Background and aims. Concerns exist about outcomes of liver transplantation (LT) from low volume centres, especially for hepatitis C (HCV) patients. The aim of the study was to assess patient outcomes as well as their predictors post LT for HCV in a small volume Australian unit (< 25 LTs/year), comparing these with the average outcomes obtained from national and international transplant registries. Patients transplanted for HCV at the South Australian Liver Transplant Unit between 1992 and 2012 were studied. Outcomes assessed were patient and graft survival at 1,3, and 5 years. Factors independently associated with the outcomes were assessed using Cox regression model.
Results. 1, 3, and 5-year patient survival for HCV patients was 95.2, 82.9, and 78.2%, graft survival were 93.7, 80.1, and 75.5% respectively. The total follow-up time observed was 299.9 years amongst 61 patients in which there were 16 deaths. The expected number of deaths was 40.4 and the standardized mortality ratio 0.40 (95% CI = 0.24, 0.65). These results compared favourably to those obtained from the SRTR registry. Variables independently associated with lower patient survival: donor age (HR = 1.06, 95% CI 1.02 - 1.11; P = 0.003), and post LT cytomegalovirus (CMV) disease requiring treatment (HR = 4.03, 95% CI 1.48 - 10.92;P = 0.06).
Conclusion. In conclusion, high rates of patient and graft survival for HCV liver transplantation can be obtained in a small volume unit. Young donor age and lack of CMV disease post-transplant were associated with better outcomes. Institutional factors may be influential determinants of outcomes.
Chronic hepatitis C (HCV) related liver cirrhosis is the most common indication for liver transplantation (LT) in developed countries including Australia, where 21% of LT recipients have HCV as their primary diagnosis according to the Australia and New Zealand Liver Transplant Registry (ANZLTR).1
Although HCV is the most common indication for LT, outcomes for HCV patients post LT are inferior relative to other indications for LT.2–4 The poorer outcome of HCV transplantation has been attributed to HCV recurrence post LT and accelerated progression of hepatic fibrosis.5 Another concern with HCV transplantation has been the absence of improved survival over time, despite advances in many facets of post LT care and improved outcomes for most other indications for LT. A potential influence on HCV post LT outcomes may be transplantation by low volume centres. Concerns about performing technically complex procedures such as LT, in low volume LT centres have been raised by multiple investigators.6–8 However, few studies have examined the influence of volume on outcomes for the specific diagnosis of LT for HCV.
AimsAs HCV is the most common indication for LT, concerns about inferior outcomes at low volume transplant centres and the relatively low volume nature of most Australian LT centres, the primary aim of this study was to examine patient and graft survival post LT in HCV patients in a small volume unit. A further aim was to compare HCV patient LT outcomes from this unit with a non-HCV cohort within the same unit and to also compare the HCV LT outcomes with average LT outcomes obtained using large database registries including the ANZLTR, the European Liver Transplant Registry (ELTR) and the Scientific Registry of Transplant Recipients (SRTR) over comparable time periods. Secondary aims were to identify factors associated with HCV patient and graft survival following LT.
Material and MethodsWe performed a retrospective cohort study using electronic and paper medical records of LT patients admitted to the South Australia Liver Transplant Unit (SALTU). The SALTU was established in 1992 and provides liver transplantation services to the state of South Australia and the Northern Territory (1.9 million population). It is one of the smallest volume centres amongst the five transplant centres in Australia. Patients studied were all who received a LT between 1st January 1992 (commencement of program) and 1st October 2012. A total of 252 transplants on 243 patients were performed during this period for all indications. All patients were followed until death or 1st October 2012. The HCV cohort was defined as patients who remained HCV-PCR positive at LT. Most patients received a standard triple immunosuppression protocol including tacrolimus, azathioprine and short term (3 months) prednisolone.
