Atopic eczema affects 5–10% of the Spanish paediatric population, and has increased in frequency over the last few decades, probably due to changes in the environment and lifestyle. Phase II of the ISAAC (International Study of Asthma and Allergies in Childhood) uses a standardised methodology to establish the prevalence of allergic disorders and factors linked to them in each centre.
ObjectivesTo assess the prevalence and severity of atopic eczema, and to establish factors linked to atopic eczema in 10–11 year-old school children in the city of Almeria (South-East coast of Spain).
Material and methodsAn ecological study was carried out as part of ISAAC II, using homologated questionnaires and allergic tests in 1143 schoolchildren. Statistic association was assessed by means of χ2 test, and then logistic regression analysis was performed with the most significant variables from the univariant analysis.
ResultsThe prevalence of atopic eczema was 11.4%. The risk factors found in the multiple logistic regression analysis were: personal antecedents of severe asthma (OR 19 CI 95% 1.35–266) and severe rhinitis (OR 7.7 CI 95% 1.79–33), fungi in bedroom during the first year of life (OR 4.2 CI 95% 1.17–15.1) and atopic eczema in one parent (OR 5.2 CI 95% 2.69–10.1).
ConclusionsThe prevalence of atopic eczema is similar to that found in other studies within ISAAC Phase I. The most important risk factors for atopic eczema are family and personal history of other atopic diseases and the presence of fungi in the home.
In recent decades there has been a global increase in allergic diseases.1 Specifically, a continuous increasing of eczema and eczema medication has been reported in developed countries.2 However, variability in the methodology and the diagnostic criteria used in the numerous studies on prevalence of allergic disorders complicate their comparison.
The ISAAC3 (International Study of Asthma and Allergies in Childhood) was created in 1991 with the aim of establishing and comparing the prevalence of asthmaand other allergic disorders in each country and to study tendencies in allergies over time, using a standardised methodology. To this effect, the study used a questionnaire comprising simple questions, aiming to prevent the reported differences from being attributable to variation of methodology and diagnostic criteria. As a result of various studies based on ISAAC, the incidence of atopic eczema (AE) in the infant population can be calculated at 15–20%, showing an increase in prevalence.4 Like asthma and rhinoconjunctivitis, which it often precedes or with which it coexists, atopic eczema has important social and economic consequences.
Assessment of children with AE proves difficult due to its fluctuating nature and the existence of different corporal distribution patterns according to age. In consequence, it is difficult to predict evolution of the disease in a single examination. For this reason, a universal, simple and efficient method to assess AE has not yet been established, and given that neither specific lesions nor laboratory diagnostic or predictive markers exist, diagnosis is based on clinical examination and at present there are no internationally accepted criteria for use in epidemiologic studies, all of which explains why the aetiology and physiopathology of AE have not been studied as much as those of asthma and rhinitis. The criteria of Hanifin and Rajka5 must be used by specialist personnel and are not useful in epidemiological studies. In 1994 the UK Working Party6 proposed some simpler diagnostic criteria which were more operative and validatable, becoming the choice for prevalence studies as they can be identified by personnel with basic training. However, some authors consider that they are not suitable for epidemiological studies or for clinical practice because although the specificity is elevated (over 90% in all studies) the sensibility is low (10–88% in different populations).7
Different factors, such as high levels of IgE and eosinophilia and the coexistence with asthma and/or rhinitis support the considering of AE as an allergic disease. Moreover, association has been observed between severity (using the SCORAD method) and levels of IgE and sensitivity to pneumo-allergens and food.8 However, although allergy is a common denominator it is not an essential requirement for disease development, in which factors such as scratching and microbial colonisation of the skin play a key role. In this way, it is relatively frequent to find children with AE who present positive cutaneous tests to food or aeroallergens, even before entering into contact with them or after having gained tolerance to them, with test positivity being a simple associated epiphenomenon. Indeed, the role of allergens in atopic eczema is controversial, with some authors even doubting the allergic nature of the disease.9 The suggestion that AE can be divided into two types, atopic and non-atopic10, has few practical consequences: on the one hand, there is no phenotype differentiation between them and, on the other, the presence of sensitisation depends on age and on the criteria used to establish test positivity, together with the number and quality of allergen extracts tested.
