The International Study of Asthma and Allergies in Childhood (ISAAC) is the largest epidemiological study ever performed and the only truly global allergy study. This review summarises the childhood eczema-related findings from ISAAC and discusses how these fit into our current understanding of eczema aetiology, with particular emphasis on worldwide time trends in eczema prevalence, climatic and dietary risk factors, breastfeeding, the role of skin barrier impairment and allergic sensitisation.
With close to two million children from 106 countries, ISAAC is the biggest and only allergy study that has taken a truly global approach. ISAAC's strength is the use of uniform validated methods, allowing direct comparison of results between paediatric populations and providing invaluable data on the worldwide burden of allergic disease, time trends in allergy prevalence and severity, as well as major disease risk factors. ISAAC started in 1991 and has so far completed three phases. Phase One measured the symptom prevalence of asthma, rhino-conjunctivitis, and eczema, using a validated questionnaire tool among children aged 6–7 and 13–14 years.1 This allowed the creation of the first world map of allergic disease prevalence, revealing significant variations in disease burden between countries.2 Reasons for these variations have been explored first in ecological and then in cross-sectional risk factor analyses as part of Phase Two. Phase Two included around 63,000 children aged 8–12 years from 30 centres in 22 countries. Data was collected through symptom and risk factor questionnaires. Participants were also skin prick tested to environmental allergens and physically examined for flexural eczema. In addition, blood was collected for genetic analyses.3 Subsequently, Phase Three looked at time trends in disease burden through comparison of prevalence figures with Phase One. As in Phase One, participants were schoolchildren aged 6–7 and 13–14. This review article discusses, in the light of other work, what we have learned from ISAAC about childhood eczema (syn. atopic eczema, atopic dermatitis).4
Global prevalence surveys and time trendsPrior to the ISAAC Phase One survey, very little was known about the prevalence of childhood eczema outside of Northern Europe. Phase One collected data from 256,410 children aged 6–7 years in 90 centres and 458,623 participants between 13 and 14 years of age from 153 centres.5,6 The validated ISAAC eczema questions, which were used in all study centres, are shown in Box 1. There were significant prevalence differences between paediatric populations for all eczema outcomes in both age groups. For instance, the prevalence of flexural eczema in the past 12 months ranged from less than 2% in Iran to over 16% in Japan and Sweden in the 6–7 years age group and under 1% in Albania to over 15% in a number of Northern European countries among 13 and 14-year-old children. With a few exceptions, prevalences tended to be higher in affluent European and Australasian settings (Japan, Australia, and New Zealand) compared to children in Eastern and Central Europe as well as East Asia.
Eczema symptoms questionnaire used in ISAAC Phases One to Three (for 6 and 7 year olds the parents answered the questions, whereas older children answered the questions themselves (Phases One and Three). In Phase Two (children aged 8–12) questions were answered by parents):
- 1.
Has your child (have you) ever had an itchy rash which was coming and going for at least six months?
- 2.
Has your child (have you) had this itchy rash at any time in the last 12 months?
- 3.
Has this itchy rash at any time affected any of the following places: folds of the elbows, behind the knees, in front of the ankles, under the buttocks, or around the neck, ears, or eyes? (‘flexural eczema in the past 12 months’)
If ‘yes’ to question 3, then additional questions about disease severity were asked:
- 4.
Has this rash cleared completely at any time during the last 12 months?
- 5.