Donor and recipient selection processes were according to guidelines of the Transplant Society of Australia and New Zealand (https://www.tsanz.com.au/organallocation protocols/documents/CSVs1.4_V4_Final.pdf). In summary all organ allocation was from deceased donors and the principal indication for LT was a MELD score > 15. Deceased donation occurred via an “opt-in” consent system, however, organ donation rates within our region are relatively high (mean donations per million population = 19.8 for years 2009-2013) compared with the Australian national average (mean donations per million population = 14.6 for years 2009-2013) and reports from other countries.9
Monitoring of HCV recurrence post LT following the early post LT period was with 3-monthly liver function tests, clinical review and 12-monthly liver biopsy. Clinically significant HCV recurrence was defined by evidence of histologically injury in combination with abnormal liver biochemical tests and elevated HCV viral load. Histological features supportive of recurrence included, prominent interface injury and necroinflammatory activity in the lobules. Fibrosis was scored using Scheuerstage and inflammatory activity using the hepatitis activity index (HAI). The usual indication for post LT HCV therapy was a histological Scheuer fibrosis stage of ≥ 2. Differentiation of HCV recurrence from changes of acute cellular rejection was done by an experienced liver histopathologist with rejection changes scored using Banff criteria. A predominance of HAI score over Banff score (ie HAI 7, Banff score 3) was used to favour HCV recurrence over acute cellular rejection, in conjunction with overall morphological and clinical features.
Steatosis was assessed both visually and histologically for all grafts. Grossly fatty donor livers were rejected prior to donor hepatectomy. Donor grafts considered mildly steatotic underwent biopsy and frozen section prior to hepatectomy and grafts with ≥ 30% steatosis were not transplanted. All grafts transplanted underwent a time zero biopsy which was used to classify HCV grafts in the study as either non steatotic or steatotic. All grafts classified as steatotic in the study had only mild histologically steatosis (<15% of hepatocytes affected).
Statistical analysisAll analyses were performed using Stata version 12.0 (StataCorp, Texas, USA). Univariate and multivariate analysis of survival and graft outcomes were assessed using Cox regression. Factors assessed in the univariate analysis for association with patient and graft survival were those listed in table 1. These factors were selected on the basis of prior literature identification and/or biological plausibility. All factors except for age, Child-Pugh and MELD scorewere included in the models as categorical variables and a global test of significance were performed for factors with more than 2 categories. Factors considered for inclusion in forward stepwise multivariate analysis were those that were significant at p<0.2 in univariate analysis. We also plotted crude survival rates using Kaplan-Meier curves and presented the associated log-rank statistics. Survival rates of HCV patients from our own unit were compared with HCV patients from the SRTR using direct standardization. Each patient’s follow-up time was split by calendar year and the total follow-up time in each year was multiplied by the known calendar year specific survival rates from SRTR or the same calendar period (1995 to 2012) to provide the expected number of deaths. The standardized mortality ratio was calculated as the observed/expected number of deaths with 95% confidence intervals obtained using the Breslow and Day approximation. All hypotheses were performed as two-tailed tests with a type 1 error rate of p < 0.05 considered significant.
Factors assessed for potential association with patient survival post LT in hepatitis C.
| Factors (variables) |
|---|
| Donor |
| Gender |
| Age |
| ABO compatibility |
| Graft steatosis |
| Recipient |
| Gender |
| Age |
| MELD score at LT |
| Child Pugh score at LT |
| Cofactors (alcohol, HCC) |
| Comorbidities (diabetes, hypertension, dyslipidaemia, BMI) |
| Viral |
| Genotype 1 |
| Prior interferon therapy |
| Perioperative |
| Cold ischaemic time |
| Era of LT |
| Graft type (whole vs. other) |
| Postoperative |
| Choice of CNI |
| CNI level at 6 months |
| Prednisolone use for more than 6 months |
| Steroid bolus for treatment of biopsy proven acute cellular rejection |
| Azathioprine or Mycophenolate use at 12 months post LT |
| Treated CMV disease |
| New onset diabetes post LT |
MELD: Model for End-Stage Liver Disease. HCC: hepatocellular carcinoma. BMI: body mass index. CNI: calcineurin inhibitor. CMV: cytomegalovirus. ALT: alanine transaminase.
