ALLERGOL. ET IMMUNOPATHOL., 1998;26(1):27-33
SPECIAL
Novel approaches to immunotherapy: epitopes, determinants, activators, or modulators?
L. Berrens
CBF. Leti, S.A. Madrid. Spain.
Conferencia pronunciada en el Congreso Interasma''97, Las Palmas, 3-5 diciembre 1997. Abstract publicado con motivo del congreso en J Invest Alleg Clin Immunol 1997;7/5:360-1.
SUMMARY
The immunological mechanism through which immunotherapy (IT) acts is not certainly known. The participation of the so-called "blocking antibodies" has not been proved and how it intervenes in the regulation of the production of IgE is still to be cleared, as it affects the action of lymphocytes Th1 and Th2. Nowadays, IT is based in the concept that the allergic reaction is somehow an antigen (allergen)- antibody (reagin) reaction. The possible modifications of IT are also based on the possibility to interfere in the antigen-antibody interaction.
It has been proved in vitro that aqueous extracts of some allergens produce the consumption of complement, by its usual via, in which the C1 component is involved, whithout the mediation of antibodies, generating anaphylotoxin C3a, which is a powerful releaser of histamine, as well as C3b, which participates in the regulation of the cellular immunitary system.
The term atopen should therefore be used when referring to the unspecific activation or adjuvant activity of antigenically different allergens, due to their common structural or functional characteristics. The term allergen should then be used when describing those structural traits of the carrier molecule, which preferently produces the induction, and possible recognition of IgE antibodies. The action atopen and allergen as separate characteristics of a same molecule could theoretically become important in the future of therapeutics.
The receptor of membrane CD21 for C3b, in B cells is a link for lectine CD23 of lymphocytes B, which has been identified with the low affinity receptor IgE (Fce RII-CD23). In summary, the basic defect in atopy may reside in a defect of the receptor portion of reagin for atopen, but not in IgE. This means that in the nearby future, this fact should be taken into account in the search of drugs which participate in this kind of activation of allergic reaction, trying to modify or modulate the low affinity receptor IgE or even the high affinity receptor IgE.
Key words: Allergen. Atopen. Immunotherapy. IgE. Receptor IgE. Immunomodulation.
Allergol et Immunopathol 1998;26:27-33.
INTRODUCTION
The first question that comes to order in the context of "Novel Approaches to Immunotherapy of Allergic Diseases" is of course: What is Immunotherapy? The most recent definition of the WHO-Position Paper on Allergen Immunotherapy (1), Draft 7, Geneva, January 27-29, 1997 reads as follows: "Allergen immunotherapy consists of administering gradually increasing quantities of an allergen vaccine to an allergic subject up to a dose effective to ameliorate the symptoms associated with the subsequent exposure to the causative allergen."
The most striking point about this definition is the use of the term "vaccine", which in the field of allergy is quite uncommon and which has only rarely and mistakenly been used in the past. The WHO report defines vaccines as follows:
"Vaccines (allergen extracts) which modify or down-regulate the immune response for allergic disease are part of the broad-based category of therapies currently utilized and being developed to treat other immunologic and infectious diseases."
This looks like a deliberate attempt to bring allergen immunotherapy, for which desensitization or hyposensitization used to be the terms of choice, into the acknowledged fields of immunology and classical vaccine development for infectious diseases, based on a few novel but unproven ideas about the mechanism of action of allergenic extracts in man. But allergy is not an infectious disease, for which vaccines are commonly used, and although almost everyone nowadays strongly believes allergy to be an immunological disease, many dermatologists and chest physicians continue to remain sceptical. However this may be, it should be clear to everyone that the administration of allergenic extracts for immunotherapy certainly is not an inoculation, nor a regular immunization comparable to the situation with bacterial and viral vaccines. Allergen immunotherapy in actual fact looks like a reversed form of homoeopathy, or at the very best some form of tolerance induction, be it immunological or toxicological. The truth is that nobody really knows how it works, as voiced by a recognized authority in the field:
"For severe cases, immunotherapy (also called allergy shots or desensitization), which was introduced in 1911, may provide relief... I wish I could describe how immunotherapy confers resistance to an allergen, but no one has put forward a definitive explanation" (2).