In this study, we aim to describe the prevalence and severity of atopic eczema in 10- and 11-year-old children from Almeria (South-east Spain), and investigate the factors associated with the presence of eczema in this patient population.
Population and methodsIn the spring and autumn of 2001 a cross-sectional study was made to analyse the relationships between different environmental conditions, personal and familial factors, and the frequency of atopic eczema (AE). After approval of the study had been obtained from the Clinical Research Ethics Committee of Torrecárdenas Hospital, as well as from the Regional Health and Education Authority, a questionnaire was distributed together with the parental authorisation form to 2293 schoolchildren aged 10–11 years in the 29 state schools in the city of Almeria, with a final study population of 1143 (49.8%) children (Figure 1).
The methodology used was that of the ISAAC project in its Phase II, in which the parents completed homologated questionnaires on bronchial, nasal and skin symptoms manifesting in the previous year and at other times in the past. Questions were also included relating to the use of treatments and healthcare services.
After obtaining informed consent, physical examination was performed to find eczemas in any of the characteristic body locations and skin prick tests were made to establish the presence of allergic sensitisation. The battery of test allergens included the following reagents (ALK-Abelló®, Madrid, Spain): Dermatophagoides pteronyssinus, Dermatophagoides farinae, cat dander, Alternaria, grass pollen mixture (Dactylis glomerata, Lolium perenne, Festuca pratensis, Poa pratensis, Phleum pratense and Avena eliator), tree pollen mixture (Betula verrucosa, Alnus glutinosa and Corylus avellana), positive control (histamine) and negative control (saline solution). For the present study, atopy was taken to be synonymous of allergic sensitisation, the latter being understood as representing positivity to at least one allergen, considered as being a wheal 3mm or greater than the negative control.
The SPSS® version 12.0 statistical package for Microsoft Windows® was used for data analysis. Calculations were made of the prevalence of symptoms and the frequency of the rest of the variables based on the ratio between the number of positive responses and the number of questionnaires completed for each question. The chi-squared test (χ2) was used to evaluate the association between the dependent variables and dichotomic variables, while logistic regression analysis was used to assess the association with polychotomic variables – defining which factors are correlated to atopic eczema (assigning risk or protective character). Statistical significance of associations was accepted for p<0.05. Later, unconditional multiple logistic regression analysis was made with all the variables yielding p<0.20.
Definition of atopic eczemaAtopic eczema was considered when a positive response to the question “Has your son/daughter had a rash at any time affecting any of the following places, knees, elbows, neck, ears or eyes?” was obtained.
ResultsA total of 1143/2293 (49.8%) schoolchildren participated in the study with a mean and median age of 10.7 years (95% of the sample between 9.50 and 11.85 years) and a slight male predominance (52%), and 98.7% were Spanish children. Ninety percent weighed over 2500g at birth, and 71.8% started breastfeeding at birth (maintained for over 6 months in 28.2% of the cases) and 74.7% began intake of solid food before the age of 4 months.
The majority (69.4%) of the families included in the sample live at present in urban or suburban areas with few gardens (73.3% during the first year of life). One-half of the children had siblings (median one), and 77% attended nursery school. The families were seen to acquire pets over the years as the children grew older. During the first year of life and currently, the most frequent domestic animals are birds (14.2% vs. 26.2%), followed by dogs (10% vs. 23%) and cats (3.3% vs. 9.8%). At the time of the study, 43% of the children came into contact with dogs outside the home at least once a week, and 18% with cats.
At the time of the response, 60% of the children were exposed to tobacco smoke in the home and 46.6% of the mothers were smokers (39% smoked during the first year of life of the child, and 19% did so during pregnancy). Smoking was abandoned in 14.8% of the homes due to the child's problems with allergy or asthma and this and other changes in the home were made when the child was between 3 and 6 years of age.
Gas was the most commonly used cooking fuel (94.4%) during the first year of life; this percentage is currently decreasing as a result of the introduction of other energy sources, specifically electricity, which is also the main energy source used for home heating purposes. The great majority of bedrooms have single glazing and 80.8% have no carpeting, with predominance of synthetic fibres in bedding. At present, 5% of the homes presented damp spots, and 1.5% had visible fungal presence on the walls or ceilings (4.5% and 10.5%, respectively during the first year of life of the children).