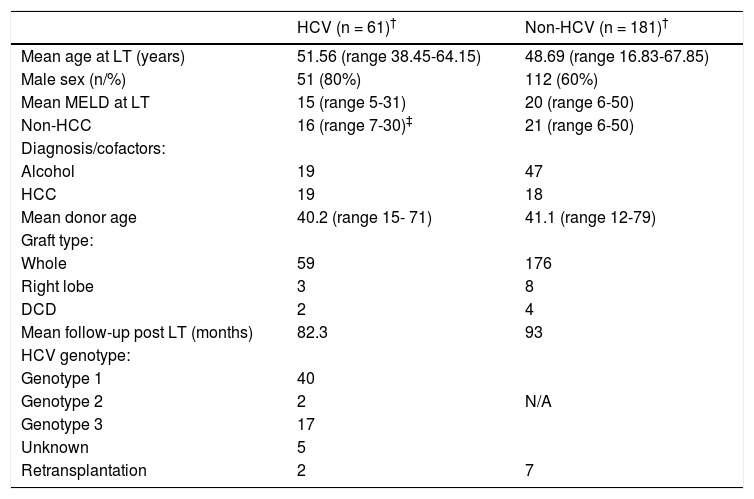
A total of 61 patients were HCV-PCR positive at LT, representing 24% (61 of 252) of all patients transplanted during the study period. Patient clinical characteristics comparing HCV and non-HCV cohort are shown in table 2. The volume of LTs increased steadily over the period of study (Figure 1) from 6 per year in 1992 - 1996 to 22 per year in 2007 - 2011.
Patient clinical characteristics comparing HCV and non-HCV cohort.
| HCV (n = 61)† | Non-HCV (n = 181)† | |
|---|---|---|
| Mean age at LT (years) | 51.56 (range 38.45-64.15) | 48.69 (range 16.83-67.85) |
| Male sex (n/%) | 51 (80%) | 112 (60%) |
| Mean MELD at LT | 15 (range 5-31) | 20 (range 6-50) |
| Non-HCC | 16 (range 7-30)‡ | 21 (range 6-50) |
| Diagnosis/cofactors: | ||
| Alcohol | 19 | 47 |
| HCC | 19 | 18 |
| Mean donor age | 40.2 (range 15- 71) | 41.1 (range 12-79) |
| Graft type: | ||
| Whole | 59 | 176 |
| Right lobe | 3 | 8 |
| DCD | 2 | 4 |
| Mean follow-up post LT (months) | 82.3 | 93 |
| HCV genotype: | ||
| Genotype 1 | 40 | |
| Genotype 2 | 2 | N/A |
| Genotype 3 | 17 | |
| Unknown | 5 | |
| Retransplantation | 2 | 7 |
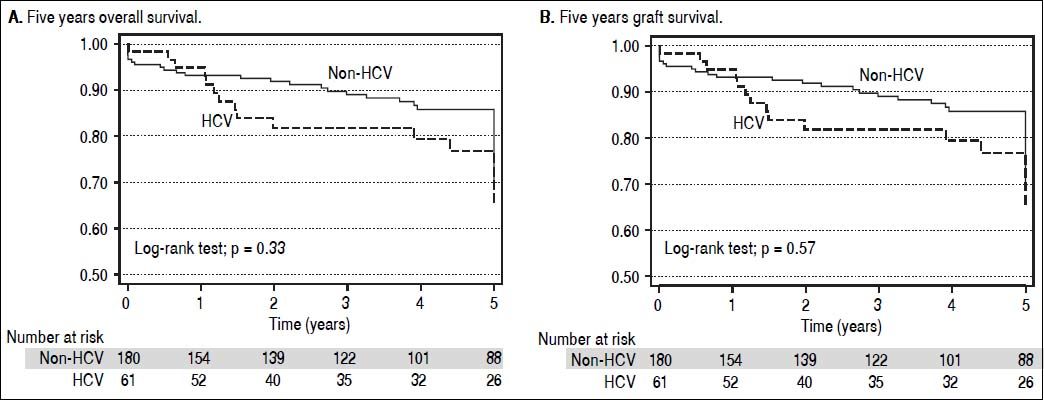
Patient survival during the first 5 years post LT for HCV and non-HCV patients is shown in figure 2A. Patient survival at 1, 3, and 5 years in the HCV cohort was 95.2, 82.9, and 78.2% respectively. Patient survival for the non-HCV cohort at 1, 3, and 5 years was 93.0, 88.8, and 85.4% respectively. There was no significant difference between the cohorts (p = 0.23).
