In recent years, laryngopharyngeal reflux (LPR) in children has been taken into consideration.
ObjectiveThe aim of this study was to assess the laryngoscopic findings in children diagnosed LPR and/or gastro-oesophageal reflux (GERD).
MethodsThe findings of 49 patients with at least one or more respiratory complaint such as chronic cough, wheezing, hoarseness, recurrent laryngitis, and throat clearing/postnasal discharge suggesting LPR were evaluated retrospectively. The diagnosis of LPR+GERD or GERD was done by the clinical history and 24h double-probe pH monitoring and/or scintigraphy.
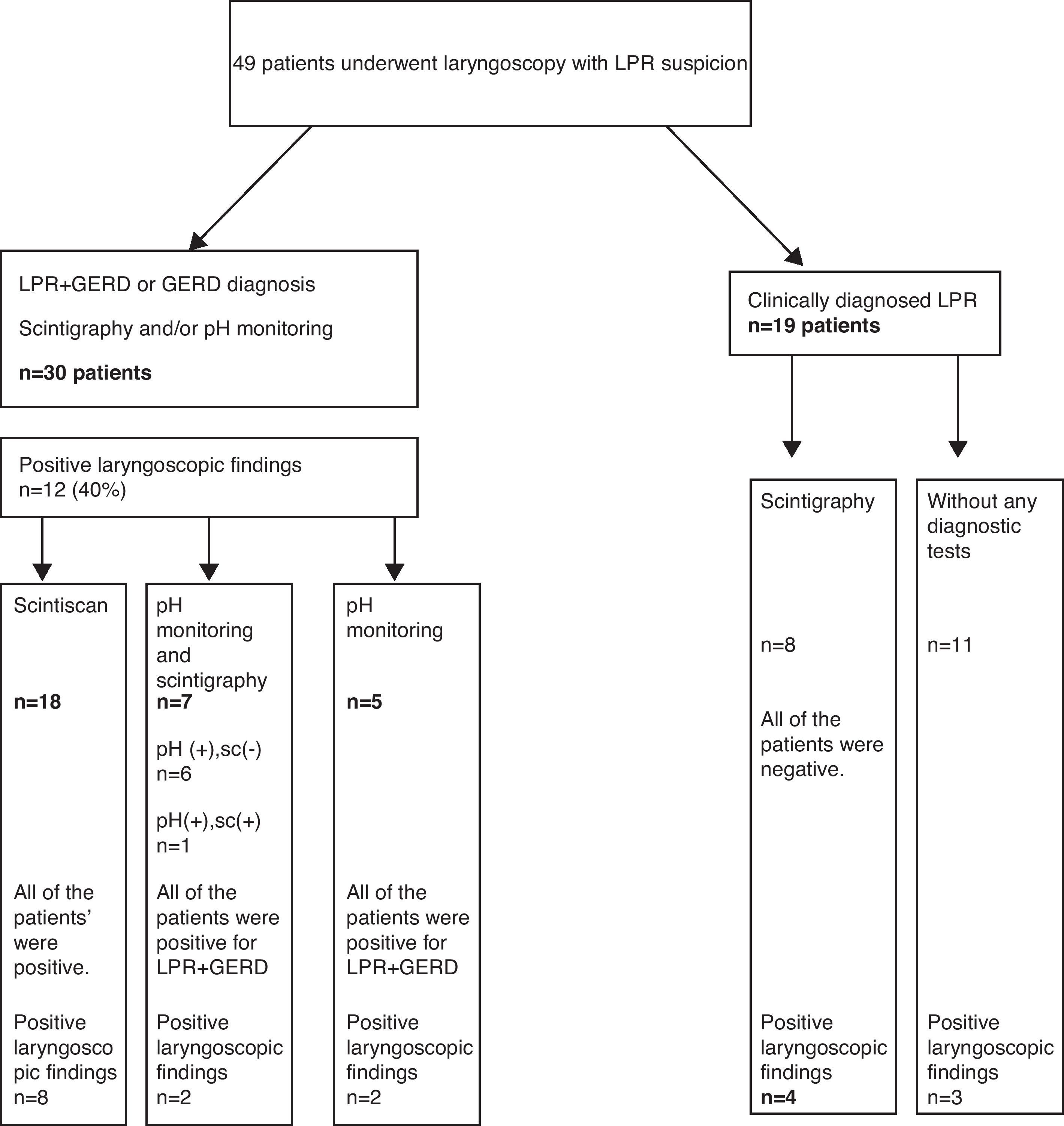
ResultsThirty eight out of 49 patients examined by laryngoscopy underwent 24h double-probe pH monitoring and/or scintigraphy. Thirty of them were diagnosed as LPR+GERD or GERD by any test positivity. Twelve of 30 patients diagnosed with LPR+GERD or GERD had a positive laryngeal finding on the examination of fibre optic laryngoscopy. The most common finding with eight cases was arytenoid erythema A sensitivity of 40% and specificity of 50% for the laryngoscopy in the diagnosis of LPR/GERD were found.
