In recent years, thanks to advances in molecular biology, allergological diagnosis has improved and specific IgE (sIgE) against an allergenic source has been transformed into sIgE against an allergenic protein or glycoprotein. This change, which has resulted in a more precise diagnosis of sensitisation, could explain the different dangers of certain molecular sensitisations and in many cases cross-reactivity phenomena, and could change indications for immunotherapy or clinical management. Here, we present two cases of children where the indication for immunotherapy and management of the disorder changed due to component-resolved diagnosis. However, the clinical history and skin prick tests should complement molecular in vitro diagnosis to improve routine clinical practice.
Recent years have witnessed great advances in the understanding of allergens and in allergological diagnosis, especially in the field of in vitro diagnosis. This advance, together with the development of molecular biology and nanotechnology have resulted in the appearance of new technologies which make the study of allergic patients easier and have lead to the so-called component-resolved diagnosis. To date the in vitro diagnosis of sensitisations has been carried out using the determination of specific IgE against the allergenic sources. The systems currently available for the determination of specific IgE consist of the exposure of the patient's serum to a system (a paper disc or aliquot) onto which the whole allergens such as extracts of dog epithelium; mites such as Dermatophagoides pteronyssinus; or cow's milk, are fixed. Recently, protein microarrays have become commercially available which allow specific IgE to be detected simultaneously against multiple allergens using a reduced supporting medium.
Initially tests were performed with a limited number of allergens and using the extracts from the whole allergenic sources which allowed various sensitisations to be identified simultaneously.1–3 However, currently knowledge of allergens has increased and the term ‘component’ has arisen as the real trigger of the allergic reaction and as one element of the allergenic source which is now seen as a mixture of several allergenic components. A particular allergenic source, such as grass pollen, in reality contains several allergenic proteins or allergenic components. Indeed, in the pollen of the graminea Phleum pratense, 10 allergens (Phl p 1, Phl p 2, Phl p 5...) exist in isolation or produced in a recombinant fashion. Currently, thanks to advances in molecular biology these protein allergenic components are available and they can be obtained in two ways: either by isolation from the rest of the proteins in the allergenic source (purified natural allergens) or by producing them in vitro from a DNA sequence (recombinant allergens). Thus, a few years after the design of protein microarrays which were performed based on extracts from the whole allergenic source (where the pollen extract itself was incorporated into the microarray) the first protein microarrays using recombinant and purified allergenic components appeared.4
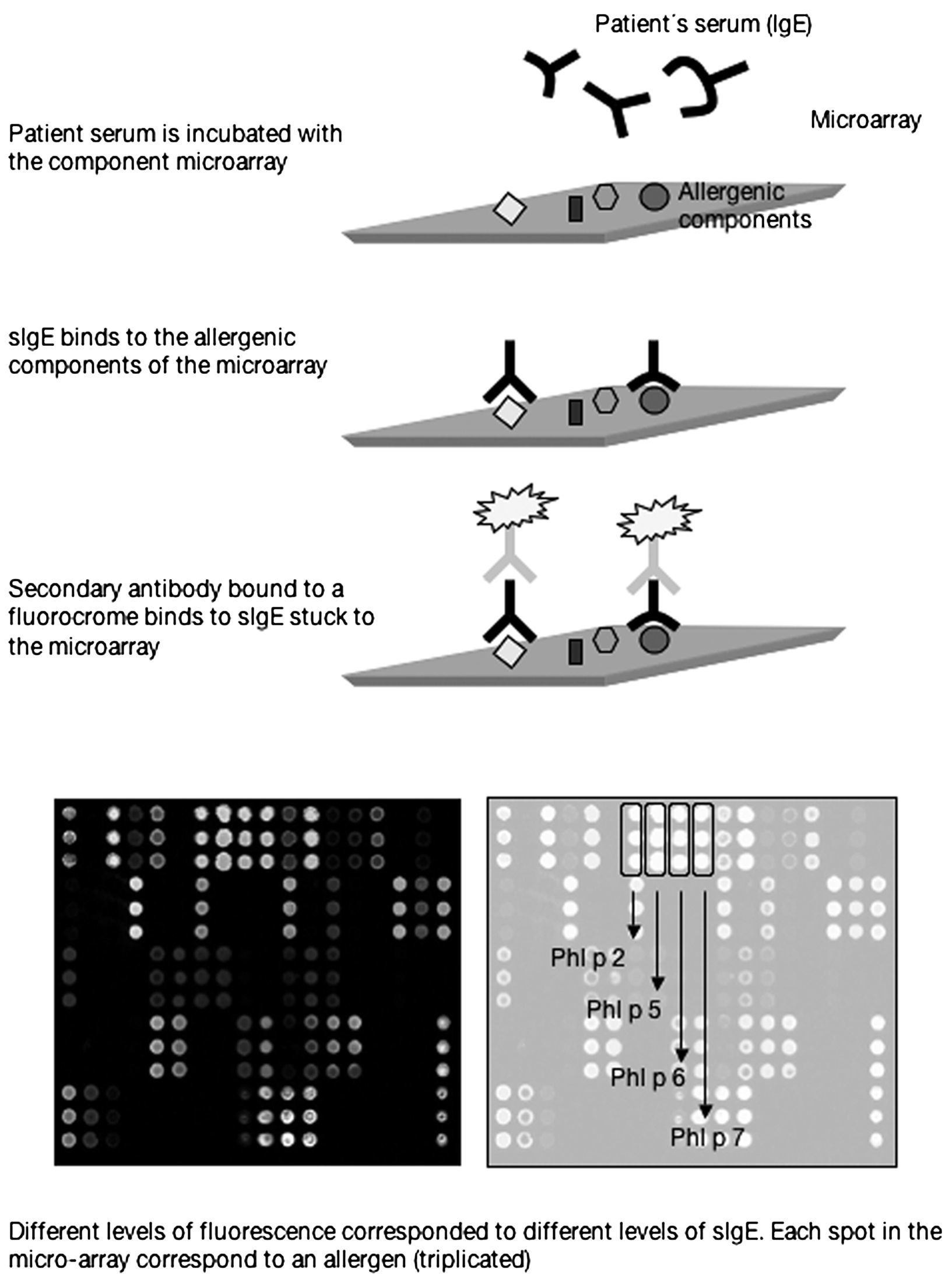
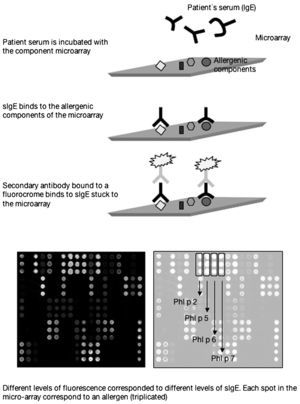
The first commercially available component-based allergen microarray currently offers 103 proteins or allergenic components represented in triplicate which are printed in a 1cm2 area of a slide of standard dimensions (2.5cm×7.5cm). Each slide in fact contains 4 microarrays, that is to say the serum from 4 patients can be studied in each one. The technique is performed using 25 microlitres of serum which are added to the microarray and incubated for 2h. After successive washings to remove the serum proteins unbound to the microarray, a detection antibody containing a fluorochrome is added which recognises the IgE and which is incubated for a further hour. After several washings to remove the excess detection antibody, a laser is used to measure the different levels of fluorescence which by means of software based on known standards included in the microarray allows the relationship to be calculated between fluorescence and arbitrary units termed ISUs (Figure 1).