Graft survival at 1, 3, and 5 years in the HCV cohort was 93.7, 80.1, and 75.5% respectively. Graft survival for the non-HCV cohort at 1, 3, and 5 years was 89.6, 84.3, and 81.1% respectively. There was no significant difference between the survival curves (p = 0.35) (Figure 2B).
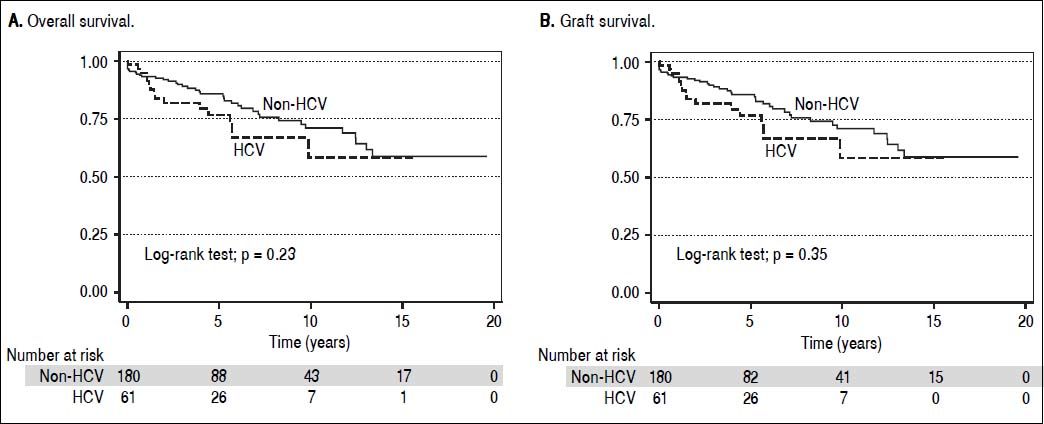
Overall patient and graft survival for both HCV and non HCV cohorts are shown in figure 3.
There were 16 deaths in the HCV cohort and causes were as follows; recurrence of HCV infection with subsequent end stage liver failure (13), primary non-graft function (1), recurrence of hepatocellular carcinoma (1), sepsis (1). There were 38 deaths in the non-HCV cohort and causes were as follows; sepsis (9), de novo malignancy (8), HCC recurrence (3), cardiovascular disease (3), intraoperative death (3), multi-organ failure (2), disease recurrence for primary biliary cirrhosis and alcoholic liver disease (2), miscellaneous (3) and unknown (5).
HCC was an indication for LT in 19 (31%) of HCV patients and in 18 (10%) of the non-HCV cohort. HCC recurrence was seen in 2 (11%) of the HCV patients and 5 (28 %) of the non-HCV patients. The overall HCC recurrence rate was 19%.
Three patients experienced primary graft non-function, one HCV patient and two in the non-HCV cohort. Two of these patients underwent re-transplantation (1 HCV, 1 non-HCV) with only the non-HCV patient surviving the peri-operative period.
Causes of graft loss in the 2 patients in the HCV cohort, not accompanied by patient death due to re-transplantation, were recurrence of HCV infection in one patient and hepatic artery thrombosis in the other patient. In addition to the two re-transplants in the HCV cohort, there were 7 re-transplants performed in the non-HCV cohort for the following indications; hepatic artery thrombosis (2), chronic rejection (2), hepatic artery aneurysm (1), primary graft non-function (1), and graft sepsis in primary sclerosing cholangitis (1).