ConclusionIn children with unexplained respiratory symptoms, laryngopharyngeal reflux should be suspected. Therefore, until enough data on this issue in the literature accumulates, the history and the laboratory findings of the patients obtained from various techniques to document paediatric LPR should be evaluated together.
Gastro-oesophageal reflux (GER) is a common physiological situation frequently causing discomfort in children. Up to 50% of healthy infants have regurgitation which spontaneously resolved itself by the age of two in most cases. On the other hand, gastro-oesophageal reflux disease (GERD) causes a backward flow of gastric content into the oesophagus, showing gastrointestinal and respiratory symptoms.1
In recent years, laryngopharyngeal reflux (LPR) has been taken into consideration in paediatric patients. It refers to retrograde flow of gastric contents to the laryngopharynx and upper aero digestive tract through upper gastro-oesophageal sphincter. Its symptoms are non-specific and the most common symptoms are laryngeal and pharyngeal symptoms. The majority of LPR patients do not show classic GERD symptoms. It is often difficult to make LPR diagnosis in children due to its intermittent pattern even if the symptoms such as chronic cough, hoarseness, repetitive throat clearing with postnasal drainage, and laryngeal spasm are seen.2,3 There are also a few researches on this subject particularly concerning children with respiratory complaints.4,5 However, recent publications related to LPR in children have been notable.
Although there is no ideal diagnostic test for LPR due to some limitations, one of the most commonly-used techniques to document LPR is the ambulatory 24h double-probe pH monitoring. The other diagnostic tools in paediatric LPR are scintigraphy, barium oesophagogram, laryngoscopy, and multichannel intraluminal impedance monitoring.2,3 Especially, the fibre optic laryngoscopy in competent hands may be a minimal invasive and less time-consuming method for detecting LPR. However, which test is the best choice in diagnosing LPR is still controversial. In addition, the correlation between LPR and endoscopic findings of larynx in children is not known very well.
ObjectiveThe aim of this study was to assess the findings of laryngoscopy in children with respiratory complaints who were diagnosed LPR and/or GERD by ambulatory 24h double-probe pH monitoring and/or scintigraphy, the relationship between the laryngoscopic findings and respiratory symptoms, and to investigate whether the results of laryngoscopy and those of the other diagnostic methods were parallel or not.
MethodsIn this retrospective study, we evaluated the findings of 49 patients, who underwent fibre optic laryngoscopy, with respiratory complaints suggesting LPR such as chronic cough, wheezing, hoarseness, recurrent laryngitis, and throat clearing/postnasal discharge. The study was carried out in the Clinic of Paediatric Allergy and Asthma, Dr. Sami Ulus Research and Training Hospital of Women's and Children's Health and Diseases. The diagnosis of LPR/GERD was done by the clinical history and 24h double-probe pH monitoring and/or scintiscan.