This situation, namely that we do not really know how allergen immunotherapy works, has persisted since the days of Noon and Freeman in 1911. It is also precisely the reason why allergen standardization has never been properly achieved, because we have in actual fact not yet succeeded in identifying the molecular nature of the active principle or principles in allergenic extracts, even though most people seem to be happy with the description of so called "major" IgE-binding antigens. But the fact of the matter is that very little progress has been made since 1911 in allergen immunotherapy, apart from some novel administration routes like oral, sublingual or nasal formulations. In terms of fundamental innovation, however, and in spite of a few fascinating new ideas about the possible mechanisms of immunotherapy, there is massive speculation but very little hard evidence. What is needed first and foremost is a critical appraisal of the basic issues in allergy. After all, only a few mechanistic explanations have ever been offered for the beneficial effects of allergen immunotherapy, one of these being the induction of the socalled "blocking antiboides". I will not dwell on this point, because what Sherman said in 1957 is still valid today: "If the blocking antibody is the protective factor, the fact still remains to be proved" (3), although of course the confusion has grown considerably due to the unresolved question of the precise role of IgG and IgG4 immunoglobulins as being either "anaphylactic" o "blocking" antibodies.
The other explanation, now widely favoured, is based on extrapolations from experiments in mice on the balance between CD4+ T-lymphocytes of the Th1 and Th2 types and their corresponding cytokine profiles. It is now widely thought that the effect of immunotherapy is on T-cell responses to the allergen. In this option, it is considered that the down-regulation of Th2-responses or an increase of Th1-response could lead to a shift in the cytokine pattern of Interleukin4-/Interferon-* secretion, thereby causing an allergen-specific Th2 "anergy" or "tolerance". I will not enter too deeply into this, because the matter has been covered in several excellent review articles by the protagonists themselves. Briefly, the induction of an immune response to foreign antigens requires the cooperation of various categories of cells, first and foremost the "antigen-presenting cells" (APC) and the T- and B-lymphocyte populations. The first signal, namely between APCs and T-lymphocytes, is composed of antigenic peptide fragments which arise during the intracellular processing of a foreign antigen internalized by APCs, e.g. macrophages. These antigenic fragments or "T-cell epitopes" are subsequently recycled to the outer membranes of the APC where they then become exposed in the form of ligands associated with membrane-bound Class II antigens of the Major Histocompatibility Complex. From among the populations of T-(helper) lymphocytes recognizing such T-cell epitopes on APCs and cooperating by themselves with immunoglobulin-secreting B-lymphocytes, activation of the allergen-specific Th2-cell clones in particular is considered to exercise a pivotal role in the development of human atopic diseases. Such activated Th2-cells produce relatively large amounts of the interleukins-4 and -5, which act as cytokine signals promoting IgE biosyn-thesis by B-lymphocytes. Based on this, a popular theory of "immediate Type I" allergy purports that in atopic patients there occurs a relative preponderance of Th2 over Th1 lymphocytes, resulting in an increased IgE synthesis, and that active therapy should therefore aim at restoring the balance of allergen-specific T-cell activity (4).
The interaction between APCs and Th-cells requires co-stimulatory factors involving several membrane receptors. Evidence also exists that if only the first signal, i.e. T-cell epitope recognition takes place, T-cell anergy may result. Hence, T-cell anergy can be induced in allergen-specific Th2 cell clones following stimulation with allergen-derived peptides. Such anergic Th-cells fail to provide help to IgE-producing B-cells, leading to down-regulation of IgE levels. Deliberate manipulation of allergen-specific T-cell clones by way of T-cell epitopes derived from allergenic molecules thus suggests itself as a possible new approach to successful immunotherapy, with the restriction that such epitopes should not bind, nor induce allergen-specific IgE antibodies. This idea for new therapies in allergy has been expressed as follows: "The future course of specific immunotherapy as treatment for allergic diseases will to a large extent be dependent on the results of clinical trials that use synthetic peptides, recombinant allergens, degraded allergenic extracts, or modified allergens that selectively tolerize Th2-type or activate Th0-type or Th1-type responses to allergens" (4).
Unfortunately, recent clinical trials with some synthetic T-cell peptide epitopes structurally defined from the amino acid sequence of selected IgE-binding "major" antigens and applied in huge doses have so far failed to achieve the desired beneficial effects. Also, there is a serious flaw here: current opinion maintains that T-cell epitopes are peptides of linear amino acid sequence which once identified may be produced synthetically. But this point of view wholly negates that naturally occurring allergens most often carry post-translational side-chains composed of carbohydrates or other organic compounds conjugated chemically to the protein backbones. Such chemically conjugated structures or "haptens" often represent immunodominant sites which are retained on physiologically produced fragments of allergens, but are absent from synthetic peptides or recombinant allergen transcripts. In the nearby future one would therefore have to use mixtures of fragments produced from the natural allergens.