HCV recurrence and antiviral treatment following LTClinically significant HCV recurrence occurred in 25 (41%) of patients. In three (5%) of these patients the recurrence pattern was consistent with focal cholestatic hepatitis (FCH), with early (< 12 months), severe biochemical and histological cholestasis associated with high viral load. The remaining patients had a recurrence pattern more consistent with chronic HCV related progressive histological damage (Scheuer fibrosis score ≥ 2, elevated transaminases).
Twenty-five patients were treated with pegylated-interferon and ribavirin based therapy post LT. The majority (60%) of patients treated had HCV genotype 1 and the mean time to commencement of antiviral therapy post LT was 30.5 months. The overall sustained virological response (SVR) rate at 24 weeks, assessed by intention to treat analysis, was 52% (13/25).
A significant side effect profile was associated with treatment of this cohort. Most (17/25 patients, 68%) patients developed adverse events that included; cytopaenias requiring supportive therapy (absolute neutrophil count 500-750/mm3 or platelet count < 25,000mm3 or haemoglobin < 10 g/dL), neuropsychiatric symptoms and decompensated liver failure. Nine patients had treatment ceased prematurely due to significant side effects.
Comparison of post LT HCV outcomes between SALTU and large transplant registriesAlthough difficult to statistically compare our figures with available large databases, patient survival at both 1 and 5 years for SALTU patients (95.2 and 78.2%) appears noninferior to survival rates from ANZLTR (89 and 78%), ELTR (81 and 64%) and SRTR (81 and 75%) databases for an equivalent era.
Direct, time-standardized comparison was possible between our HCV cohort and the SRTR HCV cohort. During the follow-up period the first HCV patient was operated on in 1995 and the last in 2012. The total follow-up time observed was 299.9 years amongst 61 patients in which there were 16 deaths. The expected number of deaths was 40.4 and the standardized mortality ratio 0.40 (95% CI = 0.24, 0.65). This analysis demonstrated a significantly, lower mortality rate for SALTU HCV patients undergoing LT relative to the SRTR HCV cohort.
Factors predicting patient and graft survival in HCV patients post LTTable 1 shows 22 clinically relevant variables that were assessed for association with patient death and graft loss for the HCV cohort.
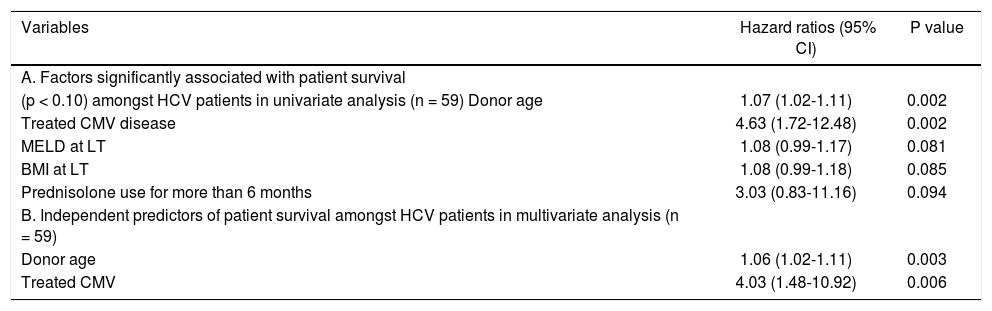
Table 3 shows variables that were significantly associated with patient death in univariate (Table 3A) and multivariate analysis (Table 3B) respectively. Donor age (HR = 1.06, 95% CI = 1.02-1.11, p = 0.003) was independently associated with patient death, with each 1 year increase in donor age associated with a 6% increased risk of patient death. Post LT CMV disease that required treatment (HR = 4.03, 95% CI 1.48-10.92; P = 0.06) was also independently associated with patient death, with an approximately four fold increased risk of death for patients treated for CMV disease.