After overnight fasting, ambulatory 24h double-probe pH monitoring was performed with the MMS Orion II device using a trans-nasally placed catheter (UPS-2020/ORION, Medical Measurements System BV, Enschede, The Netherlands), pH recorder (software version 6.7 for Windows, 95 MMS, USA) with antimony electrode (Medtronic Zynetics 24 pH catheter). According to a standard protocol 24h pH monitoring was applied.6 The pH probes were calibrated using buffer solutions of pH 7.0 and pH 4.0 just before and at the end of each examination. Following calibration, the double-sensor arm was introduced trans-nasally and advanced until gastric pH was reached by the distal sensor. The probe was then withdrawn slowly until the distal sensor showed an abrupt increase in pH value (suggesting the gastro-oesophageal inversion point), and then the probe was withdrawn another 5cm and fixed to the nose so that the distal sensor was positioned approximately 5cm above the lower oesophageal sphincter (LES) and the proximal sensor was positioned 10–20cm above the LES adjusting to the age and heights of patients, just below the upper oesophageal sphincter. All the patients were fed with their normal formulas or usual diet during pH monitoring. Oesophageal pH was recorded in supine, upright, and postprandial positions. Positive test criterion for diagnosis of GERD was considered ≥5% for the percentage of total time pH less than 4. At least three episodes of pH below 4 in the proximal probe with a simultaneous drop or a preceding decrease of pH<4 in the distal probe or ≥1% for the percentage of total time pH<4 in the proximal probe were accepted as LPR. The numbers of acid reflux lasting longer than 5min and duration of the longest reflux to proximal and distal probes were also recorded. Ambulatory 24h double-probe pH monitoring was applied by the same paediatric gastroenterologist (FD).
Scintigraphy was performed as previously described.7 The test was accepted as positive if the tracer was seen throughout the entire oesophagus at least during one reflux episode.
The ear–nose–throat (ENT) examination was done by the same otorhinolaryngologist (YB). After the ENT exam, the patients underwent laryngoscopy (4mm flexible optic fibre, Storz Videolaryngoscope 11001R01 Karl Storz (Tuttlingen, Germany)). Five minutes before going to laryngoscopy, local anaesthesia with a 10% lidocaine spray was applied on the nose and throat per one puff. Intravenous infusion of propofol 1mg/kg was given to the patients who were unable to co-operate in the laryngoscopic examination. The nasal fossa, rhinopharynx, torus tubarius, oropharynx, hypopharynx and larynx were assessed by laryngoscopy. The patients and/or their parents gave the approval for diagnostic tests and the study was approved by the Hospital's Ethical Committee.
Statistical analysisThe SPSS version 10.0, Chicago, IL, USA software was used for the statistical analysis of the obtained data. The definitions were provided as number and percentage for discrete variables and mean and standard deviation for continuous variables. The comparisons were made using the chi square test with significance defined as p<0.05.
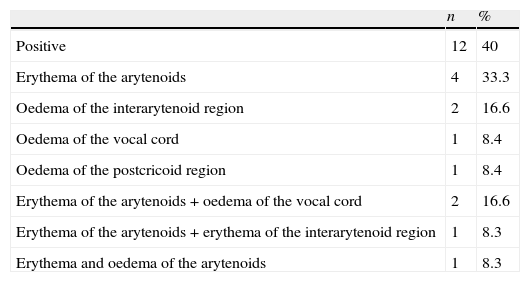
ResultsThe study included 25 (51%) boys, aged between 1 and 16 years (7.26±2.8 years). All 49 children underwent fibre optic laryngoscopy. Thirty eight out of 49 patients examined by fibre optic laryngoscopy underwent 24h double-probe pH monitoring and/or scintigraphy. Thirty (79.8%) of these 38 patients, 18 boys, ranging in age from 1 to 13 years (6.6±2.8 years), were diagnosed with LPR+GERD or GERD either by pH monitoring and/or scintigraphy. The distribution of the patients taken in the study is seen in Fig. 1. At laryngoscopic examination, whereas 19 of 49 patients in study group had a positive finding in favour of LPR, 12 of 30 patients (40%) diagnosed with LPR+GERD or GERD had a positive finding in favour of LPR. Arytenoid erythema was the most common finding in eight cases. Vocal cord oedema in three patients, interarytenoid oedema in two patients, interarytenoid erythema in one patient, arytenoid oedema in one patient, and postcricoid oedema in one patient were the other laryngeal abnormalities. In some cases more than one finding coexisted. The laryngoscopic findings are shown in Table 1. We found a sensitivity of 40% and specificity of 50% for the laryngoscopy in the diagnosis of LPR/GERD. All the patients had respiratory symptoms (cough 24, wheezing 10, hoarseness 9, recurrent laryngitis 7, and throat clearing/postnasal drip 7). In 25 cases (83.3%), there were also gastrointestinal complaints associated with these respiratory complaints (Table 2). Accompanied diseases were observed in 16 out of 30 patients with LPR/GERD: 13 bronchial asthma, 7 adenoid hypertrophy, 3 Helicobacter pylori gastritis. Proton pomp inhibitors (PPIs) were given to 30 patients diagnosed with reflux by 24h double-probe pH monitoring and/or scintigraphy.8
Positive findings of laryngoscopy in 30 LPR+GERD or GERD children.
