (TSA),61 have provided guidelines for the use of S. enterica as a carrier of these antigens for prophylactic and therapeutic purposes.8
Once S. enterica arrives at the tumor microenvironment, the infected tumor cells have Salmonella antigens and are removed by T-cells specific against this bacteria.62
In this process an important event is the cross-presentation of the tumor antigens. This alternate pathway of antigen presentation is favored by overexpression of proteins such as connexin 43, which induces the formation of adherens junctions between cells and dendritic cells. This event allows the tumor cells to transfer pre-processed peptides towards the dendritic cell for proper submission by the major histocompatibility (MHC) complex class I63 and the consequent induction of a response of antigen-specific CD8 + T lymphocytes. Once in the tumor microenvironment, S. enterica induces the antitumor expression of inducible nitrous oxide synthase (iNOS) and interferon-g (IFN-g), inhibits the expression of arginase-1, interluekin-4 (IL-4), transforming growth factor-b (TGF-b), and vascular endothelial growth factor (VEGF), and reduces the suppressor capacity of the intratumoral myeloid cells, increasing the antitumor host response.29 Various reports have documented the importance of the NK cells (natural killer), neutrophils,28 macrophages,29 T lymphocytes30 and B lymphocytes31 in antitumor activity.
However, all of this immunological machinery induced by the presence of S. enterica in the tumor microenvironment was not sufficient to allow tumor regression in clinical studies.47 One alternative for potentiating the antitumor immune response is the expression or release of tumor antigens from the surface of S. enterica, taking advantage of its affinity for antigen-presenting cells.8 In this regard, release of TAA/TSA by S. enterica has been evaluated for vaccine purposes using the I and II secretion systems. The type I secretion system (TISS) was used by Fensterle et. al. for releasing the prostate-specific antigen (PSA) through the HlyA system. Their results showed that in mice immunized with S. Typhimurium who expressed the PSA antigen, an immune response mediated by CD8 T-lymphocytes was activated, which inhibited tumor development.48 Another antigen that has been coupled to this system is the C-Raf protein, a molecule that plays a central role in signal transduction and that when overexpressed or mutated induces carcinogenesis. Immunization of a mouse model of pulmonary adenoma with S. Typhimurium that over expresses CRaf-induced antibodies against C-Raf, T-cell response, and inhibited tumor growth.64
The type III secretion system (TTSS) was used by Panthel et al. using a murine model of fibrosarcoma that overex-pressed peptide 217-225 of protein p60 of Listeria monocytogenes, which simulates a tumor antigen.49In vivo studies demonstrated that 80% of the mice immunized with S. Typhimurium that expressed the p60 antigen through the TTSS were protected against the fibrosarcoma tumor cell challenge expressed by the p60217-225 peptide.. In mice that resisted the fibrosarcoma challenge, a response of antigen-specific CD8+ T-lymphocytes was observed, which inhibited tumor development.49,65 The tumor antigen NYESO-1 (germ cell protein that is found overexpressed in lung cancer, melanoma, esophagus, ovarian, bladder and prostate cancer) has also been released through the TTSS in TTSS in S. enterica. Oral administration of this attenuated strain expressed by the NY-ESO-1 protein induced tumor regression in a murine fibrosarcoma model previously established and the regression was mediated by antigen-specific CD8+ lymphocytes.50 Studies carried out in a murine melanoma model reported that orogastric immunization with S. Typhimurium that translocates the immunogenic epitope of the protein of the endothelial growth factor receptor 2 (VEGFR-2) murine through the TTSS induced a response of antigen-specific CD8+ T-lymphocytes and reduced metastasis in up to 60%.66 It was recently demonstrated that oral administration of S. Typhimurium that releases the fusion of the E7 protein of the human papilloma virus type 16 (HPV16) coupled to the protein SipB of the SSTT of S. enterica inhibited tumor growth in 45% and promoted a survival rate of up to 70% in a murine model of cervical cancer.67
S. enterica has also been used for carrying plasmids containing the sequence of tumor antigens. An example of this is the gene L1HPV16 that encodes for the capsid protein of HPV16, where immunization of mice with S. Typhimurium carrying this plasmid induced regression of the tumor and increased survival in cervical cancer.68 Similar studies were done with the gene that codifies for the protein MTDH/AEG1-1, an oncogene associated with angiogenesis that is found overexpressed in 40% of patients with breast cancer. Transport of this gene by S. Typhimurium induced tumor regression and increased survival in a murine model of breast cancer.69
The promising results of the prophylactic activity induced by the immunization with S. enterica, which transports sequences of antigens associated with tumors, have allowed for this system to be scaled to phase I clinical studies in patients with Stage IV pancreatic cancer. In this study, which is currently in progress, four doses of 106 CFU of S. Typhi Ty21a will be administered orally (a safe strain approved for human use as a vaccine against typhoid fever), which carries a plasmid containing the human sequence VEGFR-2, a protein that is overexpressed in the endothelial microenvironment of the tumor. The objective of this study is to induce antiangiogenic activity and to generate a memory response against endothelial cells to eliminate tumor vascularization.70
6. Salmonella enterica and immunomodulator and inductor proteins of apoptosis in cancer
The success of S. enterica as a carrier of tumor antigens for the activation of antitumor CD4+ and CD8+ T-lymphocytes is limited to those tumors that express tumor associated or specific antigens.16
To overcome this problem, S. enterica has been used as a vehicle to transport molecules that modulate the immune response in the host, facilitating the process of tumor elimination.