Factors significantly associated with patient survival.
| Variables | Hazard ratios (95% CI) | P value |
|---|---|---|
| A. Factors significantly associated with patient survival | ||
| (p < 0.10) amongst HCV patients in univariate analysis (n = 59) Donor age | 1.07 (1.02-1.11) | 0.002 |
| Treated CMV disease | 4.63 (1.72-12.48) | 0.002 |
| MELD at LT | 1.08 (0.99-1.17) | 0.081 |
| BMI at LT | 1.08 (0.99-1.18) | 0.085 |
| Prednisolone use for more than 6 months | 3.03 (0.83-11.16) | 0.094 |
| B. Independent predictors of patient survival amongst HCV patients in multivariate analysis (n = 59) | ||
| Donor age | 1.06 (1.02-1.11) | 0.003 |
| Treated CMV | 4.03 (1.48-10.92) | 0.006 |
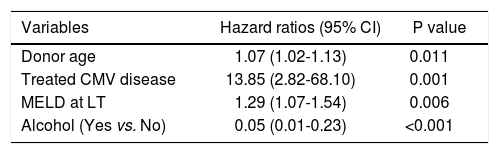
Findings were similar for the analysis of variables associated with graft loss, shown in table 4, with donor age (HR=1.06, 95% CI = 1.02-1.11, p = 0.009), and treated CMV disease (HR = 4.9, 95% CI = 1.8-13.2, p = 0.002), both independently associated with graft loss. In addition the absence of alcohol as a cofactor for liver injury prior to LT (HR = 0.02, 95% CI = 0.04-0.87, p≤0.03) was a furthervariable independently associated with graft loss.
Factors independently associated with graft survival in multivariate analysis (n = 52).*
| Variables | Hazard ratios (95% CI) | P value |
|---|---|---|
| Donor age | 1.07 (1.02-1.13) | 0.011 |
| Treated CMV disease | 13.85 (2.82-68.10) | 0.001 |
| MELD at LT | 1.29 (1.07-1.54) | 0.006 |
| Alcohol (Yes vs. No) | 0.05 (0.01-0.23) | <0.001 |
† Also adjusted for gender a priori.
A total of 8 (13%) HCV patients experienced single episodes of biopsy proven acute cellular rejection of sufficient histological and biochemical severity to require high dose steroid boluses. Although steroid bolus has been associated with adverse outcomes in multiple prior studies, there was no association with either poor patient or graft survival in this study.
DiscussionThe main purpose of this paper was to examine outcomes for HCV transplantation in a low volume unit given the importance of this condition and concerns about poor outcomes associated with LT for this indication and for small volume units.3,7
The important finding from this paper was that outcomes in HCV patients in our low volume unit compared favourably to those from the large SRTR database, using a standardized mortality comparison. Indeed our HCV cohort had a significant, 60% lower standardized mortality rate relative to the SRTR HCV cohort.
Some caution is required in drawing direct comparisons between outcomes in our centre with the SRTR database. Although this analysis was standardized by year, it was not standardized by other factors such as MELD score and donor age and we cannot exclude that these factors, rather than structural and process factors discussed below, accounted for the better survival of our cohort. Although beyond the scope of our study, further studies adjusting for the effects of MELD score, donor age, and other relevant factors would be highly desirable when comparing outcomes between small and large units.