| n | % | |
| Positive | 12 | 40 |
| Erythema of the arytenoids | 4 | 33.3 |
| Oedema of the interarytenoid region | 2 | 16.6 |
| Oedema of the vocal cord | 1 | 8.4 |
| Oedema of the postcricoid region | 1 | 8.4 |
| Erythema of the arytenoids+oedema of the vocal cord | 2 | 16.6 |
| Erythema of the arytenoids+erythema of the interarytenoid region | 1 | 8.3 |
| Erythema and oedema of the arytenoids | 1 | 8.3 |
Symptoms of 30 LPR+GERD and GERD cases.
| Complaints | n | % |
| Respiratorya | 30 | 100 |
| Cough | 24 | 80 |
| Wheezing | 10 | 33 |
| Hoarseness | 9 | 30 |
| Recurrent laryngitis | 7 | 23 |
| Throat clearing/postnasal drip | 7 | 23 |
| Frequent URTI | 5 | 17 |
| Nasal congestion | 4 | 13 |
| Snoring | 4 | 13 |
| Gastrointestinala | 25 | 83 |
| Abdominal pain | 17 | 57 |
| Halitosis | 10 | 33 |
| Emesis-vomiting | 8 | 27 |
| Loss of appetite | 7 | 23 |
| Regurgitation | 5 | 17 |
URTI, Upper respiratory tract infection.
LPR was first reported by Koufman et al. as an entity distinct from GERD.9 These distinctions between the two are based on their pathophysiology, symptoms, and physical sequelae.10 Most patients with LPR do not show classic symptoms of GERD, particularly regurgitation and heartburn. On the other hand, they often present with laryngopharyngeal symptoms due to vulnerability of the upper-airway epithelium to gastric reflux. LPR may present a wide range of otolaryngological and respiratory symptoms or seen without any clinical signs in children. It is thought to be responsible for various conditions such as refractory asthma, recurrent bronchitis, laryngomalacia, subglottic stenosis, and otitis media.2,11–19 When LPR is co-morbid with asthma, it may affect the treatment and follow-up of the patients in paediatric allergy clinics. Even though there are no clinical presentations specific to LPR, hoarseness, dysphonia, postnasal drip with repetitive throat clearing, chronic cough, laryngeal spasm and dysphagia are common symptoms of LPR. In our study, all of the patients diagnosed LPR+GERD or GERD had respiratory tract system complaints and the most common one was chronic cough (80%). In childhood there are no data about the role of non-acid reflux on LPR in English literature. However, Agrawal et al.20 reported that different mechanisms might play role in the occurrence of the symptoms of acid and non-acid reflux.
Some 16 out of 30 patients with LPR/GERD had accompanied diseases such as bronchial asthma, adenoid hypertrophy and H. pylori gastritis. However, 14 (47%) patients with respiratory complaints had only LPR/GERD without any co-morbid disease. The patients with LPR do not have gastrointestinal complaints. However, 83.3% of our patients had gastrointestinal symptoms including abdominal pain, nausea, vomiting, anorexia, regurgitation and halitosis. This implies that some patients in our study, as shown by the double-probe pH monitoring, had LPR+GERD and some had only GERD.