Loeffler et al. expressed diverse immunomodulating molecules such as cytokine LIGHT,54 interleukin IL-18,55 chemokine CCL21,25 in S. Typhimurium, and observed growth reversion in the primary tumors and their lung metastasis in murine models of breast and colon carcinoma. In these studies, proteins were coupled to a signal peptide to ensure the discharge of the molecule. Once in the tumor microenvironment proteins induced the chemoattraction of immune response cells like dendritic cells, macrophages, neutrophils, NK cells and lymphocytes. Sorenson et al. described that an oral dose of S. Typhimurium that expresses the human interleukin-2 prevented the formation of lung metastasis in a murine model of osteosarcoma. In this process, NK cells were probably responsible for tumor regression.57,58 Agorio et al. demonstrated that the single dose of the strain S. enterica with a plasmid that encodes for IL-4 or IL-18 was sufficient to delay tumor growth and prolong survival of mice with melanoma. In either case, the antitumor effect was accompanied by a systemic increase of gamma interferon (IFN- g).59
Other works have explored the induction of apoptosis of the tumor cell mediated by molecules that are expressed and secreted from S. enterica. Such is the case for the Fas ligand56 or of the TNF-a71 in murine models of colon carcinoma and melanoma, respectively. S. enterica has also been used for transporting the gene that encodes the ligand of death TRAIL (Tumor necrosis factor-Related Apoptosis-Inducing Ligand), causing a significant regression of the tumor in a murine model of gastric cancer.60
7. Salmonella enterica and antitumor cytotoxic molecules
One of the principal problems in antitumor therapy is the tumor resistance that cells have to chemotherapeutic agents. S. enterica offers a viable alternative to resolve this problem. This was documented by Hiroshima et al. in a murine model of xenotransplant of human pancreatic cancer resistant to chemotherapy where comparison of the antitumor activity of this bacteria with chemotherapeutic agents demonstrated that the attenuated strain of S. Typhimurium A1-R had greater antitumor activity than the chemotherapeutic agents 5-fluorouracil (5-FU), cisplatinum (CDDP) and gemcitabine (GEM). A synergistic antitumor effect was also observed when S. enterica was combined with 5-FU, suggesting that treatment with S. enterica induces chemosensitivity in chemotherapy-resistant cells.72 These results are consistent with the studies by Chang et al.73 in murine models of melanoma and breast cancer in which S. enterica sensitized the tumor cell to the action of the chemotherapeutic agent CDDP. An additive therapeutic effect was observed for retarding the tumor and prolonging survival of the mice with the tumors. The chemosensitivity induced by S. enterica was associated with overexpression of the protein connexin 43, a molecule that favors the adherent unions between the tumor cells allowing a better communication and homogeneous distribution of the chemotherapeutic agent.