There are several other new approaches to allergen immunotherapy, some of which are listed in table I.
| Table I |
Some novel approaches to allergen immunotherapy |
| * Mixtures of allergenic extracts and human allergic serum ("immune complexes"). |
| * Individual "major" allergens. |
| * Recombinant whole allergens, or recombinant/ synthetic T-cell stimulating peptides. |
| * Non-anaphylactic allergens or hypoallergenic isoforms of major allergens, which lack IgE-binding epitopes but retain some T-cell stimulating peptide structures, produced by site-directed mutagenesis in vitro. |
| * Non-IgE-binding (monovalent) "haptens" from immunodominant IgE-binding peptides defined by epitope mapping, in an attempt to passively saturate effector cells. |
| * Immunization with plasmid DNA encoding for allergens with the aim of modulating the cytokine production profiles of allergen-specific T-cells. |
| * Recombinant allergen-specific human IgG-F(ab'')2 fragments, or the corresponding parts of humanized mouse monoclonal antibodies, for passive abrogation of Type I allergy. |
| * Humanized anti IgE monoclonal antibodies, or "IgE-mimotopes". |
It is worthy of note that these ideas all represent immunological approaches, based on attempts to interfere with the allergen-antibody interaction, whereby the allergens are considered no different from normal antigens (5, 6). However, this presumption neither explains why allergens specifically induce the formation of antibodies of the IgE-isotype (in tandem with IgG-antibodies), nor does it help to understand why it is only a small segment of the human population that produces elevated levels of such IgE-antibodies. Obviously, additional factors must be considered. One of these ancillary factors concerns the complement system and its activation by by-standing components in allergenic extracts.
It has indeed long been known that aqueous extracts of allergenic source materials consume haemolytic complement in dilute human serum, but not in guinea pig serum. Careful studies in several institutes have confirmed that the mechanism of complement activation by particular components in allergenic extracts mainly involves the components C1 of the classical pathway, but it is not mediated by antibodies. It has also been shown that the local activation of complement by activators in allergenic extracts may lead to the generation of the powerful histamine-liberating anaphylatoxin C3a and the fragment C3b, a key factor in the regulation of the cellular immune system. Next, chemical studies of numerous allergenic extracts uncovered the almost universal presence of organic decomposition products, which include > 10 kDa Maillard-type reaction products of proteins or peptides with carbohydrates (i.e. melanoidins) on the one hand, as well as of soluble melanins in extracts of house dust and animal danders and, more particularly, of flavonoid-type condensed tannins in extracts of pollens on the other. These represent the components responsible for in vitro complement activation and partly also for the in vivo positive skin reactions in atopic allergic subjects. These complement activators are not identical to the major IgE-binding allergens, but they may occur in firm physical association with them. All allergenic extracts thus contain complement activators, but to a differing degree (7).
This observation provides is a very good explanation for recent observations showing that many wellknown allergens and compounds in allergenic extracts will trigger mast cell mediator secretion even in the absence of IgE sensitization. It has furthermore been demonstrated that complement in the serum of "atopic" individuals tends to be more sensitive to activating factors than observed with the sera of healthy control persons. It has also recently been shown in the case fo ragweed pollinosis that this serum susceptibility to complement activation is better related to the degree of skin test sensitivity than the immunological parameter of specific IgE-binding (8).
The problem now becomes how to integrate these various findings. To answer this question we will have to return to the very foundations of allergy. In the first quarter of this century, the understanding of allergy was founded on the presence of unidentified eliciting substances termed "allergens" in aqueous extracts of environmental substrates, and on the occurrence in the patients'' blood serum of a specific receptor protein termed "reagin" which could be passively transferred to the skin of a normal recipient and was thought to be an antibody (although it did not exhibit the physcicochemical and serological properties of the then known classical antibodies). The theoretical basis of allergy therefore comprised one equation with two unknown variables, which by definition cannot be solved.