The better than expected outcomes within this small volume unit are likely to have a number of explanations. Good donor and recipient selection with young donor age (mean of 40.2 years) and low recipient MELD score at the time of LT (mean of 15.1) were likely to be important contributors to good outcomes, and the importance of these factors on severity of HCV recurrence and patient survival has been well described.10 It should be noted that low MELD scores seen in this population are in part related to patients with HCV who were transplanted predominantly for HCC. The mean MELD score of non-HCC patients transplanted for HCC was above 15, the recognized break point favouring transplantation.The deleterious effects of CMV infection on patient and graft survival has also been well described, and it remains unclear if these effects are mediated via CMV induced enhanced immunosuppression or enhanced HCV replication.11
The low volume of LTs performed at our centre did not appear to adversely influence patient outcomes. Effects of centre volume on patient outcomes following LT and non-transplant procedures are controversial. Although some studies have suggested an association between low volume and poorer patient and graft outcomes,6–8 two recent large scale cohort studies have challenged these findings.12,13 Reese, et al.were unable to find a significant difference in 1-year graft survival following liver retransplantation between low (< 50 LT per year), intermediate (50-88 LT per year) and high volume (>88 LT per year) units in a study assessing the recorded outcomes of over 4,000 patients within the Organ Procurement Transplantation Network registry.12 Similarly, Kilic, et al. found that centre volume was a relatively minor contributor to 1 and 5-year mortality in orthotopic heart transplantation, in a study involving approximately 20,000 patients from the United Network for Organ Sharing registry.13
In view of these studies and our own data, it is plausible that there are important institutional factors beyond patient selection and centre volume that impact on patient outcomes. Indeed, Donabedian principles, which are widely recognized as a valid methodology for assessing quality in health care, require an assessment of institutional structure and processes, in addition to outcomes.14
Structural factors that authors believe to have contributed to good outcomes include; rapid access to on-site intensive care, interventional radiology, liver biopsy and drug assay services. Continuous clinician education is considered another important structural factor for our institution with clinicians from all groups (including hepatology, surgery, anaesthetic, intensive care, and transplant co-ordination) receiving periods of external training in large volume centres. A final important structural factor within our institution is a strong commitment to quality and safety principles. Examples of this include; continuous audits, external reviews, availability of evidence based transplant protocols to standardize care and weekly multidisciplinary meetings.
A process factor that authors consider to be strength of our institution is the ability to rescue sick patients following LT from complications, this being increasingly recognized as an important measure of institutional quality and an explanation for higher mortality following complex surgeries in low volume centers.15 The two main process factors contributing to this ability are high clinician staffing ratios across all areas of patient care and a high number of Intensive Care beds and staff providing rapid 24-h Intensive Care access for deteriorating LT patients. Of relevance are high staffing levels of registered nurses who are at the front line of patient care and can provide high levels of vigilance for deteriorating patients. Indeed, the association of registered nurses staffing levels with lower patient mortality, adverse events and improved care has been noted previously by several investigators.16,17
The second factor that authors consider critical to the ability of our institution to rescue sick patients is the availability of a rapid access-to-care pathway for deteriorating patients in the community. This pathway is available to all LT patients via transplant coordinators, who provide a 24-h 7-day per week telephone contact service for urgent patient problems. The coordinators, who develop strong therapeutic relationships with patients, can help efficiently navigate complex medical structures for sick patients by contact with on call clinicians and prompt organization of inpatient beds under the care of specialized clinicians.
A limitation of this study is that it represents a small single centre report and cannot provide definitive conclusions about outcomes for HCV transplantation and their relationship to centre volume. In addition while the results compare favourably with those from large transplant registries, a direct comparison that standardizes for all relevant factors beyond era was not possible. However, the report confirms that excellent outcomes for complex surgical procedures can be achieved in small volume centres and reinforces findings from other larger studies that volume is not always a powerful determinant of outcome.12,13
ConclusionWe present outcome data for patients with HCV following LT in a small volume unit. We demonstrated that better than expected and above average outcomes are feasible in this setting. While acknowledging the influence of patient selection in achieving these outcomes (young donor age and low recipient MELD score), we also speculate about other important institution specific contributors to successful outcomes for HCV patients. Such institutional structural and process factors are also likely to help overcome the potential negative effects on outcomes of low volume units. Quantifying these institutional factors and studying their effects in larger, multicenter studies would contribute further to our understanding of outcomes following LT and is an important area for further research given the common situation of low volume units performing complex medical procedures in the Australian Healthcare system.
Abbreviations- •
CMV: cytomegalovirus.
- •
HCC: hepatocellular cancer.
- •
HCV: chronic hepatitis C.
- •
LT: liver transplantation.
- •
MELD: Model of End Stage Liver disease.
- •
SALTU: South Australia Liver Transplant Unit.
Authors acknowledge all staff involved with the care of transplant patients at Flinders Medical centre. Authors specifically acknowledge the contributions of Shabnah-Ratnarajah with collection of data and Sonja Jamieson and Monica Beattie for secretarial support required with preparation of the manuscript.