Although the results of the synergistic effect mediated by the administration of S. enterica and the chemotherapeutic agent are encouraging, implementation of better strategies that allow for the selective destruction of the tumor tissue without damaging healthy tissue is still necessary. An alternative to this dilemma is the use of enzymes that once localized in the tumor microenvironment activate cytotoxic compounds (pro-drugs), which eliminate the tumor cell.52 This strategy has been explored using the attenuated strains of S. enterica as carriers. It has been described that for them to be effective at least two stages are required: the first is the administration of the attenuated bacteria that will carry the enzyme of interest to the tumor microenvironment and the second is the administration of the inactive cytotoxic compound (pro-drug), which will be strictly activated in the tumor microenvironment in the presence of the enzyme.52 Examples of this strategy are the works of Chen et al. where expression of the gene that encodes for phosphor-
ylase enzyme of the purine nucleoside (sPNP) in S. Typhimurium strain VNP20009 activated the non-toxic compound 6-methylpurine-2’-deoxyriboside (6MeP), a potent antitumor drug. 6MeP slows the growth of the tumor and increases infiltration of CD8+ T-lymphocytes in a mouse model of melanoma.53 Massa et al. documented the antitumor activity of S. Typhimurium SL3262 that expresses an antibody of only one domain against the CD20 antigen and, at the same time, expresses the thymidine kinase enzyme, which activates such drugs as gancyclovir. This recombinant bacteria increased the specificity of the tumor microenvironment in a xenographic model of human non-Hodgkin’s lymphoma due to the presence of the anti-CD20 antibody and induced an antitumor activity that increased survival in mice deficient in the specific immune response.74
In order to improve the anti-tumor activity mediated by S. Typhimurium VNP20009 in phase I clinical trials, Nemunaitis et al. expressed in this strain the gene for the enzyme deaminase cytosine from Escherichia coli. This enzyme is responsible for converting 5-fluorocytosine to 5-fluorouracyl, a cytotoxic metabolite that is used in treatments of gastric cancer, breast cancer, prostate cancer and head and neck cancer.75 With this new recombinant strain a pilot clinical trial was carried out and included patients with refractory cancer (one with squamous cell carcinoma of the head and neck and two with adenocarcinoma of the esophagus). The results demonstrated that the strain VNP20009 colonized the tumor tissue in two patients. Activity of the deaminase cytosine was observed when the concentration of 5-fluorouracil was measured in the tumor tissue.75 This work confirms the capacity of S. enterica to colonize tumor tissue in humans and its utility as carrier of molecules that activate cytotoxic compounds with antitumor activity.
The search for better antitumor therapeutic alternatives has allowed for the evaluation of a new generation of molecules, among which are found bacterial proteins with cytotoxic activity. Examples are the diphtheria toxin of Corynebacterium diphtheria, the exotoxin A of Pseudomonas aeruginosa, the a-hemolysin of Staphylococcus aureus, the parasporine-4 of Bacillus thuringiensis, the listeriolysin O of Listeria monocytogenes, the aerolysin of Aeromonas hydrophila, the cytolysin A of E. coli, among others.76-78 The selective transport of these cytotoxic proteins towards the tumor cells without damaging the normal cells has been resolved by coupling them with specific antibodies against tumor antigens generating the compounds called “immunotoxins” or by coupling them with some molecule that has an overexpressed ligand in the tumor cell. Such is the case of certain growth factors or cytosines. These proteins are called “toxin specific.”78
However, the bigger challenge is to transport these compounds to hypoxic areas and areas with poor tumor vascularity. To overcome this obstacle, some cytotoxic proteins have been expressed in attenuated strains of S. enterica and in this way are transported to the tumor microenvironment in an effective and selective manner. Studies by Ryan et al. using a murine model of breast cancer demonstrate that the intravenous administration of an S. Typhimurium that expresses hemolysin E (Hly E), a protein of E. coli that forms pores in the cellular membrane under a promoter that is induced in anaerobiosis, allowed for the colonization of the tumor, induced necrosis and decreased the tumor mass,79
Another protein of E. coli that has been evaluated for its antitumor activity is cytolysin A (ClyA), which also forms pores in the membrane and recently has been expressed in S. Typhimurium under an inducible system of tetracycline to avoid damage to normal cells. Administration of this recombinant Salmonella as an antitumor therapy in a murine model of colon carcinoma allow for the tumor to regress, decreased metastasis to the lung and promoted survival of the mouse.77,80,81 The encouraging results in the preclinical models using cytotoxic bacterial proteins expressed in S. enterica constitute a promising alternative that should be explored in detail.