In the past 12 months, how often, on average, has your child (have you) been kept awake at night by this itchy rash? (Never in the last 12 months, less than 1 night per week, 1 or more nights per week)
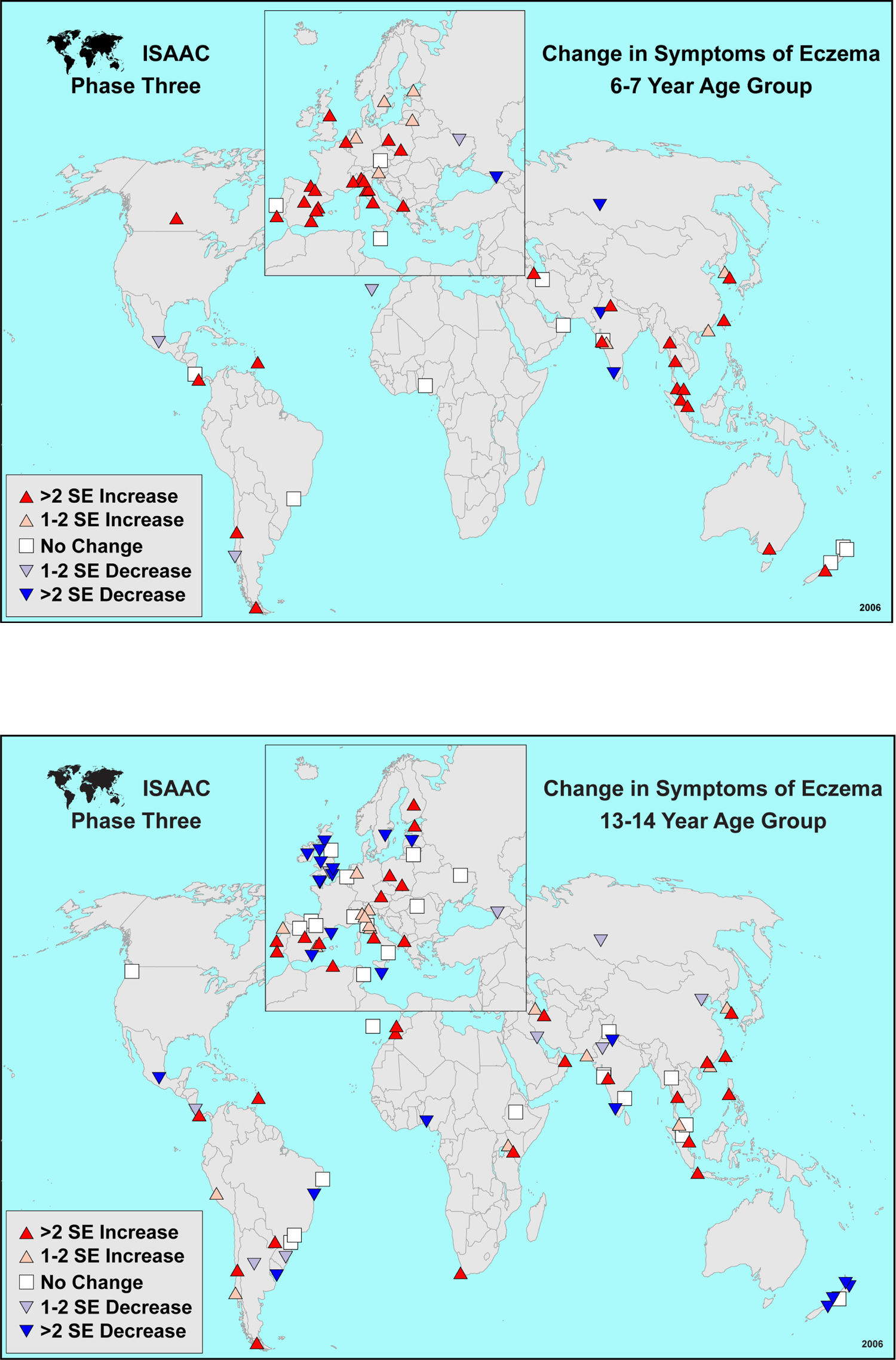
Phase Three added valuable information on time trends in disease distribution. 302,159 children aged 13–14 years in 105 centers from 55 countries and 187,943 children aged 6–7 years in 64 centres from 35 countries were surveyed from the Phase One study centres, using the same validated questionnaire tool 5–10 years after the initial survey.7 Overall, in affluent country centres where eczema among 13 and 14 year olds was common, prevalences did not increase further or even decreased, whereas the eczema burden continued to rise in most developing country settings (Figure 1). As for 6–7 year olds, the majority of centres showed an increase in eczema symptoms.
Apart from generating world maps of eczema prevalence, the main outcome of Phases One and Three was that eczema prevalence did not only vary between countries, but there were also differences within populations of the same ethnic background, suggesting that environmental influences play an important role in disease risk. Consequently, a number of ecological and cross-sectional analyses based on the ISAAC data set have examined individual risk factors for eczema.