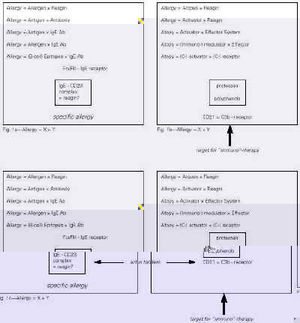
What happened historically is that reagins became subsequently and unconditionally defined as antibodies, albeit of a special nature. With this parameter fixed, the scene was set for regarding allergens as antigens in a specific and immunological context, which in the course of time developed into the present-day concept of specific allergy as being based on the interaction of IgE-antibodies with their eliciting allergens, as shown in figure 1a.
If, on the other hand, the identity of reagin is left open and undecided, for example as some sort of mediator protein or even an entire physiological mediator system, the search for the nature of the allergenic modulator or activator can proceed in an unbiased fashion. Historically, this approach has led to a concept of allergy as being founded on an intrinsic (or "atopic") lability of some physiological mediator system, i.e. on a mechanism of non-specific hyperreactivity as delineated in figure 1b.
Considerations of this kind have led to an early appeal for re-introduction of the term "atopen" at the INTERASMA Congress in 1975: "The term ''atopen'' should therefore be reintroduced when describing the nonspecific activating or adjuvant activity of antigenically distinct allergens by common structural or functional characteristics. The term ''allergen'' should then be reserved for describing those structural features of the carrier molecule which give rise to the preferential induction of, and possible recognition by, antibody of the IgE class. The segregation of an atopen aspect and an allergen aspect as separate characteristics of a single molecule carries fundamental theoretical implications. It implies that the recognition partner for allergen, i.e. (IgE) antibody, might not be identical to the receptor for atopen" (9).
At the present moment in time, both lines of thought are of course wholly valid and open to experimental verification. Indeed, there now exists a situation in which both mechanisms may co-exist, i.e. atopic allergy in specific immunological mode based on a non-specific intrinsic hyperreactivity as a manifestation of an underlying genetic abnormality, as summarized in figure 1c.
According to current theory the exposure of human beings to environmental allergens causes the formation of allergen-specific antibodies of the IgG- (and IgM-) isotype. Within an immunological context, allergens may therefore be regarded as ordinary IgG-antibody inducing foreign antigens. However, for predisposed "atopic" individuals, these antigens are endowed with the ancillary property either due to special molecular characteristics or to the simultaneous presence in natural allergenic substrates of appropriate modulators to additionally promote the biosynthesis of antibodies of the IgE class.
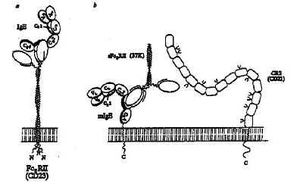
Modern insights into what happens biochemically at the cell membrane nowadays allow us to carry the integration a little further. Agents like C3b derived from the complement system activated by atopens truly exhibit adjuvant properties known to enhance the immune response to specific antigens, including the IgE response. The incriminated C3b outer membrane receptor CD21 on B-cells is a ligand for the lectin CD23 on B-lymphocytes, which in its turn has been identified as the low-affinity IgE (Fc*)-receptor, as shown schematically in figure 2 taken from Sutton and Gould (10).
Although the details of the chain of T-cell / B-cell and complement receptor interactions are far from resolved, it is already evident that the stimuli for allergen-specific IgE antibody synthesis and for non-allergen-specific complement activation must in some way be linked. Investigators actively engaged in the study of these associations have in fact shown that CD23 can be split directly by extraneous proteolytic action, for example by the enzyme antigens Der p 1 and Der p 3 from house dust mites, thereby releasing the soluble lectin domain sCD23 that can still bind IgE. Indeed, the rather attractive suggestion has been made that an appropriate future treatment for allergy might be based on inhibition of extrinsic or intrinsic proteolytic enzymes capable of cleaving off sCD23 from the low-affinity IgE-receptor (10), or by peptide inhibition of the receptor-IgE interaction (11).
In summary, the basic defect in atopy or hyperreactivity may reside in the receptor portion of reagin for atopen, but not in IgE, and this means that for basic drug development we will have to consider an across-the-board treatment by attempting to modify or modulate Fc*RII-CD23 or perhaps even the high-affinity receptor IgE-Fc*RI. This, indeed, represents a purely biochemical approach to drug design, for which the term "vaccine technology" is entirely our of order.