The children eat fruit, fresh and cooked vegetables more than three days a week: 67%, 51.9% y 22.5% respectively; fish 28.2% and meat 62.8%. On the other hand, 33.7% consume hamburgers, 55.5% fizzy drinks, 62.3% factory baked goods and 70.4% sweets more than once a week. 78.3% of the children do physical exercise outside school at least 2 days a week.
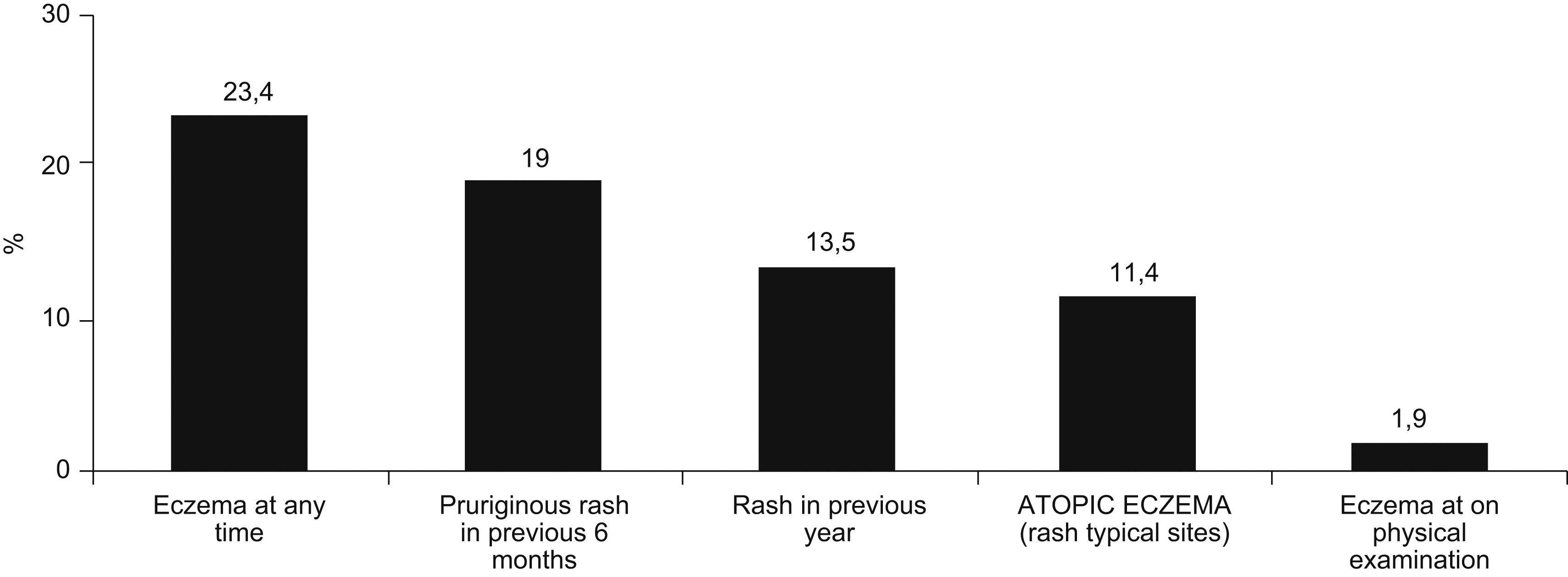
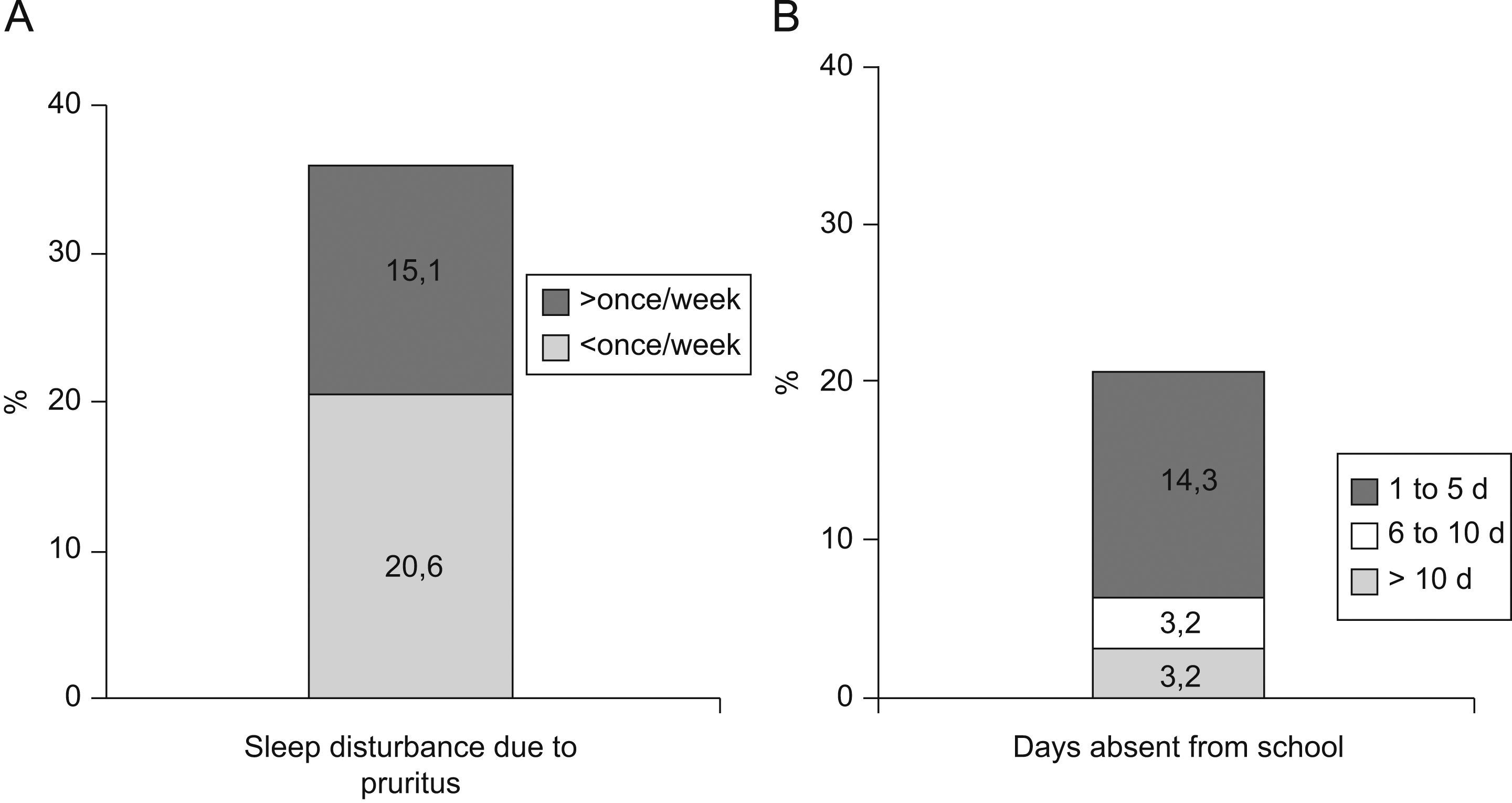
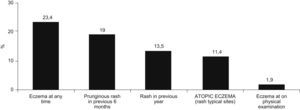
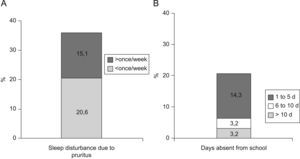
The prevalence of atopic eczema was 11.4% (126/1106). Affirmative answers to the questions “Has your son/daughter had pruriginous skin eruptions during the last year?” and “Has your son/daughter ever had eczema?” (equivalent to prevalence of eczema at present and medical diagnosis of atopic eczema respectively) were given by 13.5% and 23.4% of parents, respectively. In 50% of cases atopic eczema was present before the age of four years, with complete remission during the previous year in 79% of these. In 35.7% of the children presenting atopic eczema the lesions were sufficiently severe to disturb sleep occasionally and 20.7% lost at least one day of school attendance during the previous year. Only in 20/1077 cases (1.85%) eczemas were found in any of the characteristic sites at the moment of examination. The prevalences of asthma, rhinitis and atopic eczema were 9.3%, 14.9%, and 16.7% among mothers; and 5.8%, 10%, and 10.6% among fathers, respectively (Figure 2).