ClimateOne potential explanation for prevalence differences between populations is climate; an area that had previously received little attention with regard to eczema. Based on the Phase One data set, an ecological analysis was conducted using information on long-term climatic conditions in the different study areas from the World Weather Guide.8 Variables that were examined included latitude, altitude, average outdoor temperature and relative outdoor humidity. The results, which were adjusted for countries’ gross national per capita income (GNP), suggest that eczema symptoms correlate positively with latitude and negatively with annual outdoor temperature but none of the other factors. These findings have been supported by cross-sectional studies in Spain and Taiwan9,10 and could be due to direct climatic influences. Alternatively, behavioural changes triggered by weather are a potential explanation, such as time spent outside in sun, especially as UV light has well-established immuno-suppressive effects and is used as a treatment for eczema.11 Work that has looked at flare factors in established eczema supports this notion, as lower outdoor temperatures, especially in combination with skin irritants, can contribute to disease worsening, whereas indoor climate seems less important.12,13 However, the relationship between outdoor climate and disease flares is complex with some children reporting worsening in summer and others in winter, as suggested by a small longitudinal study among German children.13 Outdoor temperature and humidity as well as seasonal changes in pollen counts are likely to interact,10,12 and further studies, which also take skin barrier function and hydration status as well as bacterial skin colonisation into account, are required.14
DietAnother potential explanation for prevalence differences between countries are dietary factors. Given how uncommon eczema and other allergies still are in most developing nations, an important question is whether consumption of a ‘western’ affluent diet (i.e. high intake of refined grains, cured and red meats, as well as saturated and unsaturated fatty acids) is associated with an increase in eczema risk. This was explored in another ecological analysis from ISAAC Phase One, looking at the association between eczema prevalence and per capita consumption of vegetables, olive oil, dietary fibre, fat (total, saturated and unsaturated), protein from various dietary sources, carbohydrates, as well as a number of vitamins. There was a consistent negative association between eczema prevalence and per capita consumption of vegetables, protein from cereal and nuts as well as all fresh and frozen fish, even after adjustment for GNP.15
However, such ecological analyses do not allow to directly extrapolate findings from the population to the individual level. A number of longitudinal studies which examined individual dietary components have suggested that a high fish intake during pregnancy has a protective effect on eczema risk in the offspring up to 5 years of age with risk reductions ranging between 25–43%.16–18 Similar risk reductions have been described in children with a high fish intake during late infancy.6,19 These findings have been attributed to fish's rich content in anti-inflammatory n-3 polyunsaturated fatty acids (n-3 PUFA). Over past decades, western diets have become low in n-3 PUFAs (e.g. alpha-linoleic acid, eicosapentaenoic acid, and docosahexaenoic acid) with a corresponding increase in pro-inflammatory n-6 polyunsaturated fatty acids (n-6 PUFA), such as linoleic acid.20 An increase in n-6 PUFA leads to higher levels in arachidonic acid and consequently prostaglandin E2, which, in turn, could promote expression of Th2-mediated cytokine profiles and therefore allergic disease. In keeping with this theory is that maternal intake in n-6 PUFA was associated with an increased eczema risk in Japanese children at 2 years of age and the finding that children who predominantly consume margarine rather than butter also show an increased risk in eczema development.20,21 In addition, case-control studies have demonstrated that eczema sufferers have higher blood levels of linoleic acid (n-6 PUFA precursor) and lower levels of n-3 PUFAs.22,23 However, a carefully conducted birth cohort study failed to show a significant association between maternal and cord blood n-6 PUFA, n-3 PUFA, n-6 PUFA:n-3 PUFA ratio and eczema risk at 18–30 months of age.22,23 Equally, the literature on fatty acid profiles in breastmilk as a risk factor for allergies has been rather conflicting, with some studies even reporting an increased eczema risk in association with n-3 PUFA.24,25 To complicate matters further, a randomised double-blind placebo controlled trial with fish oil supplementation among 98 atopic pregnant women found no difference in overall eczema risk in the offspring between intervention and placebo group, but a reduction in disease severity.26 Along the same lines, a small randomised double-blind placebo controlled study with docosahexaenoic acid (n-3 PUFA group) among 53 adult patients with eczema also showed a modest reduction in eczema severity.27,28 In the end, only large, adequately powered intervention studies can answer the question as to whether fish oil supplementation during pregnancy and/or lactation is able to prevent eczema development and reduce eczema severity, particularly in high risk children.