Fig. 2--Múltiple interactions between IgE, Fc*RII and CR2. a, The interaction between human Fc*RII and IgE. Two lectin-like head regions of membrane-bound Fc*RII combine with the two C*3 domains of IgE. b, interaction of trimetric 37K sFc*RII with membrane-bound IgE. The "free" head may bind to another IgE molecule, or other cell surface molecule, such as CR2. CR2 consists of a tandem array of SCR domains (refer to text), some of which are glycosylated; the locations of these sites are shown schematically. Fc*RII may bind in the region of domain 5 (refer to text) and involve the recognition of carbohydrate. Crosslinking mlgE and CR2 (which forms a signal transduction complex with CD19; on the surface of a committed B cell may provide a mechanism for specific activation of IgE synthesis by Fc*RII. (Molecules and cell membrane are drawn to scale).
RESUMEN
El mecanismo inmunológico por el que actúa la inmunoterapia (IT) no se conoce con certeza. No está demostrada la participación de los llamados "anticuerpos bloqueantes", y aún está por aclarar en su totalidad el modo cómo interviene en la regulación de la producción de IgE, al incidir en la acción de los linfocitos Th1 y Th2. En la actualidad, la IT se basa en el concepto de que la reacción alérgica, en cierto modo, es una reacción antígeno (alergeno)-anticuerpo (reagina). Las posibles modificaciones de la IT se basan igualmente en la posibilidad de interferir la interacción antígeno-anticuerpo.
Está demostrado in vitro que extractos acuosos de algunos alergenos, dan lugar a un consumo de complemento, por la vía clásica, en lo que está implicado el componente C1, sin que haya mediación de anticuerpos, pudiéndose generar anafilotoxina C3a, que es un poderoso liberador de histamina, así como C3b, que participa en la regulación del sistema inmunitario celular.
El término atopeno se referiría a la activación inespecífica o actividad adyuvante de alergenos antigénicamente distintos, por sus características estructurales o funcionales comunes. El término alergeno se reservaría para descibir aquellos rasgos estructurales de la molécula transportadora, que da lugar preferentemente a la inducción, y posible reconocimiento, de anticuerpos de la clase IgE. La acción atopeno y alergeno como características separadas de una misma molécula, teóricamente puede tener importancia en el futuro de la terapéutica.
El receptor de membrana CD21 para el C3b, de las células B es un ligando para la lectina CD23 de los linfocitos B, el cual a su vez se ha identificado con el receptor de baja afinidad para la IgE (Fc* RII-CD23). En resumen, el defecto básico de la atopia podría residir en un defecto de la porción del receptor de reagina, para el atopeno, y no pra la IgE. De ahí, que para un futuro, debería tenerse en cuenta este hecho para la búsqueda de medicamentos que intervengan en este modo de activación de la reacción alérgica, intentado modificar o modular el receptor de baja afinidad e incluso el de alta afinidad para la IgE.
Palabras clave: Alergeno. Atopeno. Inmunoterapia. IgE. Receptor IgE. Inmunomodulación.
REFERENCES
1. WHO Position Papel. Allergen immunotherapy: Therapeutic vaccines for allergic diseases. Draft 7. Geneva, January 27-29, 1997.
2. Lichtenstein LM. Scientific American 1993;269:116-24.
3. Sherman WB. Reaginic and blocking antibodies. J Allergy 1957;28:72-4.
4. Van Neerven RJJ, Ebner C, Yssel H, Kapsenberb ML, Lamb JR. T-cell responses to allergens: epitope-specificity and clinical relevance. Immunology Today 1996;17:526-32.
5. Shakib F (ed). Immunological intervention strategies in allegy. Biochem Soc Trans 1997;25:383-403.
6. Wheeler AW, Drachenberg KJ. New routes and formulations for allergen-specific immunotherapy. Allergy 1997;52:602-12.
7. Berrens L, De la Cuadra López B. Complement activating agents in allergenic extracts. Inflammation Research 1997;46:455-60.
8. Gönczi S, Varga L, Hidvégi T, Schmidt B, Pánya A, Kokai M, Füst G. The severity of clinical symptoms in ragweed-allergic patients is related to the extent of ragweed-induced complement activation in their sera. Allergy 1997;52:1110-4.
9. Berrens L. The atopen: a rehabilitation. Ann Allergy 1976;36:351-61.
10. Sutton BJ, Gould HJ. The human IgE network. Nature 1993;366:421-8.
11. Helm BA, Spivey AC, Padlan EA. Peptide blocking of IgE/receptor interaction: possibilities and pitfalls. Allergy 1997;52-1155-69.