Association was found between AE and absence of breast-feeding (OR 2.4; CI 95% 1.29–4.53; p=0.006) or breast- feeding for less than 6 months (OR 1.95; CI 95% 1.07–3.5 p=0.029). The age when solid foods were introduced did not influence the presence of AE. We did not find association with nursery school attendance, the existence of siblings, a history of measles, whooping cough or tuberculosis nor with vaccination against these diseases. However, we found a higher risk of AE (OR 1.6; CI 95% 1.05–2.42; p=0.02) in children who had had intestinal parasites in the past. Atopy, understood to be any positivity in the skin prick test, is a risk factor for AE, (OR 1.5; CI 95% 1.06–2.28; p=0.022), likewise wheezing during the previous year (OR 2.43; CI 95% 1.57–3.74; p<0.001), severe wheezing – which causes difficulty in speaking (OR 3.33; CI 95% 1.5–7.41; p=0.002) – and wheezing when exercising (OR 2.1; CI 95% 1.27–3.68; p=0.004). AE is associated to rhinitis (OR 1.98; CI 95% 1.36–2.88; p<0.001) and rhinoconjunctivitis (OR 2.5; CI 95% 1.33–4.68; p=0.003) during the previous year, with a greater risk in the case of medical diagnosis of allergic rhinitis (OR 2.95; CI 95% 1.96–4.46; p<0.001) or severe rhinitis (which disturbs daily activities OR 2.51–4.49, depending on the frequency of these interruptions).
Risk factors for AE are parental history of allergy, both maternal (OR 2 CI 95% 1.37–2.93; p<0.001) and paternal (OR 2.2 CI 95% 1.46–3.29 p<0.001), particularly atopic eczema (OR 2.98 CI 95% 1.97–4.5 p<0.001 and OR 2.47 CI 95% 1.52–4 p<0.001, respectively). The presence of atopic eczema in either parent is the variable related to family antecedents with most influence on the presence of AE in the children in this study (OR 3.46; CI 95% 2.35–5.07; p<0.001).
The domestic risk factors were the existence of visible fungi during the first year of life (OR 2.59; CI 95% 1.24–5.41 p=0.009) and current electric cooking facilities (OR 1.6; CI 95% 1.11–2.38; p=0.012) while the current use of gas for cooking (OR 0.56 CI 95% 0.38–0.83; p=0.004) was the only protective factor.
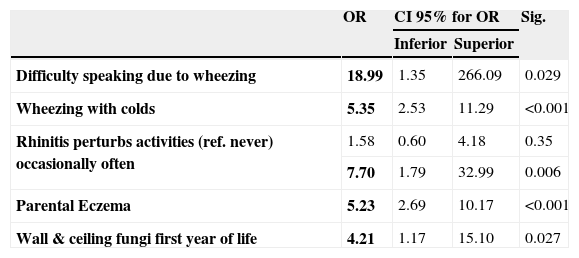
Following univariant analysis, non–conditional logistic regression for atopic eczema was made (Table 1) with all the variables exhibiting statistical significance (p< 0.20). In the resulting model, five variables were found to be associated with AE in the children from Almeria: severe asthma (OR 19 CI 95% 1.35–266; p=0.029) severe rhinitis (OR 7.7 CI 95% 1.79–33; p=0.006), presence of wheezing associated with colds (OR 5.3 CI 95% 2.53–11.29; p<0.001), eczema in a parent (OR 5.2 CI 95% 2.69–10.1; p<0.001) and fungi on walls or ceilings during the first year of life (OR 4.2 CI 95% 1.17–15.1; p=0.027). Surprisingly, atopy (a risk factor in the Chi 2 analysis) disappears as an associated factor in the multiple logistic regression analysis.
Multiple Logistic Regression Model for factors linked to Atopic Eczema
| OR | CI 95% for OR | Sig. | ||
| Inferior | Superior | |||
| Difficulty speaking due to wheezing | 18.99 | 1.35 | 266.09 | 0.029 |
| Wheezing with colds | 5.35 | 2.53 | 11.29 | <0.001 |
| Rhinitis perturbs activities (ref. never) occasionally often | 1.58 | 0.60 | 4.18 | 0.35 |
| 7.70 | 1.79 | 32.99 | 0.006 | |
| Parental Eczema | 5.23 | 2.69 | 10.17 | <0.001 |
| Wall & ceiling fungi first year of life | 4.21 | 1.17 | 15.10 | 0.027 |
OR indicates odds ratio; CI confidence interval; Sig. statistical significance (p value)
The overall participation rate was 49.8%. The main reasons for non-participation were doubts about the aim of the study, rejection to skin prick or blood test or questionnaire completion (90%), children out of age range (8%); and refusal of the pupils (2%). Surveys conducted in a school framework run the risk of participation bias, with those parents with family or personal history of allergic diseases being more prone to sign a consent, justifying a larger prevalence of atopy and atopic diseases. Another factor affecting selection bias is that repeated use of the schools to perform socio-sanitary programs (Almeria had already participated in ISAAC I) could predispose the parents to a non-collaborative attitude.