Breastfeeding and delayed weaningMany advocate breastfeeding as a way of preventing allergies, including eczema. For instance, the World Health Organization (WHO) recommends that babies are exclusively breastfed for 6 months,29 and most European ministries of health advocate at least 4 months of exclusive breastfeeding to aid allergy prevention. It is therefore conceivable that differences in the length of breastfeeding and the age infants are weaned onto solids could explain part of the eczema prevalence differences between ISAAC study populations.
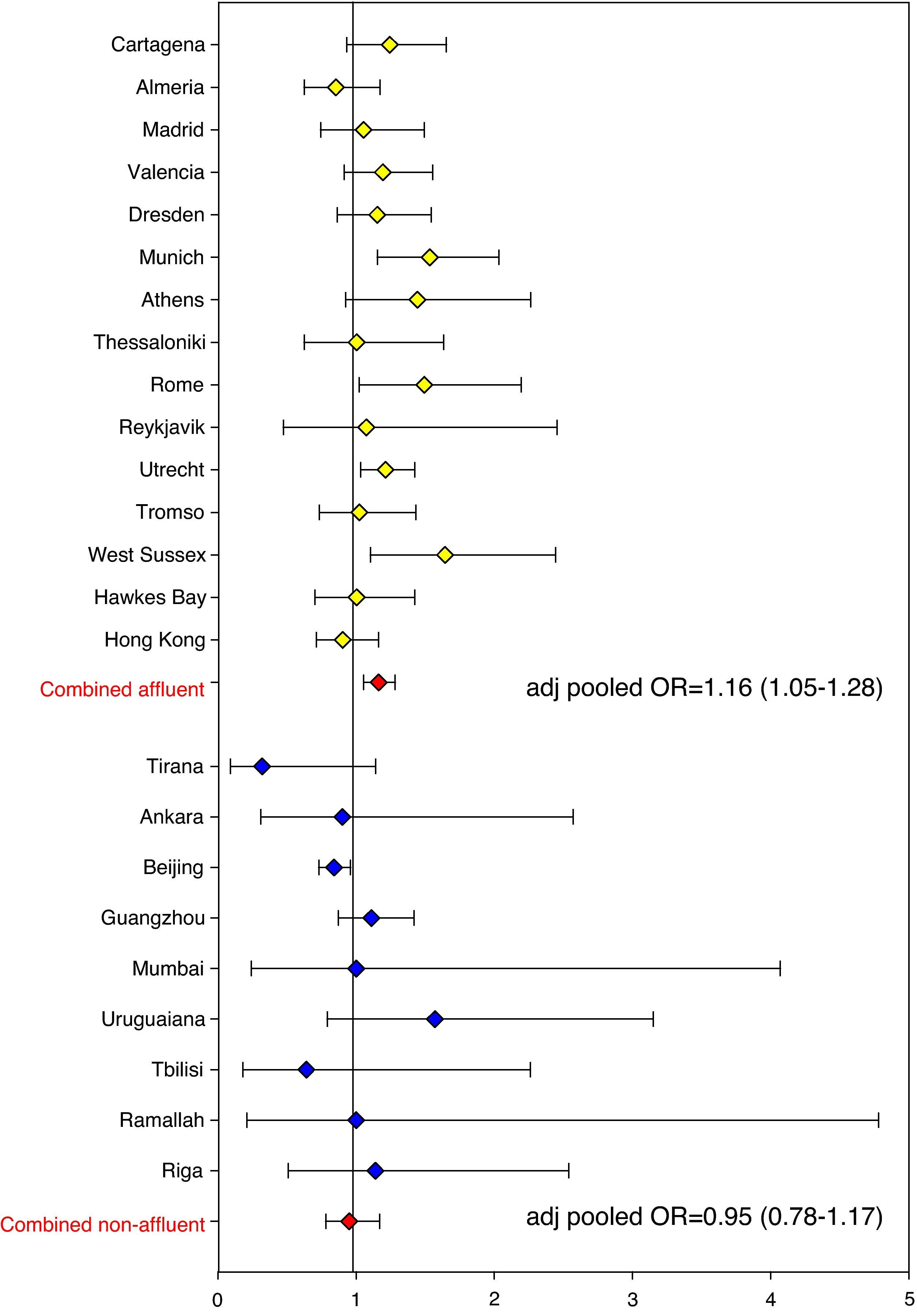
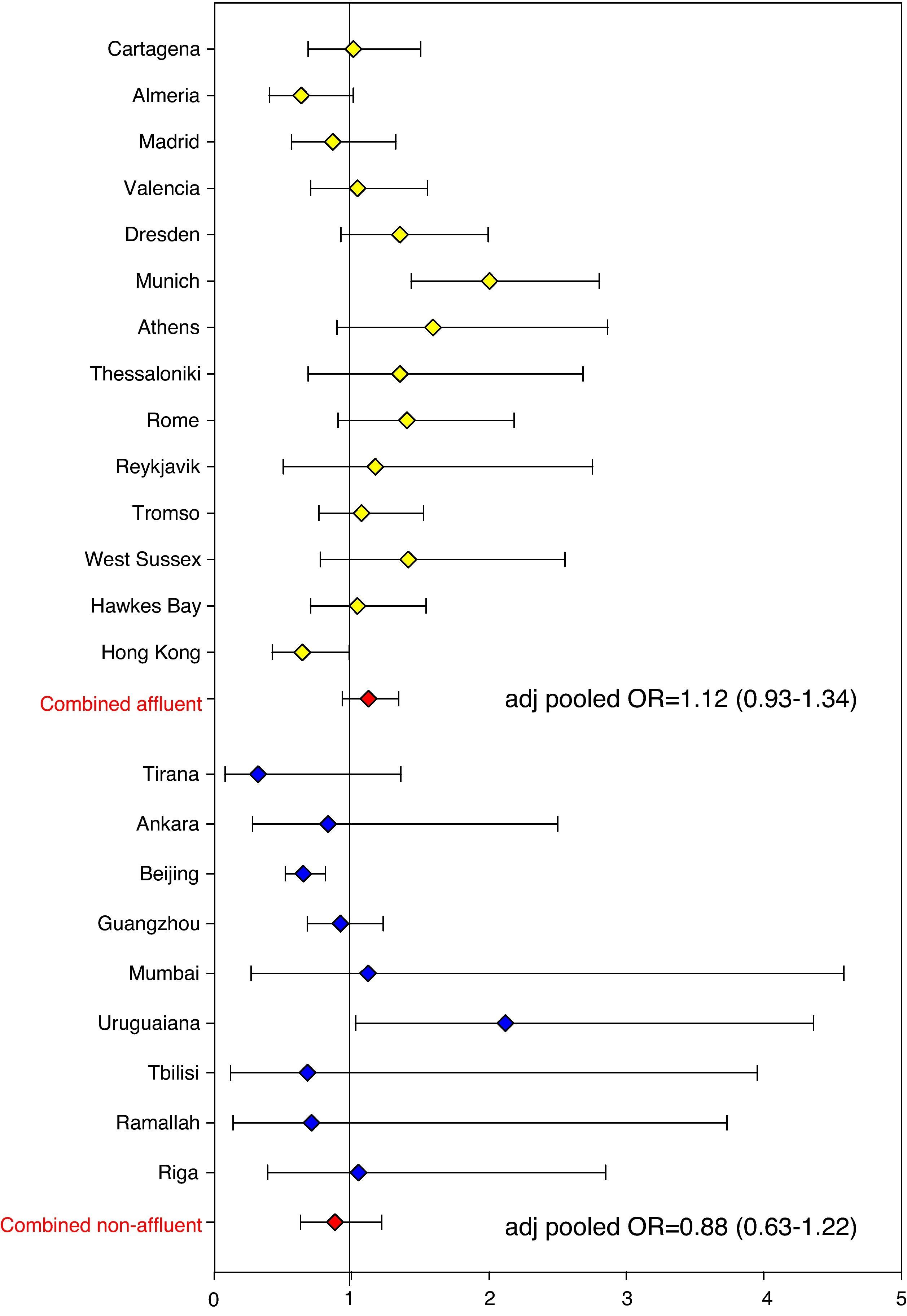
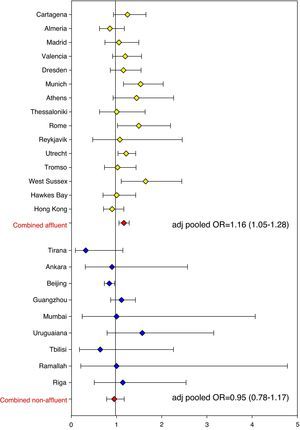
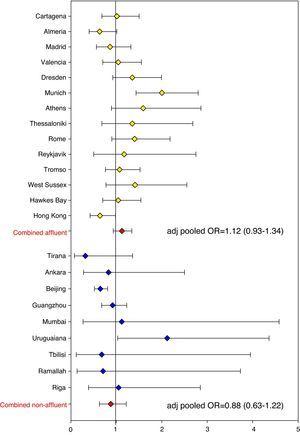
The association between eczema risk and breastfeeding was consequently explored in ISAAC Phase Two (data as yet unpublished). Odds ratios (ORs) were calculated for individual study centres. In addition, pooled ORs were calculated across all study centres and separately for affluent and non-affluent country settings (based on World Bank criteria and GNP). In the vast majority of study centres all childhood eczema outcomes were not significantly associated with breastfeeding (yes/no, < or >6 months) or length of exclusive breastfeeding (<2 months, 2–4 months, >4 months), with the pooled adjusted odds ratio (all study centres) for ‘breastfed ever’ and ‘eczema ever’ being 1.06 (95% CI 0.96–1.17; Figure 2). The pooled adjusted odds ratio for ‘exclusive breastfeeding >4 months’ and ‘eczema ever’ was 1.03 (0.89–1.18; Figure 3). These risk estimates were similar for all other eczema outcomes, including flexural eczema on skin examination. Estimates also did not change significantly following stratification for disease severity (disease persistence and sleep disturbance) and allergic sensitisation (skin prick test positivity to environmental allergens). Findings were not significantly different between affluent and non-affluent countries. Hence, there is little evidence from this substantial cross-sectional analysis that breastfeeding and delayed weaning protect against childhood eczema.