Symptoms and physical findings seen in LPR may not be sufficient for diagnosis because similar symptoms can be derived from infections, allergies, postnasal drip, exposing to toxic inhalants and passive smoking.21 Nevertheless, our patients had no signs of any infection or allergic rhinitis during fibre optic laryngoscopy. For this reason, we thought that our patients’ symptoms might be associated with LPR.Although there is no ideal diagnostic method for LPR detection, multichannel intraluminal impedance monitoring, pH monitoring, scintigraphy, ultrasound, barium oesophagogram, and oesophageal biopsy can be used for diagnosis. Barium esophagram has a low sensitivity and specificity for diagnosis of GERD.22 When any reflux episode is considered as a positive test, gastric scintigraphy has a sensitivity of 79% and a specificity of 93%.23 The correlation between scintigraphy and pH monitoring is weak.24–26
Ambulatory 24h double-probe pH monitoring is considered by many authorities as a gold standard for diagnosing LPR. However, its sensitivity is low and the incidence of false-negative results is as high as 25–50%.2,13 It may also not be tolerated well by children and it sometimes requires hospitalisation. Additionally, it is an expensive and a time-consuming test, and it cannot detect the intermittent reflux episodes, non-acidic reflux episodes and gaseous reflux. Although parameters on the distal probe for the diagnosis of GERD are well defined in the literature, the diagnosis of LPR is still under discussion. There is a wide range of parameters used to evaluate LPR. One of them is the number of laryngopharyngeal reflux episodes. It ranges from one to seven or more reflux episodes depending on researchers’ methods and analyses.3 Another parameter of LPR diagnosis, although not well defined so far, is the pH values for the hypopharynx. Although in the diagnosis of LPR the 24h double-probe pH monitoring is accepted as a gold standard by some authors, it is not commonly used by otolaryngologists.27
In our study, 12 patients were diagnosed with LPR+GERD by 24h pH monitoring. Nineteen of 30 patients were diagnosed with GERD by the scintigraphy. Six patients with negative scintigraphy had positive pH monitoring. This result supporting the literature showed us that there was not a good correlation between scintigraphy and pH monitoring. It is also known that the combination of the tests increases the sensitivity of the diagnosis of GERD.
When the patients with suspected LPR were evaluated by the fibre optic laryngoscopy, only 40% of the patients had positive findings in favour of LPR. The most common laryngeal finding was arytenoid hyperaemia in eight cases. Other findings included the vocal cord oedema, oedema on the region of interarytenoid, arytenoid oedema and oedema on the region of postcricoid. In some cases, there was more than one finding. Our study showed that sensitivity to detect reflux was 40% and specificity was 50% for any positive laryngeal findings by laryngoscopy.
Not too much research has been carried out on laryngeal findings of LPR in children so far.4,5,15,28 Carr at al.4 focused on the correlation between extraoesophageal reflux disease and direct laryngoscopy and bronchoscopy findings. In this retrospective study, 84% of the children who underwent direct laryngoscopy and bronchoscopy were diagnosed with GERD. They detected at least one laryngeal abnormality at 83% of children diagnosed with GERD. The most common laryngeal findings were postglottic oedema, arytenoid oedema, large lingual tonsils, vocal fold oedema and vocal fold nodule. The best sensitivity and specificity were obtained with the combination of postglottic oedema, arytenoid oedema, vocal fold oedema and vocal fold nodules. The authors concluded that laryngeal findings showed high predictive value in GERD.
The same authors prospectively evaluated paediatric patients who underwent direct laryngoscopy and bronchoscopy for various reasons. In their study, severe arythenoid oedema, postglottic oedema or enlargement of the lingual tonsils were found as pathognomonic for GERD.5 In another study, it is stated that the laryngeal pseudosulcus is the most common finding in children and therefore, it should be included in the scoring system.28 The basic difference between Carr et al.’s study5 and our study was in terms of endoscopy indications. Although they evaluated various indications such as tracheostomy surveillance, noisy breathing, dysphonia, chronic cough, laryngotracheal reconstruction follow-up, desaturations, tracheal or subglottic stenosis, bronchial biopsy, and recurrent pneumonia, we only evaluated the cases with suspected LPR. This can explain why we found a much lower rate (40%) of positive laryngeal findings than those obtained by Carr et al. Indeed, laryngoscopy findings in children with LPR may be fewer than was estimated. In a study, only 40% of the adult patients with GERD laryngitis had laryngeal findings on flexible laryngoscopy.29
In the current study, we are limited by the small size of the study group and the retrospective nature of the study. However, our study in children with respiratory complaints investigated LPR is a rare study.
ConclusionWe think that in children with unexplained respiratory symptoms paediatric laryngopharyngeal reflux should be suspected. Therefore, until sufficient data on this issue in the literature accumulate, the history and the laboratory findings of the patients obtained from various techniques to document paediatric LPR should be evaluated together.
Conflict of interestThe authors have no conflict of interest to declare.