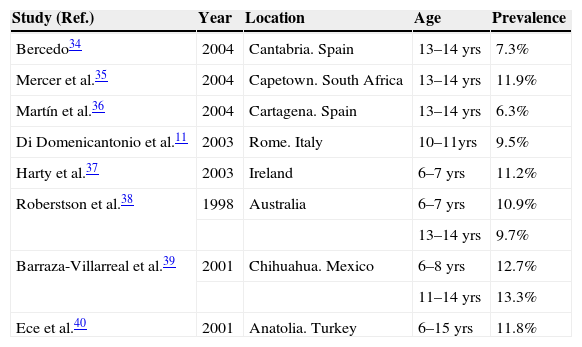
In our study the prevalence of atopic eczema was found to be 11.4% (126/1106); similar findings to those of studies with ISAAC methodology (Table 2). The coincidence with prevalence figures in adolescents could be due to the fact that AE tends to remit or disappear between the ages of 5 and 10 years in many cases, with constant prevalence from the latter age on. The disease is characteristic of early periods of infancy and normally has a favourable evolution (79% of parents answered that their children's eczema had remitted completely), which could explain the greater prevalence of prior medical diagnosis of atopic eczema (23.4%). The percentage of flexural eczemas at the moment of examination was similar to the findings of another study in Rome within ISAAC Phase II.11
Prevalence of atopic eczema in children in different countries
| Study (Ref.) | Year | Location | Age | Prevalence |
| Bercedo34 | 2004 | Cantabria. Spain | 13–14 yrs | 7.3% |
| Mercer et al.35 | 2004 | Capetown. South Africa | 13–14 yrs | 11.9% |
| Martín et al.36 | 2004 | Cartagena. Spain | 13–14 yrs | 6.3% |
| Di Domenicantonio et al.11 | 2003 | Rome. Italy | 10–11yrs | 9.5% |
| Harty et al.37 | 2003 | Ireland | 6–7 yrs | 11.2% |
| Roberstson et al.38 | 1998 | Australia | 6–7 yrs | 10.9% |
| 13–14 yrs | 9.7% | |||
| Barraza-Villarreal et al.39 | 2001 | Chihuahua. Mexico | 6–8 yrs | 12.7% |
| 11–14 yrs | 13.3% | |||
| Ece et al.40 | 2001 | Anatolia. Turkey | 6–15 yrs | 11.8% |
In our study, gender did not influence atopic eczema significantly, although association with prevalence of allergic sensitisation exists, which was greater in males (data not shown). A study on 3000 British prepubertal children showed that the prevalence of asthma and rhinitis symptoms was greater in males, but observed no difference for eczema.12 On the other hand, greater prevalence of rhinitis and atopic eczema in females has been observed in 13 and 14-year-old Spanish adolescents.13 This suggests the implication of hormonal factors on the presence of allergic disorders.