Forest plot showing risk estimates (ORs) and corresponding 95% CIs for the association between ‘eczema ever’ and ‘breastfed ever’ for all ISAAC Phase Two study centres. Risk estimates were pooled, separately for affluent and non-affluent countries (based on World Bank criteria and GNP). All ORs were adjusted for age, sex, bedroom sharing, and maternal atopy.
Forest plot showing risk estimates (ORs) and corresponding 95% CIs for the association between ‘eczema ever’ and ‘exclusive breastfeeding > 4 months’ for all ISAAC Phase Two study centres. Risk estimates were pooled, separately for affluent and non-affluent countries (based on World Bank criteria and GNP). All ORs were adjusted for age, sex, bedroom sharing, and maternal atopy.
The ISAAC findings related to breastfeeding are also consistent with a recent meta-analysis of prospective cohort studies.30 As for delayed weaning, two studies have suggested an increased risk for eczema in infants exposed to solid foods during the first few months of life,31,32 while a number of other studies have shown either no association or even the opposite, i.e. delayed introduction of solids was associated with a higher risk in eczema development.33–37 Reverse causation has been proposed as an explanation, but no convincing evidence of parental allergy playing a role in feeding practices has been found.33 Furthermore, observational data from the UK suggests that the gradual decrease in the proportion of young infants given solids at an early age has coincided with an around three-fold increase in childhood eczema.38,39 There is also mounting evidence from animal research that early introduction of potentially allergenic foods, such as cow's milk, might induce tolerance rather than allergy.40,41