Thus, as component-resolved diagnosis (CRD) determines specific IgE against the particular component responsible for the allergy, it allows the allergy to be diagnosed with greater accuracy. Furthermore, sensitisation to particular allergenic components has been associated with different clinical patterns5 as a result of which CRD could predict the risk of specific clinical pictures of varying severity. Moreover, if only CRD is used, we can identify the specific sensitisation to allergic components common to different allergenic sources which give rise to cross-reactivities (for example, a fruit or a pollen). CRD may also explain some of the reactions observed with immunotherapy in patients with the same sensitisation to an allergenic source6, since patients sensitised to the same allergenic source may present a different pattern of sensitisation at molecular level. In fact, some companies have begun to quantify certain allergenic molecules, those considered to be most relevant, in their extracts for both immunotherapy and diagnosis in skin tests. Those considered to be less relevant because they affect a minority of the allergic population and whose concentration may vary from vial to vial are not quantified. Thus, the incorporation of CRD into our daily practice could even modify indications for immunotherapy in some cases.
However, when faced with a suspected IgE-mediated reaction, skin tests continue to be the diagnostic technique of choice. In fact, recent studies7,8 show the efficacy of skin tests for the diagnosis of allergic sensitisations in children and propose, for European countries, a battery of standard air-borne allergens to which certain allergens may be added or removed depending on the age of the child or the clinical history of the patient. Currently in vivo techniques use natural allergens, that is, the whole allergenic source which offers the advantage of including all the proteins but with the disadvantage of not allowing individualisation of the sensitisation profile.
With the aim of better understanding the application of CRD, we present two cases in which the diagnosis, and as a result the indication of immunotherapy and clinical management, were modified on the basis of results from molecular diagnosis using microarrays.
Case 1The subject was a five-year-old male diagnosed with moderate and persistent bronchial asthma, persistent rhinitis and anaphylaxis to shell fish. To determine the sensitisation, skin tests against airborne and food allergens and determination of specific IgE against whole extracts of D. pteronyssinus, prawn and recombinant prawn tropomyosin were performed using InmunoCap (Phadia, Sweden) and against 89 allergenic components using the ISAC CRD89 microarray (VBC-genomics, Phadia, Sweden).
Skin tests were positive to D. pteronyssinus and shell fish with a specific IgE of 67.7kU/L for D. pteronyssinus, >100kU/L for prawn and >100kU/L for prawn tropomyosin. Microarray analysis revealed sensitisation of the tropomyosin of D. pteronyssinus (a specific IgE Der p 10 of 26.77ISU) with no sensitisation to the remaining mite allergens analysed (D. pteronyssinus -Der p 1, Der p 2-, D. farinae - Der f 1, Der f 2- and D. siboney –Der s 1-). The specific IgE values against the other 5 tropomyosins (prawn, cockroach, snail and Anisakis) were high but very similar to each other.