There was no significant association between birth-weight and AE. A lower prevalence of rhinitis and atopic eczema has been observed at the age of one year in ex-premature babies of very low weight at birth.14 Given that Th2 predominance during pregnancy gives protection against premature births, allergy could constitute an evolutionary benefit.15
We have found that schoolchildren who were breast-fed for less than 6 months had a greater risk of atopic eczema, in coincidence with the results of Kramer et al.16 In contrast, Bergmann et al.17 have found breast-feeding to be associated with a greater risk of AE during the first 7 years of life (3% more for each additional month), even after adjustment for possible confounding factors. Caution is required in interpreting studies which report a greater prevalence of allergy in children who have received prolonged breast-feeding, since such data can be explained by inverse causality, that is to say, such children could have an important familial burden of atopy or an early start of atopic eczema, with mothers more aware of the protective factor of breast-feeding, who therefore tend to prolong it as much as possible.18
We have not found association between the age of introduction of solid foods and the existence of atopic eczema. Some authors consider the introduction before the age of 4 months to constitute a greater risk of AE at the ages of 2,3 and 10 years19,20, increasing this association in relation to the number of foods introduced early. A meta-analysis found that the introduction of solid foods before the age of 4 months increases the risk factor for eczema, but there is a lack of data to support association with other allergic disorders.21 The intake of elevated doses of alimentary antigens during the first year of life is inductive to tolerance, while small, intermittent doses can lead to sensitisation in atopic children. For this reason, measures that recommend the avoidance of the early introduction of solid foods are being reconsidered. An interesting hypothesis suggests that sensitisation may be produced by environmental paths through eczematous lesions, and that early, intensive treatment of AE could curb the probability of allergic sensitisation.22–24
In addition, we have not found significant association between the presence of atopic eczema and nursery school attendance or siblings, coinciding with the study of Ponsonby et al.25 who on the other hand found an inversely proportional relationship between the number of siblings and asthma and rhinitis rates. We did not find association with measles, whooping cough and tuberculosis, or with vaccination against these diseases.
Children who have suffered intestinal parasitation in the past (though the type is not specified) have a higher risk of AE. While it is true that parasite infections appear to give protection against allergic disorders in developed countries26, such protection may derive from the coexistence in these populations of other protective factors against allergic reactions that could act as confounding factors.
The prevalence of allergic diseases in the parents is lower than in their children, except for atopic eczema, where 16.7% of the mothers have AE vs. 11.4% of the children. This leads us to consider that the mothers could interpret other skin problems (contact dermatitis, irritant contact dermatitis, xerosis) as atopic eczema. The greater prevalence of allergic disorders among the mothers could be due to the fact that it is generally the mothers who complete the questionnaires (81.3%). They may give more consideration to their own symptoms and undervalue those of the fathers, or there may be different thresholds for perception and communication of symptoms in men and women. This could also be explained by the fact that oestrogens are proinflammatory hormones while the male steroids are immune suppressors.27 Parental history of allergopathologies in general and of AE in particular constitutes a risk factor for the children in the study. Of the variables analysed belonging to the family history group, we found that the existence of eczemas in the father or mother had greater influence on AE, which supports the role of genetic influence on the disease.
We did not find a statistically significant association between the location of the home (current or during the first year of life) and the presence of AE. There was an association between the presence of fungi patches on the walls and ceiling during the first year of life and AE. This could be related to greater exposure to house dust mites and to the coexistence of AE with asthma and/or rhinitis, which are also influenced by these factors. Cooking with gas constitutes a protective factor while cooking with electricity is a risk factor. The possibility of confounding factors must be considered as while, nowadays, the use of vitro-ceramic surfaces is accessible to a great number of families; it was associated with a higher socioeconomic level some years ago. This has been considered a possible risk factor for allergic diseases, atopic eczema among them.28,29
We have not found any relation between atopic eczema and exposure to any animal in the past or present, neither to eating habits or sports.
In accordance with the results, passive smoking is not a risk factor for AE. Although the relationship between smoking and asthma is obvious30, association with the other allergic diseases is controversial. Some authors consider there is a greater risk of atopic eczema31, while other studies find a lower risk of rhinitis and atopic eczema between smokers and their children.12,32.
Atopy does not appear as an associated factor in the logistic regression model. This finding is in agreement with the conclusions of a recent article framed into ISAAC II, about the role of atopic sensitisation in flexural eczema.33
One of the limitations of the study is the low-response rate, contributing to a selection bias by which families with atopic antecedents are more willing to enrol on the survey and to complete the questionnaires more accurately, perhaps leading to an overestimation of the atopic eczema prevalence. Another important concern is that the cross-sectional design does not permit to infer causality from the associated factors. The strength of this study is to be framed within ISAAC Phase II, and the observed associations between AE and some variables are consistent with the current body of clinical knowledge.
ConclusionsThe prevalence of atopic eczema in schoolchildren from Almeria is similar to that of other centres, with the following risk factors: personal and family history of allergic diseases and the presence of fungi on walls during the first year of life. Atopy (a risk factor in the Chi 2 analysis) does not constitute an associated factor in the logistic regression model, which suggests that atopic eczema is influenced by other non-atopic components.
Conflict of interestNone of the authors declare conflict of interest in relation to this work.