Skin barrier dysfunction and allergic sensitisationIf it is true that the early introduction of potentially allergenic foods through the oral route induces immunological tolerance, then this raises the question as to whether penetration of food and aeroallergens allergens across the impaired barrier in inflamed eczematous skin can induce allergic sensitisation. This would also suggest that atopy is a secondary phenomenon in eczema, rather than being a primary event in eczema development.
ISAAC Phase Two data has shown that the odds of allergic sensitisation in children with examined flexural eczema vs healthy controls varied significantly between populations, ranging from 0.74 (95% CI 0.31–1.81) in Pichincha (Ecuador) to 4.53 (95% CI 1.72–11.93) in Madrid (Spain).42 There was also a significant association between a country's socio-economic status (based on World Bank criteria) and the strength of the association between atopy and flexural eczema, with significantly stronger associations seen in affluent compared to non-affluent settings (combined age- and sex-adjusted ORaffluent=2.69 [95% CI 2.31–3.13] vs ORnon-affluent=1.17 [95% CI 0.81–1.70]). However, there was a linear relationship between the number of positive skin prick test responses and the probability of having flexural eczema in the majority of affluent study centres. While the ISAAC study results overall point towards an association between allergic sensitisation and childhood eczema due to shared aetiologies linked to a ‘western lifestyle’ and affluence rather than direct causality, causality cannot be excluded where a dose–response relationship between the number of positive skin prick tests and flexural eczema probability was observed.