Case 2This subject was an eight-year-old male diagnosed with repeated episodes of urticaria due to sensitisation to latex, bronchial asthma due to sensitisation to grass pollens, oral allergy syndrome due to fruit and ulceration. Sensitisations were analysed with skin–prick tests which were positive for fruits from the latex group, latex and grass pollens. Specific IgE values were: banana 0.74KU/L, chestnut 1.70KU/L, kiwi <0.35KU/L peanut 37.8KU/L, latex 11.7KU/L, lolium>100KU/L, and total IgE 876KU/L. Specific antigen immunotherapy was begun against grass pollens with partial response of seasonal symptoms although treatment with antihistamines was required during the season. No further episodes of urticaria have occurred and the patient avoids fruits of the Rosaceae family.
During the last consultation, in May, skin tests were performed and were positive for gramineae, plantago, salsola, birch, olive, profilin and peach. Using the microarray technique mentioned above, we were able to confirm sensitisation to the major gramineae and olive allergens; rPru p1 (homolog of Bet v1), nPru p3 (LTP), panalergens of peach; rHev b 8 (profilin of latex) as well as the rest of the profilins (birch, olive, mercury, etc). Results for the latex allergens rHev b1, 3, 5 and 6 were negative. Currently, the patient shows no clinical signs of sensitisation to latex.
DiscussionIn both cases our diagnosis was modified following the application of molecular diagnosis with microarrays. In the first case, sensitisation is to the panallergen tropomyosin and not to the major mite allergens. As a result, there is no indication for immunotherapy and the patient can be treated symptomatically and with avoidance measures. The second patient does not present sensitisation to the major latex allergens but to profilins (including the latex profilin) and as a result the therapeutic approach is different.
The main panallergen in the animal world is tropomyosin. Sensitisation to this allergen accounts for the cross-reactivity between–principally- shell fish and dust mites. It is a very well preserved molecule present in muscle and non-muscle cells of vertebrates and invertebrates. Allergenic tropomyosin comes from invertebrates such as crustaceans (for example, prawn, lobster, crab), arthropods (dust mites and cockroaches), molluscs (sea snails and snails) or nematode parasites (Anisakis), whereas tropomyosin from vertebrates is not allergenic9. Consequently, when confronted with skin tests positive to crustaceans, anisakis and mites, we can suspect sensitisation to tropomyosin. However, in young children in whom skin tests are performed with fewer allergens, it can be difficult to make such suppositions except when symptoms clearly emerge from the clinical history or when skin tests to shell fish or Anisakis are performed. In spite of the sensitisation to tropomyosin, in this case we initially suspected a sensitisation to mites as being responsible for the nasal and persistent bronchial symptoms in this patient. However, no sensitisation to the main dust mite allergens was detected but rather, only sensitisation to tropomyosin.
It is difficult to assess if the tropomyosin could be the culprit allergen of the persistent asthma and rhinitis. However, the fact that the natural extract of house dust mites is employed in immunotherapy and only group 1 and 2 are quantified in some commercial extracts, and no data is given regarding the concentration of the tropomyosin in each vial contraindicates the use of these extracts. Thus, in this case, the diagnosis of sensitisation to tropomyosin without major dust mite allergens modifies our therapeutic approach, especially as far as the use of immunotherapy is concerned. The question should be raised as to whether those patients receiving immunotherapy against mites with no clear response may have sensitisation to tropomyosin, and not to the main allergens present in the extract.
Furthermore, sensitisation to profilins in patients with sensitisations to pollens is much more frequent that sensitisations to other panallergens, and clinically it is usually easier to suspect such a diagnosis as it is associated with oral allergy syndrome, which consists of a series of symptoms limited to the oropharynx, such as itching of the oropharyngeal mucosa, with or without erythema or perioral wheals, which appear after the ingestion of plant foods, normally fruit from the Rosaceae family. Given the thermolabile nature and resistance to enzymatic proteolysis of this group of proteins, the presence of serious systemic symptoms is less frequent than in patients sensitised to other allergens. Profilins are well preserved proteins in eukaryotic organisms and are present in a wide variety of pollens and plant foods. For this reason, patients sensitised to profilins give multiple positive results in skin tests against different pollens and plant foods, which can give a clear indication of the diagnosis to be made. In the second of our patients, the skin test performed with the whole allergen was positive but when the analysis by components was made, it was observed that the sensitisation was not to the major allergens of latex but only to profilin. As a result, the treatment options for the patient would not be the same as it seems that the sensitisation to the latex profilin has no clinical repercussions in the exposure to this material.10
In conclusion, component-based diagnosis is a useful technique in the diagnosis of allergies when used in association with the clinical history of the patient and complemented by skin tests. Similarly, sensitisation to panallergens should be taken into account in routine practice, especially in patients with multiple sensitisations, and in this context microarrays are a useful, simple and non-invasive technique. Finally, this technique is also very useful in the diagnosis of children with multiple sensitisations, in whom the number of skin pricks which can be administered is limited due to the young age of the patients.