8. Salmonella enterica and the antitumor genetic silencing
S. enterica has a great capacity of transporting and transferring plasmid DNA to the interior of the eukaryote cells,82-84 inducing antitumor activity in murine models of melanoma, bladder cancer and adenocarcinoma of the lung.85-88 Based on these observations, attenuated S. enterica has become the ideal candidate for transport and release in tumor microenvironment of the small interference RNA (siRNA) for silencing of genes implicated in cancer.89,90 An example of this is the silencing of proteins implicated in the resistance to chemotherapy such as gp-170 encoded for the MDR (multidrug resistance) carried out in a murine model of squamous cell carcinoma of the tongue.91 The expression of the transcription factor STAT-3 has also been silenced, a molecule associated with the survival of tumor cells in murine models of prostate cancer92,93 and hepatocellular carcinoma.94 Genetic silencing of proteins has also been documented with antiapoptotics such as Bcl-2 in a murine model of melanoma.95 Oncogenes such as the gene CTNNB1 that encodes forβ-catenin have also been silenced in murine models of colon cancer.96 Recent studies in a murine model of laryngeal cancer have described the silencing of the gene that encodes for survivin, a protein implicated in the suppression of apoptosis.97 In all the cases described, release of the siRNA mediated through S. enterica in different mouse models of cancer induced tumor regression.
9. Perspectives
More than a century and a half has passed from the first time that the antitumor activity of bacteria was described.2 Although advances in this field have been slow, one of the most important achievements to date is the use of the attenuated strain of Mycobacterium bovis, BCG, as an effective immunotherapy for superficial transitional bladder cell carcinoma in humans.13
The studies described in this review demonstrated the potential use of attenuated strains of S. enterica serovar Typhi and Typhimurium as a promising alternative for resolving inherent problems in the therapy with chemotherapeutic agents because these live attenuated vectors have a great selectivity for tumor tissue and metastasis and sensitize the tumor cells to the chemotherapy.6,8,98 These properties are potentiated for the cytolytic activity that S. enterica has per se according to the capacity of inducing an innate and adaptive response in the tumor microenvironment and to the indisputable ability to transport heterologous molecules to the tumor microenvironment such as associated or tumor-specific antigens, immunomodulator molecules and inductors of apoptosis, activating proteins of cytotoxic molecules and transport of siRNA. These silence the genes implicated in the tumorigenesis and resistance to apoptosis8 (Table 2).
Although phase I clinical trials have been documented using S. enterica for eliminating tumors (Table 3), greater efforts focused on the following are still necessary.
Develop safe attenuated strains. It is important to mention that the results of phase I clinical studies with S. Typhimurium strain VNP20009 showed that the bacteria did not induce severe adverse effects and was well tolerated by patients with metastatic melanoma, metastatic renal carcinoma, carcinoma of the head and neck and adenocarcinoma of the esophagus.47,75,99 Currently a phase I clinical trial on patients with pancreatic cancer using S. Typhi strain Ty21a is underway. This strain is safe and is approved for human vaccine use.70 Other options that should be evaluated in clinical phase antitumor therapy are strains of S. Typhi CVD908, CVD908-htrA and Ty800, which have also been demonstrated to be safe in vaccine clinical trials, even in children.27,100
Develop better mechanisms of antitumor molecular transport to the interior of the cell or to the cellular micro-environment. An interesting strategy to evaluate is the use of the systems of bacterial secretion such as the V type or of self-transporters to release antitumor molecules coupled to fusogenic peptides that destabilize the membranes. This strategy would allow the antitumor molecule to reach its target within the cell, whether or not S. enterica is found within or outside the tumor cell.8,101
Improve the selectivity of the bacteria for the cell or tumor tissue. One strategy that has been evaluated by Massa et al. is the expression of an antibody of only one domain against the CD20 antigen on the surface of S. Typhimurium SL3262 to increase the specificity of the bacteria for the tumor microenvironment in a xenographic model of non-Hodgkin’s lymphoma.74 However, there are still diverse alternatives that could help to increase this selectivity. One interesting proposal is that synthetic adhesins fused to variable domains of the heavy chain of the antibodies commonly called nanoantibodies which, when expressed by Escherichia coli, have been shown to be efficient for colonizing tumors that express some recognized antigen by the synthetic adhesion fused to the nanoantibody.102
Establish clinical studies where the potential use of S. enterica in combination with chemotherapeutic agents is evaluated.52
Finally, consistent with pre-clinical and clinical studies in this review, attenuated strains of S. enterica can be considered excellent allies in the fight against cancer.
Conflict of interest
The authors declare no conflicts of interest.
Funding
The present study received funding from Fondos Federales HIM-2013-028(GAA) and HIM-2014-032 (RLP). HCN, AVE and DCBP were recipients of a scholarship from PROBEI. GAA was recipient of a scholarship of CONACYT.
Received for publication: 10-15-14;
Accepted for publication: 2-3-15
http://dx.doi.org/10.1016/j.bmhimx.2015.01.010
Correspondence: Dr. Rosendo Luria-Pérez
E-mail:rluria@himfg.edu.mx; rluria77@gmail.com