The main dilemma is that the majority of studies performed to date are cross-sectional in design. Only longitudinal and intervention studies can infer causality. A recent Australian birth cohort study among 500 at risk children found that allergic sensitisation at 18 months was not associated with eczema at 5 years (adjusted OR=0.78, 95% CI 0.23–2.64).43 In the same cohort eczema phenotype at 18 months was a predictor of allergic sensitisation at 5 years (adjusted OR=1.67, 95% CI 1.20–2.33), again suggesting that sensitisation is a secondary rather than primary phenomenon in childhood eczema. This is in keeping with findings from a UK birth cohort among almost 600 children, where no clear relationship between levels of house dust mite exposure at two months of age, and eczema and house dust mite sensitisation risk at 8 years was found.44
Skin barrier impairment may provide the missing link between allergic sensitisation and childhood eczema and could explain some of the seemingly contradictory study findings discussed above. The effect of the two common filaggrin (FLG) skin barrier gene mutations R501X and 2282del4 and three rarer variants (R2447X, S3247X, 3702delG) on eczema and allergic sensitisation risk were studied among 3099 German children recruited as part of ISAAC Phase Two in Munich and Dresden.45 FLG variants increased the risk of eczema more than three-fold (OR=3.12, 95% CI 2.33–4.27) with a corresponding population-attributable risk of 10.8%. Furthermore, the association between FLG and eczema was stronger for atopic compared to non-atopic eczema, and this has been confirmed in a number of other studies,46–48 lending support to the hypothesis that allergic sensitisation occurs secondary to an impaired epidermal barrier in eczematous skin. In this explanatory model, IgE-driven processes amplify the inflammation cascade in eczematous skin, partly by leading to reduced filaggrin expression and consequently further skin barrier breakdown.49,50 This would fit the observation that the association between allergic sensitisation and eczema is stronger in more severe disease.51
However, genetics alone cannot explain the dramatic increase in eczema prevalence over past decades.52 Environmental factors, such as frequent use of detergents and water hardness, are likely to play an important role and through interaction with genetic factors are thought to contribute to skin barrier breakdown (reduction in natural moisturising factor, increase in skin pH and subsequent increase in protease activity), which could represent the first step on the way to eczematous skin inflammation.53
What is now required are large birth cohort studies with clear diagnostic criteria to examine the intricate relationship and sequence of events between skin barrier gene mutations (there may be others than just filaggrin!), phenotypic skin barrier impairment, and the immunological changes associated with clinical eczema, its severity, age of onset, chronicity and allergic sensitisation, and one such study is currently underway in the UK (www.eatstudy.co.uk). The potential impact on clinical practice is significant, as the delineation of subtypes of childhood eczema may allow us to develop tailor-made preventative and therapeutic strategies. For example, if children with filaggrin mutations were to develop skin barrier impairment prior to clinical eczema and allergic sensitisation and given the increased allergic respiratory disease risk in children with atopy, intensive emollient therapy which prevents skin barrier breakdown could help to prevent not only eczema development but also allergic sensitisation and through this later allergic respiratory disease.54
Where do we go from here?ISAAC provides a unique cross-sectional data set to study potential risk factors for eczema development. Much of the collected ISAAC data remains to be explored, such as information on the management of allergic disease in different countries and risk factor analyses to explain urban versus rural prevalence and severity gradients between ISAAC centres in the same country. However, it is important to recognise the limitations of cross-sectional study designs, which cannot be compensated by size and resulting statistical power. Only carefully conducted longitudinal and intervention studies can ultimately provide definitive answers to the many remaining questions related to eczema aetiology.
Conflict of interestNone.
FundingCF is funded by a Clinician Scientist Award from the UK National Institute for Health Research (NIHR). The views expressed in this publication are those of the author and not necessarily those of the UK National Health Service, the National Institute for Health Research or the UK Department of Health.
This article was originally published in German in “Allergologie” and has been reproduced in English with the kind permission of Dustri-Verlag Dr. Karl Feistle GmbH & Co KG, Munich, Germany. CF is a member of the ISAAC Steering Committee.